The Link between Soil Geochemistry in South-West England and Human Exposure to Soil Arsenic
Abstract
:1. Introduction
- (i)
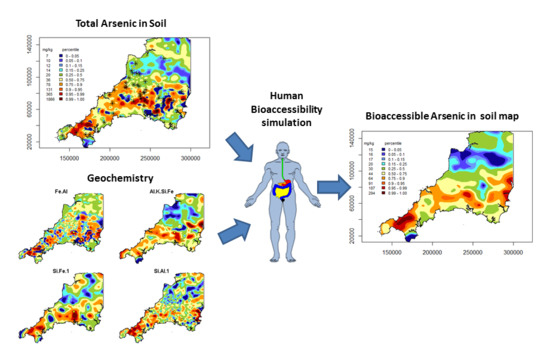
- To use the whole soil geochemistry data and selected bioaccessibility measurements on the same soils to identify the geochemical controls on As bioaccessibility;
- (ii)
- To use regression modelling, with derived data from the whole soil geochemistry as predictor variables and As bioaccessibility as the dependant variables and to predict As bioaccessibility at all soil locations to examine its regional spatial distribution.
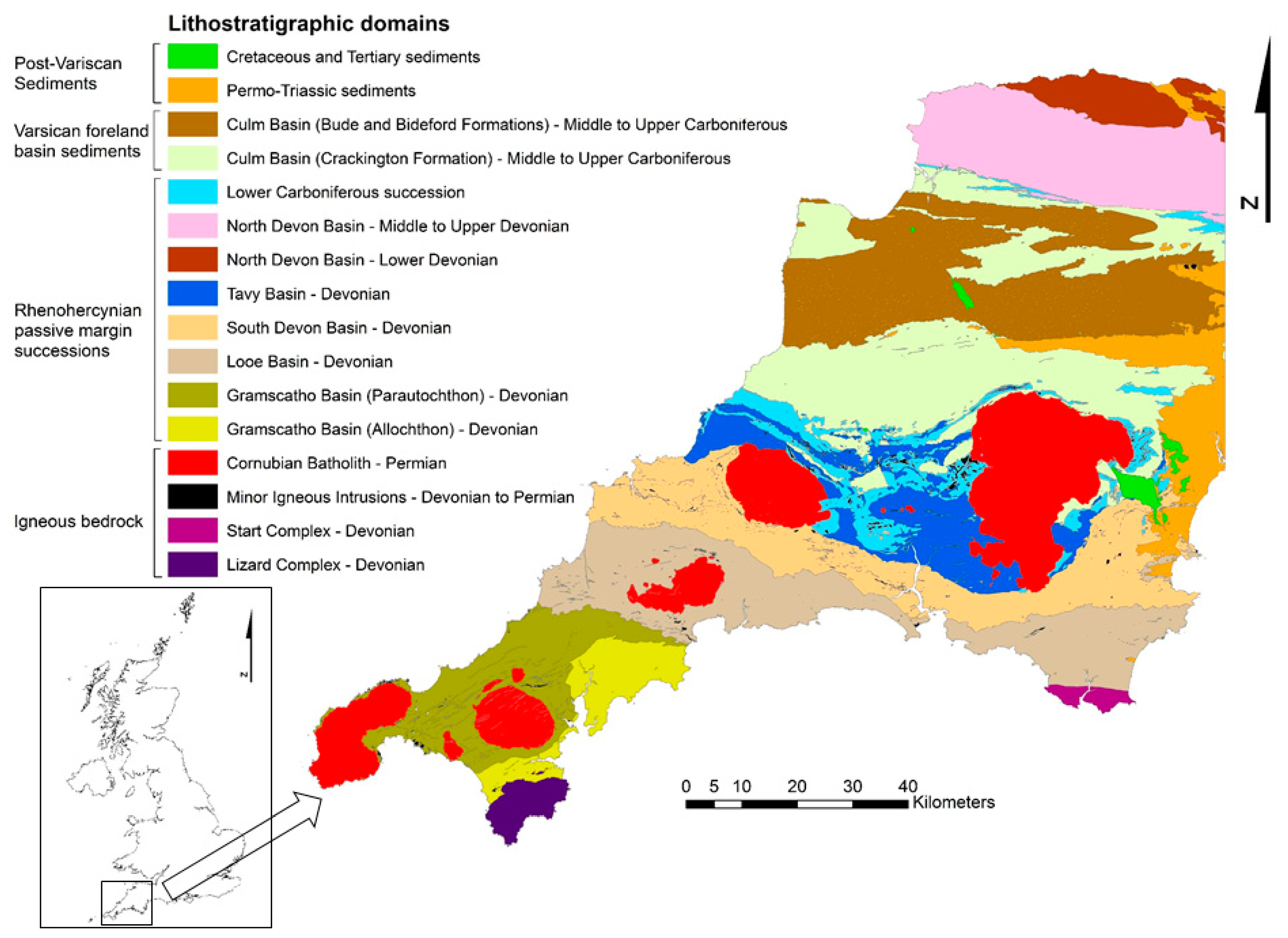
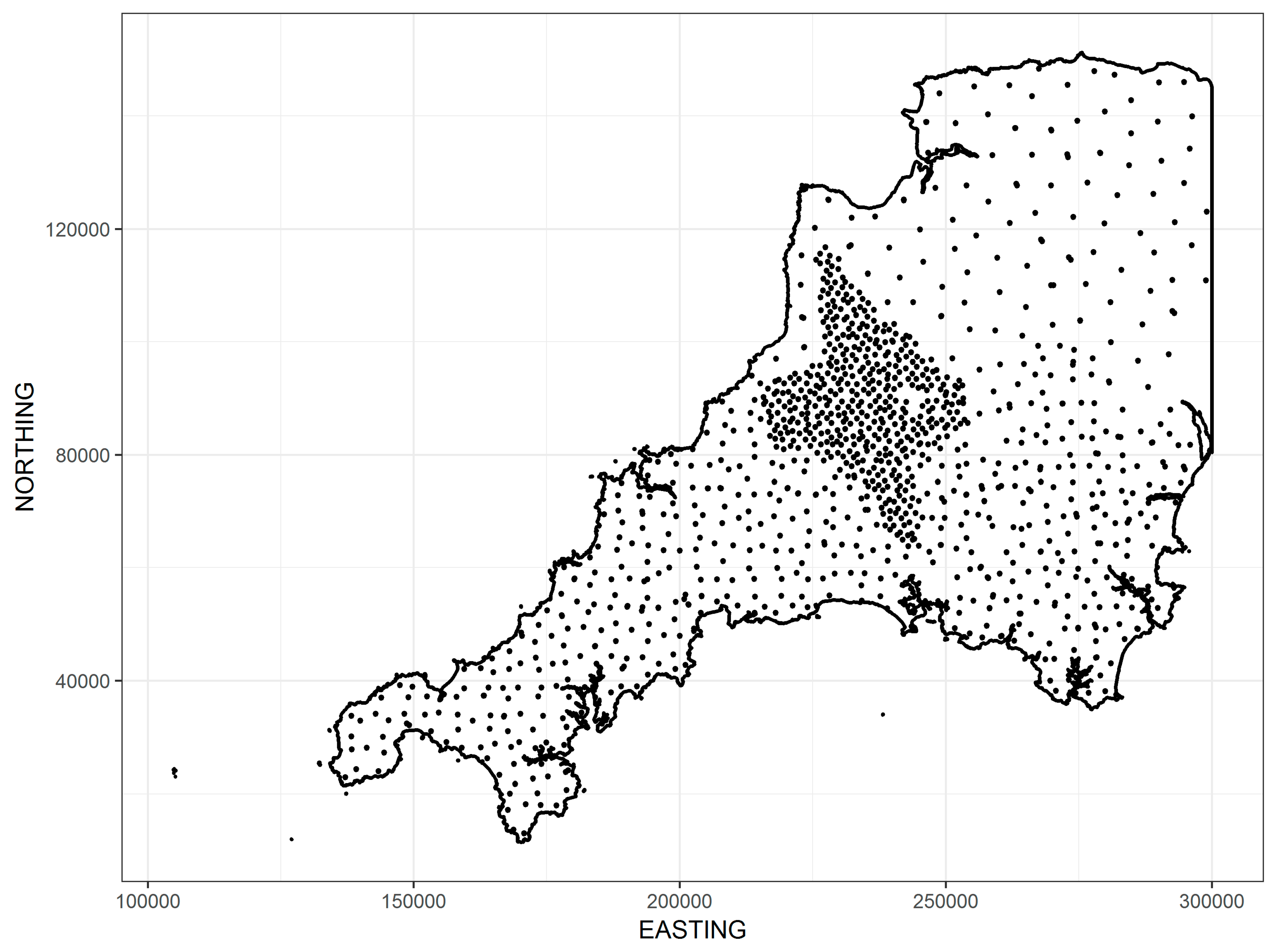
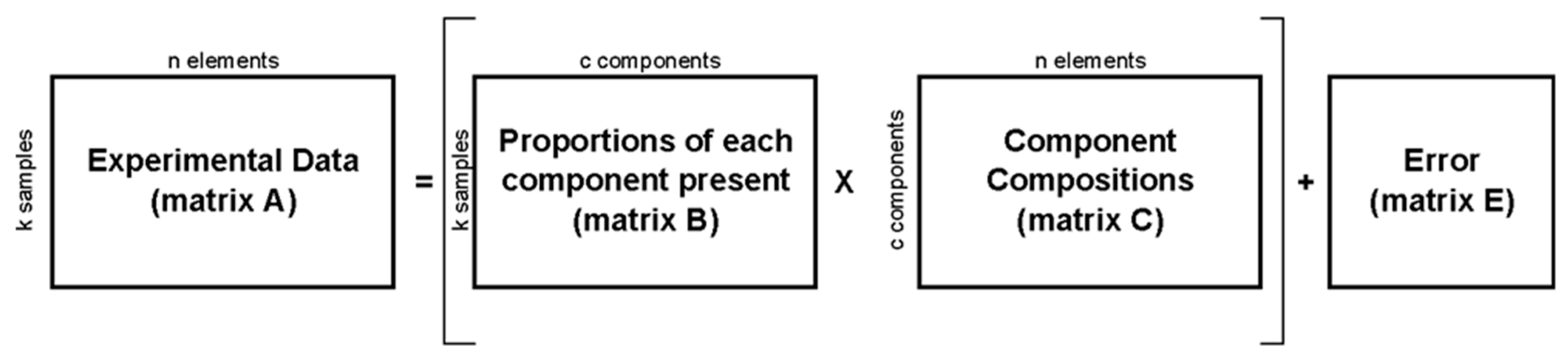
2. Materials and Methods
3. Results
3.1. Total As Concentrations
3.2. Bioaccessible As Concentrations
4. Discussion
4.1. Intrinsic Soil Constituents
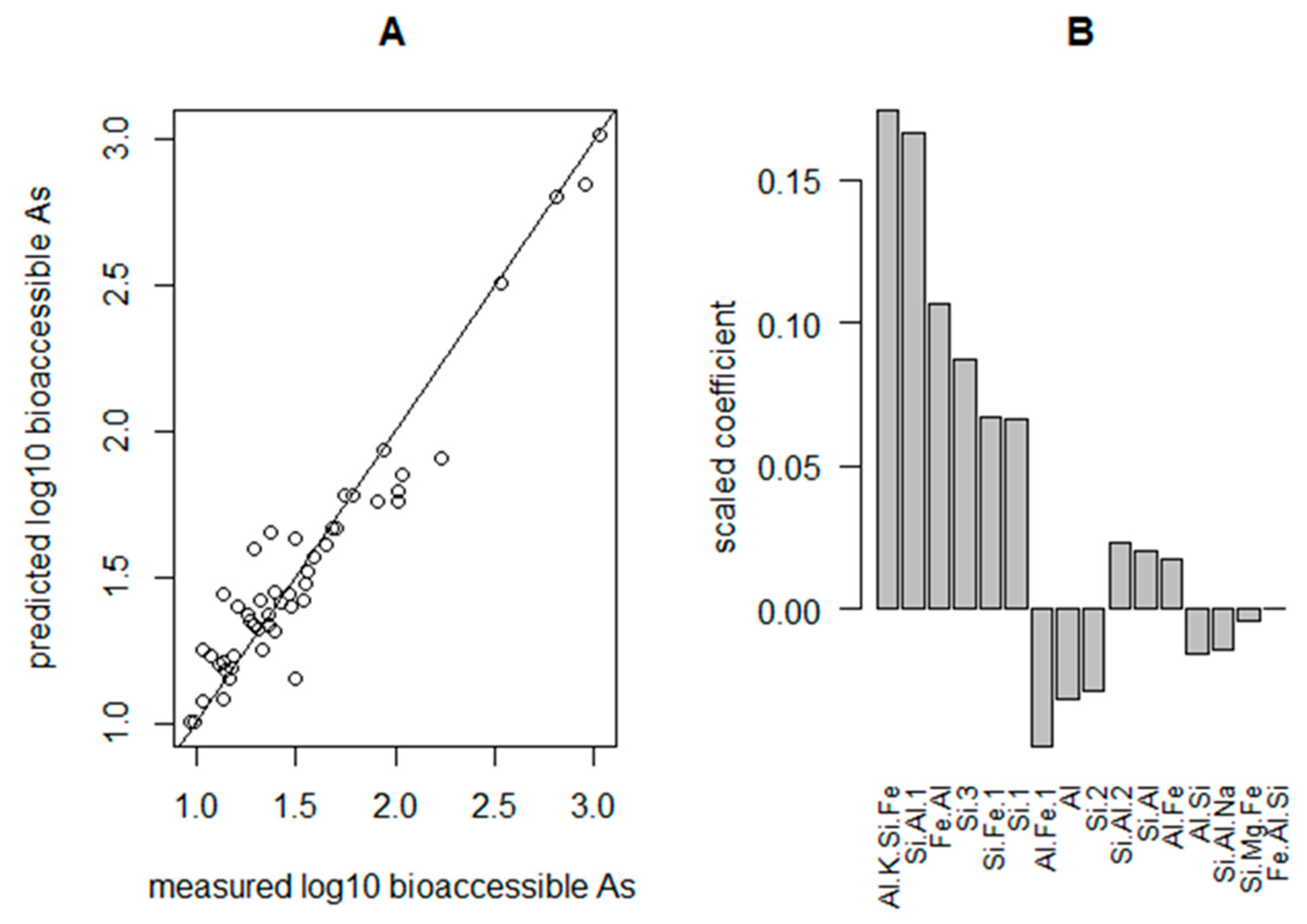
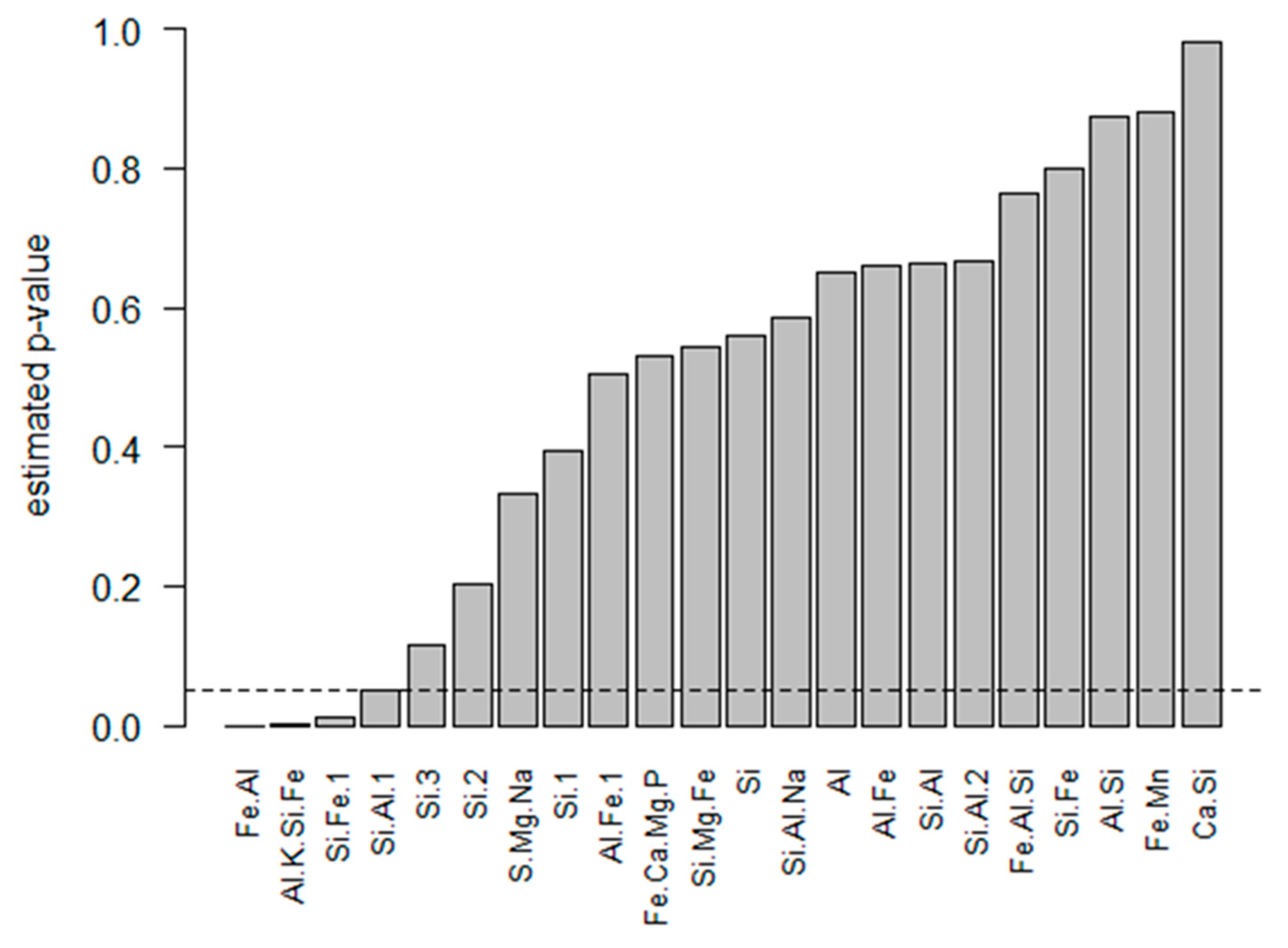
4.2. Regression Modelling of Bioaccessible As
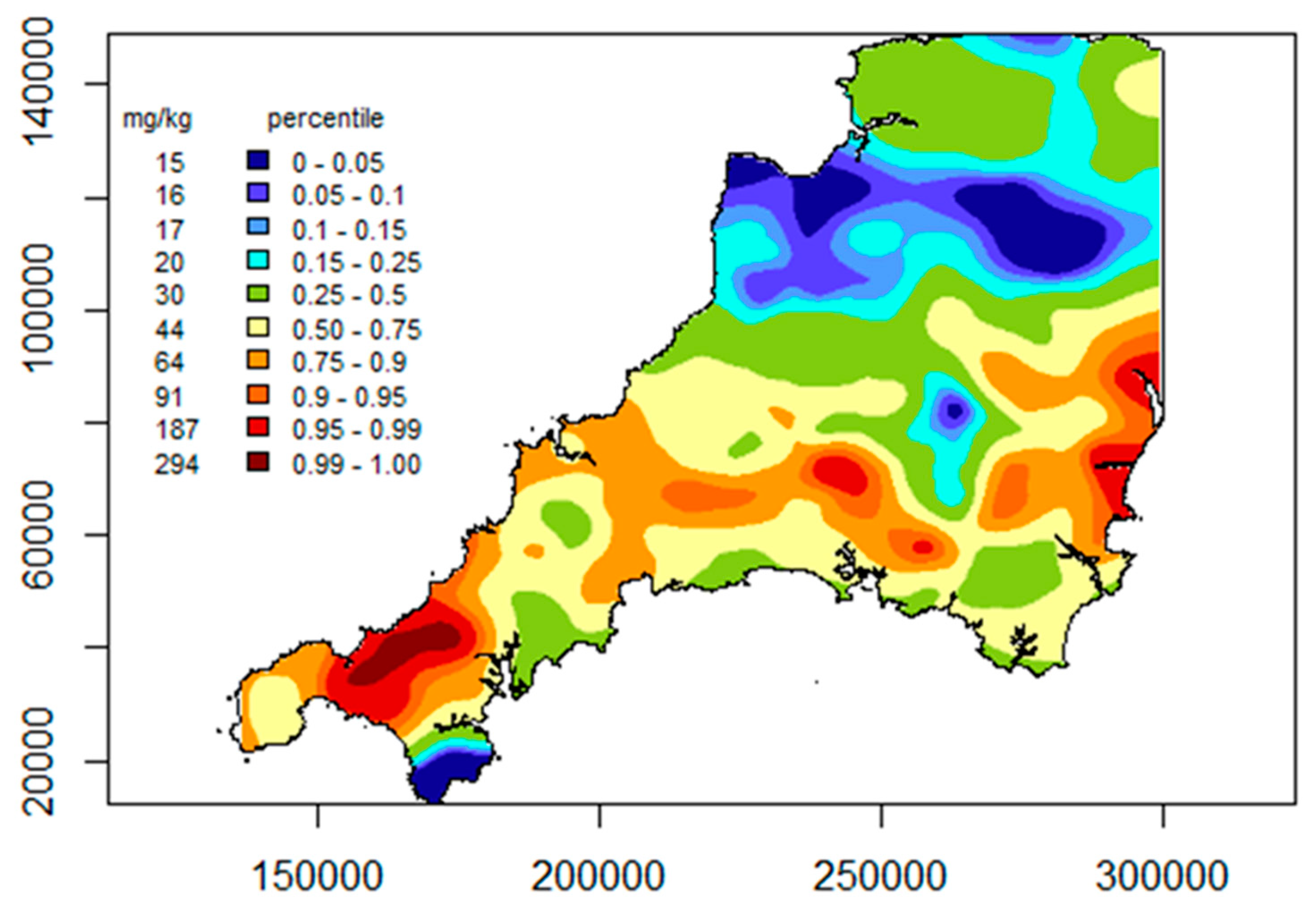
4.3. Bioaccessible Arsenic Prediction
- (i)
- The regression model only looks at linear relationships and does not take into account interaction effects;
- (ii)
- The regression model does not take into account spatial correlation;
- (iii)
- The thin plate spline is not a full spatial model which takes into account the actual spatial variability in the data (e.g., as measured by a variogram [94]);
- (iv)
- No attempts have been made to quantify the overall uncertainty in the predicted values.
5. Conclusions
- (i)
- Taking a representative selection of soil samples, based on their geochemistry, from the total geochemical survey;
- (ii)
- Measuring the bioaccessibility for the element(s) under study in the selected samples using a robust, validated and well documented method [79];
- (iii)
- Establishing a predictive relationship between the bioaccessible element concentration and the total geochemical composition of the soil using a suitable modelling method;
- (iv)
- Predicting the bioaccessible element concentration for all of the soil samples;
- (v)
- Spatially modelling or interpolating the predicted bioaccessible element concentrations over the region covered by the geochemical soil survey.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dines, H.G. The Metalliferous Mining Region of South-West England, 1st ed.; HMSO: London, UK, 1956.
- Kirkwood, C.; Everett, P.; Ferreira, A.; Lister, B. Stream sediment geochemistry as a tool for enhancing geological understanding: An overview of new data from south west England. J. Geochem. Explor. 2016, 163, 28–40. [Google Scholar] [CrossRef] [Green Version]
- ATSDR. Toxicological Profile for Lead; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007.
- ATSDR. Toxicological Profile for Arsenic; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007.
- Palumbo-Roe, B.; Klinck, B. Bioaccessibility of arsenic in mine waste-contaminated soils: A case study from an abandoned arsenic mine in SW England (UK). J. Environ. Sci. Health Part A Toxic Hazard. Subst. Environ. Eng. 2007, 42, 1251–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghasemi, A.; Manesh, S.M.T.; Tabatabaei, S.H.; Mokhtari, A.R. Geoenvironmental studies and heavy metal mapping in soil: The case of Ghohroud area, Iran. Environ. Earth Sci. 2015, 74, 5221–5232. [Google Scholar] [CrossRef]
- González-Corrochano, B.; Esbrí, J.; Alonso-Azcárate, J.; Martínez-Coronado, A.; Jurado, V.; Higueras, P. Environmental geochemistry of a highly polluted area: The La Union Pb–Zn mine (Castilla-La Mancha region, Spain). J. Geochem. Explor. 2014, 144, 345–354. [Google Scholar] [CrossRef]
- Kasimov, N.; Kosheleva, N.; Gunin, P.; Korlyakov, I.; Sorokina, O.; Timofeev, I. State of the environment of urban and mining areas in the Selenga Transboundary River Basin (Mongolia Russia). Environ. Earth Sci. 2016, 75, 1283. [Google Scholar] [CrossRef]
- Khorasanipour, M.; Esmaeilzadeh, E. Environmental characterization of Sarcheshmeh Cu-smelting slag, Kerman, Iran: Application of geochemistry, mineralogy and single extraction methods. J. Geochem. Explor. 2016, 166, 1–17. [Google Scholar] [CrossRef]
- Levitan, D.M.; Zipper, C.E.; Donovan, P.; Schreiber, M.E.; Seal, R.R.; Engle, M.A.; Chermak, J.A.; Bodnar, R.J.; Johnson, D.K.; Aylor, J.G. Statistical analysis of soil geochemical data to identify pathfinders associated with mineral deposits: An example from the Coles Hill uranium deposit, Virginia, USA. J. Geochem. Explor. 2015, 154, 238–251. [Google Scholar] [CrossRef]
- Salminen, R.; Gregorauskiene, V. Considerations regarding the definition of a geochemical baseline of elements in the surficial materials in areas differing in basic geology. Appl. Geochem. 2000, 15, 647–653. [Google Scholar] [CrossRef]
- Salminen, R.; Tarvainen, T. The problem of defining geochemical baselines. A case study of selected elements and geological materials in Finland. J. Geochem. Explor. 1997, 60, 91–98. [Google Scholar] [CrossRef]
- Caritat, P.; Reimann, C.; Team, N.P.; Team, G.P. Comparing results from two continental geochemical surveys to world soil composition and deriving Predicted Empirical Global Soil (PEGS2) reference values. Earth Planet. Sci. Lett. 2012, 319, 269–276. [Google Scholar] [CrossRef]
- Fordyce, F.M.; Brown, S.E.; Ander, E.L.; Rawlins, B.G.; O’Donnell, K.E.; Lister, T.R.; Breward, N.; Johnson, C.C. GSUE: Urban geochemical mapping in Great Britain. Geochemistry 2005, 5, 325–336. [Google Scholar] [CrossRef]
- Galan, E.; Fernandez-Caliani, J.C.; Gonzalez, I.; Aparicio, P.; Romero, A. Influence of geological setting on geochemical baselines of trace elements in soils. Application to soils of South-West Spain. J. Geochem. Explor. 2008, 98, 89–106. [Google Scholar] [CrossRef]
- Glennon, M.; Harris, P.; Ottesen, R.; Scanlon, R.; O’connor, P. The Dublin SURGE Project: Geochemical baseline for heavy metals in topsoils and spatial correlation with historical industry in Dublin, Ireland. Environ. Geochem. Health 2014, 36, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.C.; Ander, E.L. Urban geochemical mapping studies: How and why we do them. Environ. Geochem. Health 2008, 30, 511–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludden, J.; Peach, D.; Flight, D. Geochemically based solutions for urban society: London, a case study. Elements 2015, 11, 253–258. [Google Scholar] [CrossRef]
- Sierra, M.; Martinez, F.J.; Aguilar, J. Baselines for trace elements and evaluation of environmental risk in soils of Almeria (SE Spain). Geoderma 2007, 139, 209–219. [Google Scholar] [CrossRef]
- Reimann, C.; Birke, M.; Demetriades, A.; Filzmoser, P.; O’Connor, P. Chemistry of Europe’s Agricultural Soils—Part B: General Background Information and Further Analysis of the GEMAS Data Set; E. Schweizerbart Science Publishers: Stuttgart, Germany, 2014. [Google Scholar]
- Reimann, C.; Birke, M.; Demetriades, A.; Filzmoser, P.; O’Connor, P. Chemistry of Europe’s Agricultural Soils, Part A. Environ. Earth Sci. 2014, 72, 3239–3242. [Google Scholar]
- Garrett, R.G. Relative spatial soil geochemical variability along two transects across the United States and Canada. Appl. Geochem. 2009, 24, 1405–1415. [Google Scholar] [CrossRef]
- Eze, P.N.; Knight, J.; Evans, M. Tracing recent environmental changes and pedogenesis using geochemistry and micromorphology of alluvial soils, Sabie-Sand River Basin, South Africa. Geomorphology 2016, 268, 312–321. [Google Scholar] [CrossRef]
- Plant, J.; Baldock, J.; Haslam, H.; Smith, B. The role of geochemistry in environmental and epidemiological studies in developing countries. Episodes 1998, 21, 19–27. [Google Scholar] [CrossRef]
- Gamino-Gutierrez, S.P.; Gonzalez-Perez, C.I.; Gonsebatt, M.E.; Monroy-Fernandez, M.G. Arsenic and lead contamination in urban soils of Villa de la Paz (Mexico) affected by historical mine wastes and its effect on children’s health studied by micronucleated exfoliated cells assay. Environ. Geochem. Health 2013, 35, 37–51. [Google Scholar] [CrossRef]
- Hooker, P.J.; Nathanail, C.P. Risk-based characterisation of lead in urban soils. Chem. Geol. 2006, 226, 340–351. [Google Scholar] [CrossRef]
- Morrison, S.; Fordyce, F.; Scott, E.M. An initial assessment of spatial relationships between respiratory cases, soil metal content, air quality and deprivation indicators in Glasgow, Scotland, UK: Relevance to the environmental justice agenda. Environ. Geochem. Health 2014, 36, 319–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, D.; Johnson, C.C. Distribution of iodine in soils of Northern Ireland. Geochemistry 2011, 11, 25. [Google Scholar] [CrossRef]
- Wylie, C.; Shaw, D.; Fordyce, F.; Lilly, A.; McGorum, B. Equine grass sickness in S cotland: A case–control study of signalment-and meteorology-related risk factors. Equine Vet. J. 2014, 46, 64–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E. Portrait of the South West. Reg. Trends 2010, 42, 43–59. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J.; Stanek, E.J.; Barnes, R. Soil ingestion rates in children identified by parental observation as likely high soil ingesters. J. Soil Contam. 1997, 6, 271–279. [Google Scholar] [CrossRef]
- Swartjes, A. Dealing with Contaminated Sites: From Theory towards Practical Application; Springer: London, UK, 2011. [Google Scholar]
- Denys, S.; Caboche, J.; Tack, K.; Rychen, G.; Wragg, J.; Cave, M.; Jondreville, C.; Feidt, C. In Vivo Validation of the Unified BARGE Method to Assess the Bioaccessibility of Arsenic, Antimony, Cadmium, and Lead in Soils. Environ. Sci. Technol. 2012, 46, 6252–6260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basta, N.T.; Scheckel, K.G.; Bradham, K.D.; Richey, J.S.; Dayton, E.A.; Whitacre, S.W.; Casteel, S.W. Soil Chemical Controls on Arsenic Bioaccessibility and Bioavailability. Epidemiology 2008, 19, S29. [Google Scholar]
- Juhasz, A.L.; Weber, J.; Smith, E. Impact of soil particle size and bioaccessibility on children and adult lead exposure in pen-urban contaminated soils. J. Hazard. Mater. 2011, 186, 1870–1879. [Google Scholar] [CrossRef]
- Juhasz, A.L.; Weber, J.; Smith, E.; Naidu, R.; Marschner, B.; Rees, M.; Rofe, A.; Kuchel, T.; Sansom, L. Evaluation of SBRC-Gastric and SBRC-Intestinal Methods for the Prediction of In Vivo Relative Lead Bioavailability in Contaminated Soils. Environ. Sci. Technol. 2009, 43, 4503–4509. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, K.; Cave, M.; Li, H.-B.; Ma, L.Q. Lead bioaccessibility in 12 contaminated soils from China: Correlation to lead relative bioavailability and lead in different fractions. J. Hazard. Mater. 2015, 295, 55–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowney, Y.W.; Ruby, M.V.; Salatas, J.; Pastorok, R. The relative bioavailability of metals from soil to ecological receptors. Toxicol. Sci. 2003, 72, 398–399. [Google Scholar]
- Oomen, A.G.; Hack, A.; Minekus, M.; Zeijdner, E.; Cornelis, C.; Schoeters, G.; Verstraete, W.; Van de Wiele, T.; Wragg, J.; Rompelberg, C.J.M.; et al. Comparison of five in vitro digestion models to study the bioaccessibility of soil contaminants. Environ. Sci. Technol. 2002, 36, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Van de Wiele, T.R.; Oomen, A.G.; Wragg, J.; Cave, M.; Minekus, M.; Hack, A.; Cornelis, C.; Rompelberg, C.J.M.; De Zwart, L.L.; Klinck, B.; et al. Comparison of five in vitro digestion models to in vivo experimental results: Lead bioaccessibility in the human gastrointestinal tract. J. Environ. Sci. Health. Part A 2007, 42, 1203–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wragg, J.; Cave, M.; Basta, N.; Brandon, E.; Casteel, S.; Denys, S.; Gron, C.; Oomen, A.; Reimer, K.; Tack, K.; et al. An inter-laboratory trial of the unified BARGE bioaccessibility method for arsenic, cadmium and lead in soil. Sci. Total Environ. 2011, 409, 4016–4030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basta, N.T.; Juhasz, A. Using In Vivo Bioavailability and/or In Vitro Gastrointestinal Bioaccessibility Testing to Adjust Human Exposure to Arsenic from Soil Ingestion. In Arsenic: Environmental Geochemistry, Mineralogy, and Microbiology, Proceedings of the 24th Annual V.M. Goldschmidt Conference and held at the Miners Foundry, Nevada City, CA, USA, 15–16 June 2014; Bowell, R.J., Alpers, C.N., Jamieson, H.E., Nordstrom, D.K., Majzlan, J., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 2014; Volume 79, pp. 451–472. [Google Scholar]
- Cave, M.; Wragg, J.; Gowing, C.; Gardner, A. Measuring the solid-phase fractionation of lead in urban and rural soils using a combination of geochemical survey data and chemical extractions. Environ. Geochem. Health 2015, 37, 779–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, S.; Chelliah, M.M.; McKinley, J.; Palmer, S.; Ofterdinger, U.; Young, M.; Cave, M.; Wragg, J. The importance of solid-phase distribution on the oral bioaccessibility of Ni and Cr in soils overlying Palaeogene basalt lavas, Northern Ireland. Environ. Geochem. Health 2013, 35, 553–567. [Google Scholar] [CrossRef] [Green Version]
- Karadas, C.; Kara, D. Chemometric evaluation for the relation of BCR sequential extraction method and in vitro gastro-intestinal method for the assessment of metal bioavailability in contaminated soils in Turkey. Environ. Sci. Pollut. Res. 2012, 19, 1280–1295. [Google Scholar] [CrossRef]
- Li, S.W.; Li, J.; Li, H.B.; Naidu, R.; Ma, L.Q. Arsenic bioaccessibility in contaminated soils: Coupling in vitro assays with sequential and HNO3 extraction. J. Hazard. Mater. 2015, 295, 145–152. [Google Scholar] [CrossRef]
- Luo, X.S.; Yu, S.; Li, X.D. The mobility, bioavailability, and human bioaccessibility of trace metals in urban soils of Hong Kong. Appl. Geochem. 2012, 27, 995–1004. [Google Scholar] [CrossRef]
- Palumbo-Roe, B.; Wragg, J.; Cave, M. Linking selective chemical extraction of iron oxyhydroxides to arsenic bioaccessibility in soil. Environ. Pollut. 2015, 207, 256–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patinha, C.; Reis, A.P.; Dias, C.; Cachada, A.; Adao, R.; Martins, H.; da Silva, E.F.; Sousa, A.J. Lead availability in soils from Portugal’s Centre Region with special reference to bioaccessibility. Environ. Geochem. Health 2012, 34, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Pelfrene, A.; Waterlot, C.; Mazzuca, M.; Nisse, C.; Bidar, G.; Douay, F. Assessing Cd, Pb, Zn human bioaccessibility in smelter-contaminated agricultural topsoils (northern France). Environ. Geochem. Health 2011, 33, 477–493. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.P.; Patinha, C.; Wragg, J.; Dias, A.C.; Cave, M.; Sousa, A.J.; Costa, C.; Cachada, A.; da Silva, E.F.; Rocha, F.; et al. Geochemistry, mineralogy, solid-phase fractionation and oral bioaccessibility of lead in urban soils of Lisbon. Environ. Geochem. Health 2014, 36, 867–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, E.C., III; Shi, H.; Wronkiewicz, D.J.; Pavlowsky, R.T. Phase partitioning and bioaccessibility of Pb in suspended dust from unsurfaced roads in Missouri—A potential tool for determining mitigation response. Atmos. Environ. 2014, 88, 90–98. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.R. Assessment of a geochemical extraction procedure to determine the solid phase fractionation and bioaccessibility of potentially harmful elements in soils: A case study using the NIST 2710 reference soil. Anal. Chim. Acta 2012, 722, 43–54. [Google Scholar] [CrossRef]
- Kelepertzis, E.; Stathopoulou, E. Availability of geogenic heavy metals in soils of Thiva town (central Greece). Environ. Monit. Assess. 2013, 185, 9603–9618. [Google Scholar] [CrossRef]
- Pascaud, G.; Leveque, T.; Soubrand, M.; Boussen, S.; Joussein, E.; Dumat, C. Environmental and health risk assessment of Pb, Zn, As and Sb in soccer field soils and sediments from mine tailings: Solid speciation and bioaccessibility. Environ. Sci. Pollut. Res. 2014, 21, 4254–4264. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Zhang, Y.; Liu, F.; Wang, J.; Hou, Q. Changes of arsenic fractionation and bioaccessibility in wastewater-irrigated soils as a function of aging: Influence of redox condition and arsenic load. Geoderma 2016, 280, 1–7. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Hao, X.W. Effect of red mud addition on the fractionation and bio-accessibility of Pb, Zn and As in combined contaminated soil. Chem. Ecol. 2012, 28, 37–48. [Google Scholar] [CrossRef]
- Im, J.; Yang, K.; Jho, E.H.; Nam, K. Effect of different soil washing solutions on bioavailability of residual arsenic in soils and soil properties. Chemosphere 2015, 138, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Jelusic, M.; Lestan, D. Effect of EDTA washing of metal polluted garden soils. Part I: Toxicity hazards and impact on soil properties. Sci. Total Environ. 2014, 475, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Reijonen, I.; Metzler, M.; Hartikainen, H. Impact of soil pH and organic matter on the chemical bioavailability of vanadium species: The underlying basis for risk assessment. Environ. Pollut. 2016, 210, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zagury, G.J.; Bello, J.A.R.; Guney, M. Valorization of a treated soil via amendments: Fractionation and oral bioaccessibility of Cu, Ni, Pb, and Zn. Environ. Monit. Assess. 2016, 188, 222. [Google Scholar] [CrossRef]
- Appleton, J.D.; Cave, M.R.; Palumbo-Roe, B.; Wragg, J. Lead bioaccessibility in topsoils from lead mineralisation and urban domains, UK. Environ. Pollut. 2013, 178, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Malone, B.P.; Jha, S.K.; Minasny, B.; McBratney, A.B. Comparing regression-based digital soil mapping and multiple-point geostatistics for the spatial extrapolation of soil data. Geoderma 2016, 262, 243–253. [Google Scholar] [CrossRef]
- Jean-Soro, L.; Le Guern, C.; Bechet, B.; Lebeau, T.; Ringeard, M.F. Origin of trace elements in an urban garden in Nantes, France. J. Soils Sediments 2015, 15, 1802–1812. [Google Scholar] [CrossRef]
- Percival, J.B.; White, H.P.; Goodwin, T.A.; Parsons, M.B.; Smith, P.K. Mineralogy and spectral reflectance of soils and tailings from historical gold mines, Nova Scotia. Geochem. Explor. Environ. Anal. 2014, 14, 3–16. [Google Scholar] [CrossRef]
- Palmer, S.; Ofterdinger, U.; McKinley, J.M.; Cox, S.; Barsby, A. Correlation analysis as a tool to investigate the bioaccessibility of nickel, vanadium and zinc in Northern Ireland soils. Environ. Geochem. Health 2013, 35, 569–584. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yang, F.; Wei, C. Factors influencing the heavy metal bioaccessibility in soils were site dependent from different geographical locations. Environ. Sci. Pollut. Res. 2015, 22, 13939–13949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelfrene, A.; Detriche, S.; Douay, F. Combining spatial distribution with oral bioaccessibility of metals in smelter-impacted soils: Implications for human health risk assessment. Environ. Geochem. Health 2015, 37, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Cave, M.R.; Wragg, J.; Harrison, H. Measurement modelling and mapping of arsenic bioaccessibility in Northampton, UK. J.Environ. Sci. Health Part A 2013, 48, 629–640. [Google Scholar] [CrossRef] [PubMed]
- British Geological Survey, G-BASE for South West England. Available online: http://www.bgs.ac.uk/gbase/gBaseSW.html (accessed on 18 October 2018).
- Flight, D.M.A.; Scheib, A.J. Soil Geochemical Baselines in UK Urban centres: The G-BASE Project. In Mapping the Chemical Environment of Urban Areas; Johnson, C., Demetriades, A., Locutura, J., Ottesen, R.T., Eds.; Wiley-Blackwell: Oxford, UK, 2011; pp. 186–206. [Google Scholar]
- Rawlins, B.; O’Donnell, K.; Ingham, M. Geochemical Survey of the Tamar Catchment (South-West England); CR/03/027; British Geological Survey: Nottingham, UK, 2003. [Google Scholar]
- Johnson, C.C.; Breward, N.; Ander, E.L.; Ault, L. G-BASE: Baseline geochemical mapping of Great Britain and Northern Ireland. Geochemistry 2005, 5, 347–357. [Google Scholar] [CrossRef]
- Qin, J.H.; Nworie, O.E.; Lin, C.X. Particle size effects on bioaccessible amounts of ingestible soil-borne toxic elements. Chemosphere 2016, 159, 442–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duggan, M.J.; Inskip, M.J.; Rundle, S.A.; Moorcroft, J.S. Lead in Playground Dust and on the Hands of Schoolchildren. Sci. Total Environ. 1985, 44, 65–79. [Google Scholar] [CrossRef]
- USEPA. Test Methods for Evaluating Solid Waste; SW-846; U.S. Environmental Protection Agency: Washington, DC, USA, 2000.
- Middleton, D.R.; Watts, M.J.; Beriro, D.J.; Hamilton, E.M.; Leonardi, G.S.; Fletcher, T.; Close, R.M.; Polya, D.A. Arsenic in residential soil and household dust in Cornwall, South West England: Potential human exposure and the influence of historical mining. Environ. Sci. 2017, 19, 517–527. [Google Scholar] [CrossRef]
- Watts, M.J.; Button, M.; Brewer, T.S.; Jenkin, G.R.; Harrington, C.F. Quantitative arsenic speciation in two species of earthworms from a former mine site. J. Environ. Monit. 2008, 10, 753–759. [Google Scholar] [CrossRef]
- ISO. Assessment of human exposure from ingestion of soil. In Soil Quality; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Appleton, J.D.; Cave, M.R.; Wragg, J. Anthropogenic and geogenic impacts on arsenic bioaccessibility in UK topsoils. Sci. Total Environ. 2012, 435, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Wragg, J.; Cave, M.; Nathanail, P. A Study of the relationship between arsenic bioaccessibility and its solid-phase distribution in soils from Wellingborough, UK. J. Environ. Sci. Health. Part A 2007, 42, 1303–1315. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Schwarz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Nychka, D.; Furrer, R.; Paige, J.; Sain, S. Fields: Tools for Spatial Data; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Bauer, D.F. Constructing Confidence Sets Using Rank Statistics. J. Am. Stat. Assoc. 1972, 67, 687–690. [Google Scholar] [CrossRef]
- Ander, E.L.; Johnson, C.C.; Cave, M.R.; Palumbo-Roe, B.; Nathanail, C.P.; Lark, R.M. Methodology for the determination of normal background concentrations of contaminants in English soil. Sci. Total Environ. 2013, 454–455, 604–618. [Google Scholar] [CrossRef] [PubMed]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Wainwright, M. Statistical Learning with Sparsity: The Lasso and Generalizations; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Rollin, K.; Gunn, A.; Scrivener, R.; Shaw, M. Potential for Stratiform Massive Sulphide Mineralisation in South-West England; British Geological Survey: Nottingham, UK, 2001. [Google Scholar]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides—Structure Properties, Reactions, Occurences and Uses; VCH Publishers: Weinheim, Germany, 1996. [Google Scholar]
- Ghorbanzadeh, N.; Jung, W.; Halajnia, A.; Lakzian, A.; Kabra, A.N.; Jeon, B.-H. Removal of arsenate and arsenite from aqueous solution by adsorption on clay minerals. Geosyst. Eng. 2015, 18, 302–311. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Van Der Weiden, M.J.J.; Tournassat, C.; Charlet, L. Surface Complexation of Ferrous Iron and Carbonate on Ferrihydrite and the Mobilization of Arsenic. Environ. Sci. Technol. 2002, 36, 3096–3103. [Google Scholar] [CrossRef]
- Rawlins, B.G.; Lark, R.M.; O’Donnell, K.E.; Tye, A.M.; Lister, T.R. The assessment of point and diffuse metal pollution of soils from an urban geochemical survey of Sheffield, England. Soil Use Manag. 2005, 21, 353–362. [Google Scholar] [CrossRef]

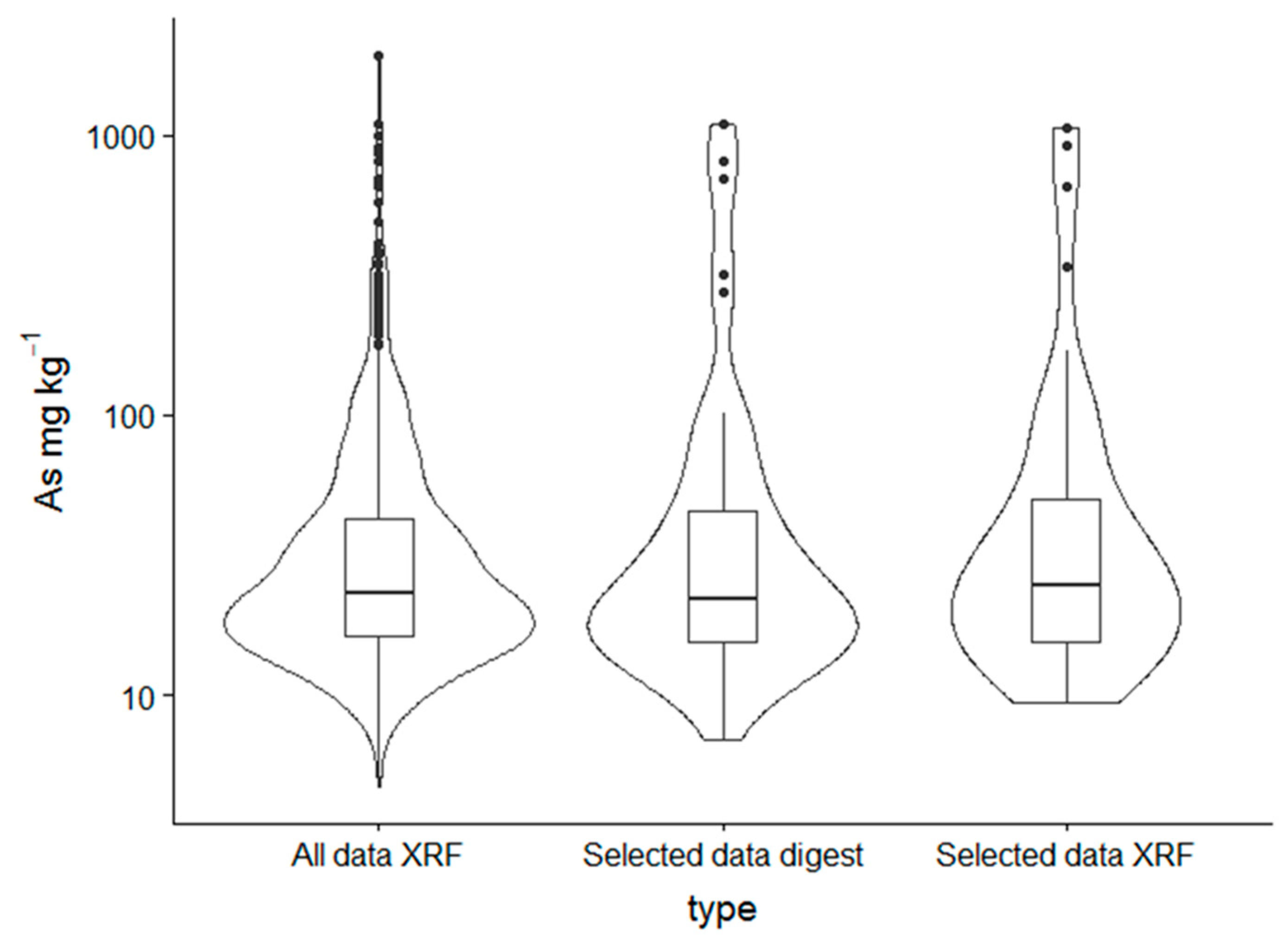
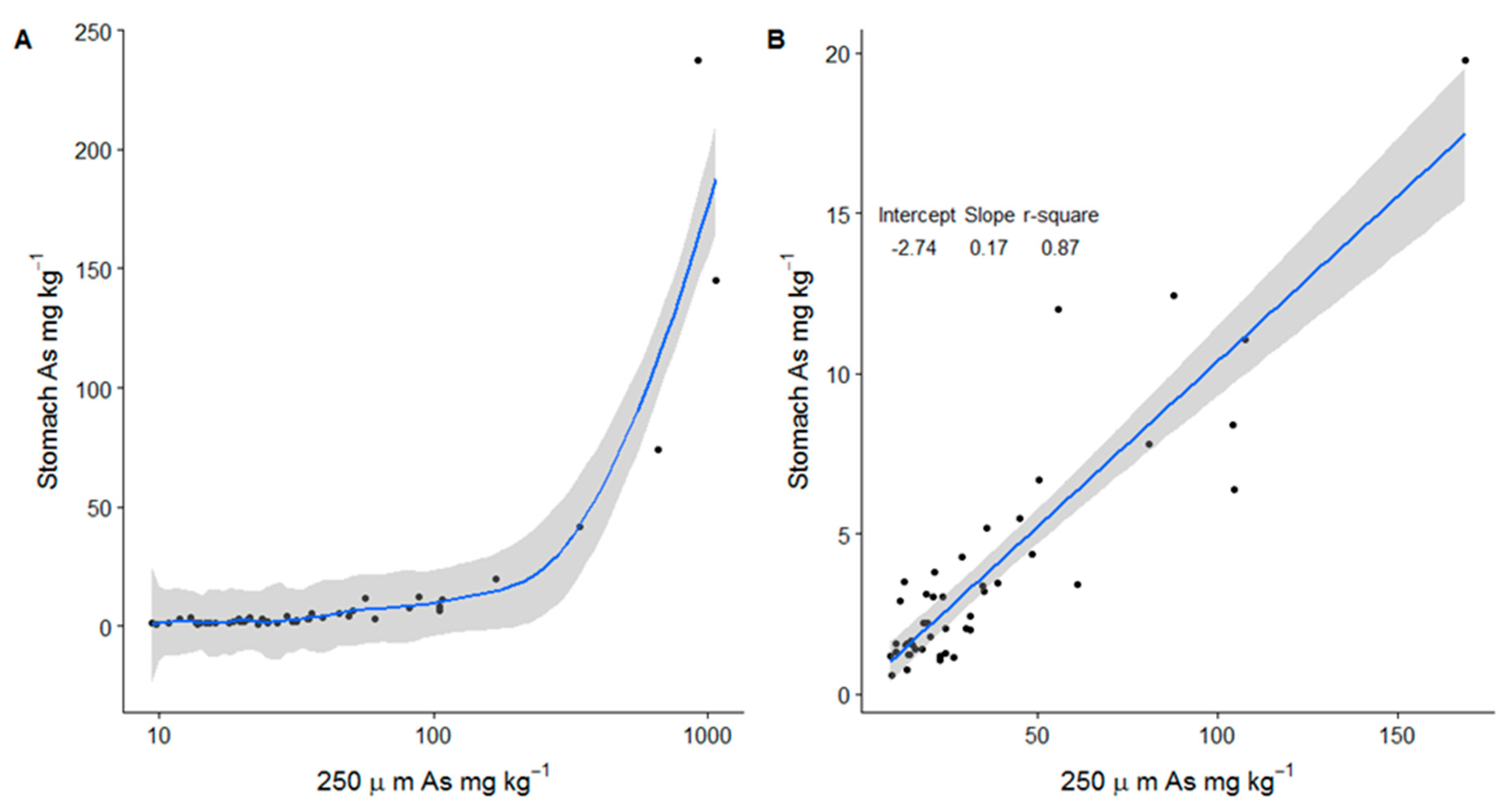
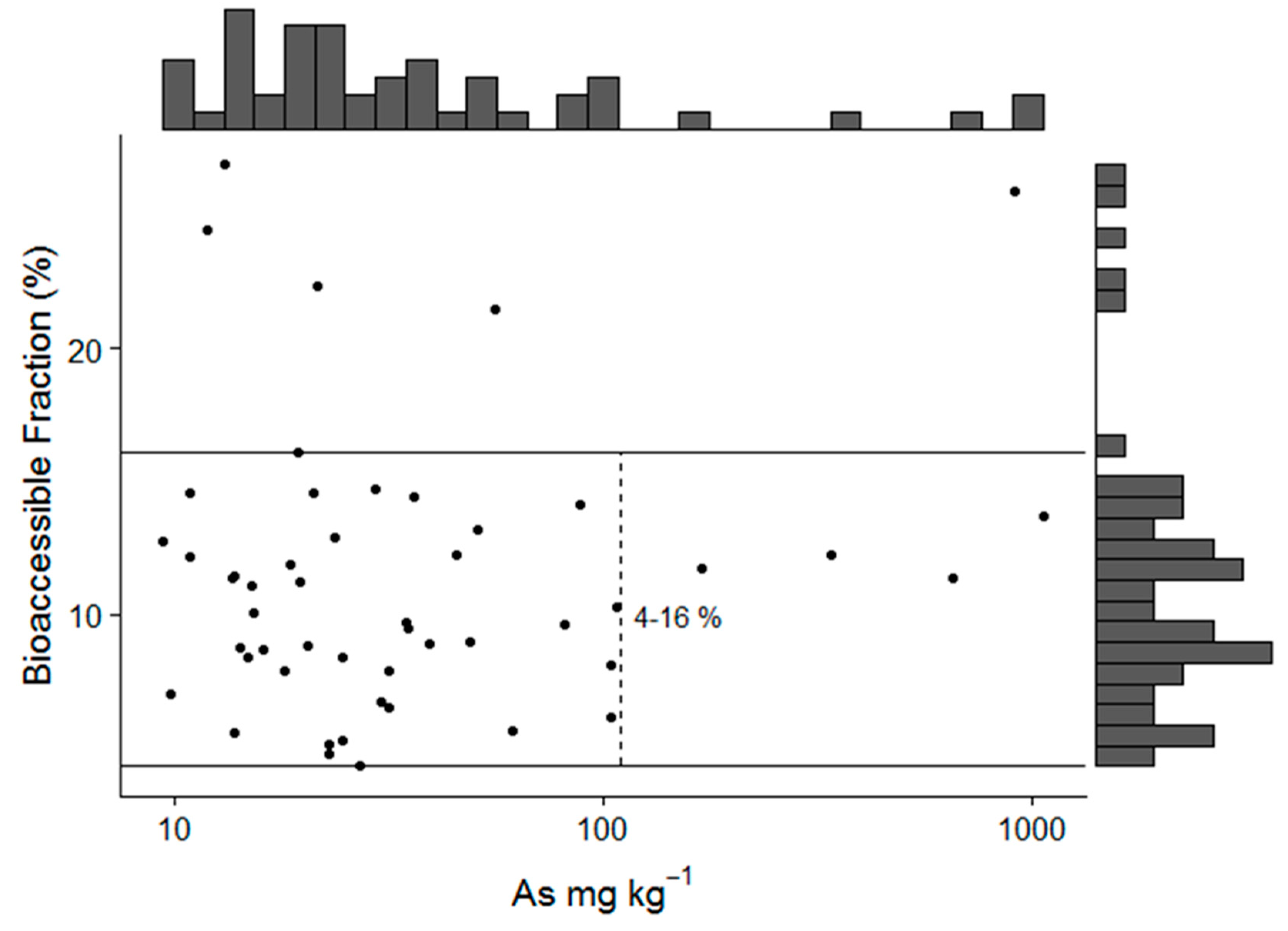
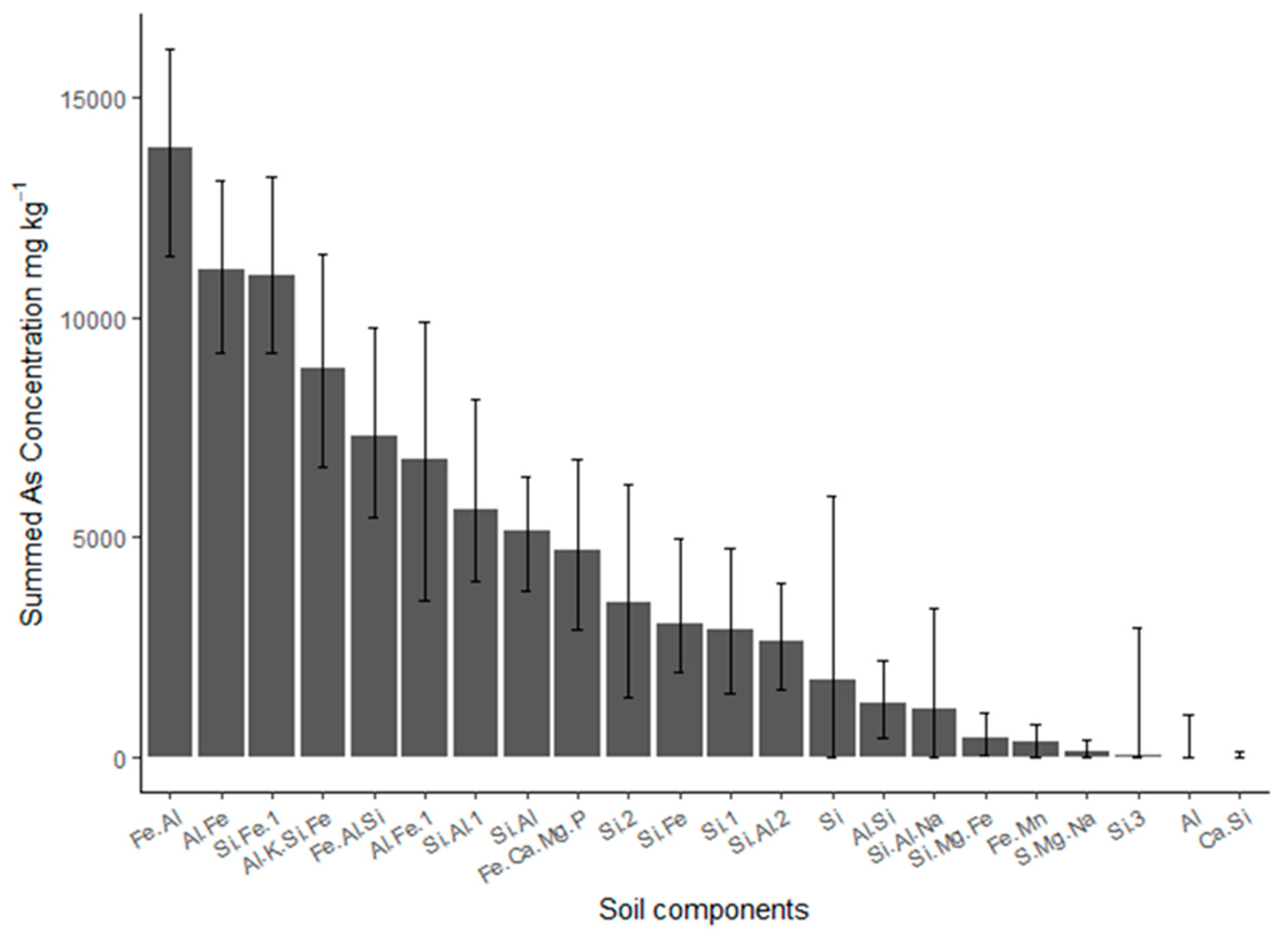

| N | Mean | Sd | Median | Mad | Min | Max | Range | Skew | |
|---|---|---|---|---|---|---|---|---|---|
| All data XRF | 1154 | 50.2 | 108 | 23.1 | 13.8 | 4.7 | 1949 | 1945 | 8.9 |
| Selected data digest | 50 | 92.4 | 211 | 24.7 | 16.2 | 9.4 | 1063 | 1053 | 3.5 |
| Selected data XRF | 50 | 91 | 211 | 21.9 | 12.6 | 6.9 | 1102 | 1095 | 3.5 |
| Bioaccessible As (Stomach) | 50 | 13.4 | 39.6 | 3 | 2.3 | 0.6 | 237 | 237 | 4.4 |
| Bioaccessible As (Intestine) | 50 | 11.4 | 33.7 | 2.5 | 2 | 0.7 | 206 | 205 | 4.5 |
| Sample Type | C4SL (37 mg·kg−1) | NBC Principal (32 mg·kg−1) | NBC Mineralised (290 mg·kg−1) |
|---|---|---|---|
| Number of samples (total As) above criteria <2 mm XRFS (n = 1154) | 340 | 408 | 25 |
| % | 29.5 | 35.4 | 2.17 |
| Number of samples (total As) above criteria <2 mm XRFS (n = 50) | 16 | 18 | 4 |
| % | 32 | 36 | 8 |
| Number of samples (total As) above criteria <250 µm acid digestion (n = 50) | 16 | 19 | 4 |
| % | 32 | 38 | 8 |
| Number of samples (bioaccessible As) above criteria <250 µm acid digestion (n = 50) | 4 | 4 | 0 |
| % | 8 | 8 | 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wragg, J.; Cave, M.; Hamilton, E.; Lister, T.R. The Link between Soil Geochemistry in South-West England and Human Exposure to Soil Arsenic. Minerals 2018, 8, 570. https://doi.org/10.3390/min8120570
Wragg J, Cave M, Hamilton E, Lister TR. The Link between Soil Geochemistry in South-West England and Human Exposure to Soil Arsenic. Minerals. 2018; 8(12):570. https://doi.org/10.3390/min8120570
Chicago/Turabian StyleWragg, Joanna, Mark Cave, Elliott Hamilton, and T. Robert Lister. 2018. "The Link between Soil Geochemistry in South-West England and Human Exposure to Soil Arsenic" Minerals 8, no. 12: 570. https://doi.org/10.3390/min8120570