Occurrence of Felsic Rocks in Oceanic Gabbros from IODP Hole U1473A: Implications for Evolved Melt Migration in the Lower Oceanic Crust
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Background and Lithology of Hole U1473A
2.2. General Description of Host Gabbros and Felsic Veins
2.2.1. Host Gabbros
2.2.2. Felsic Veins
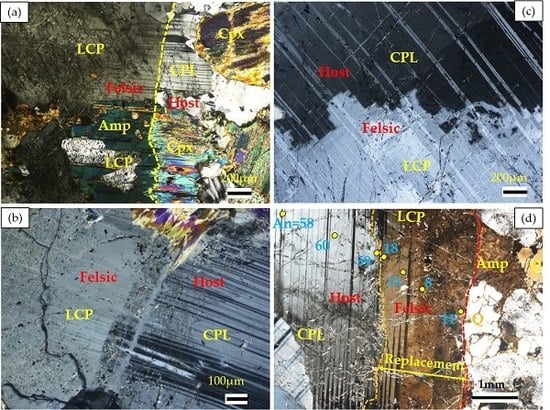
a. Diverse Occurrences of Felsic Veins in the Hole U1473A
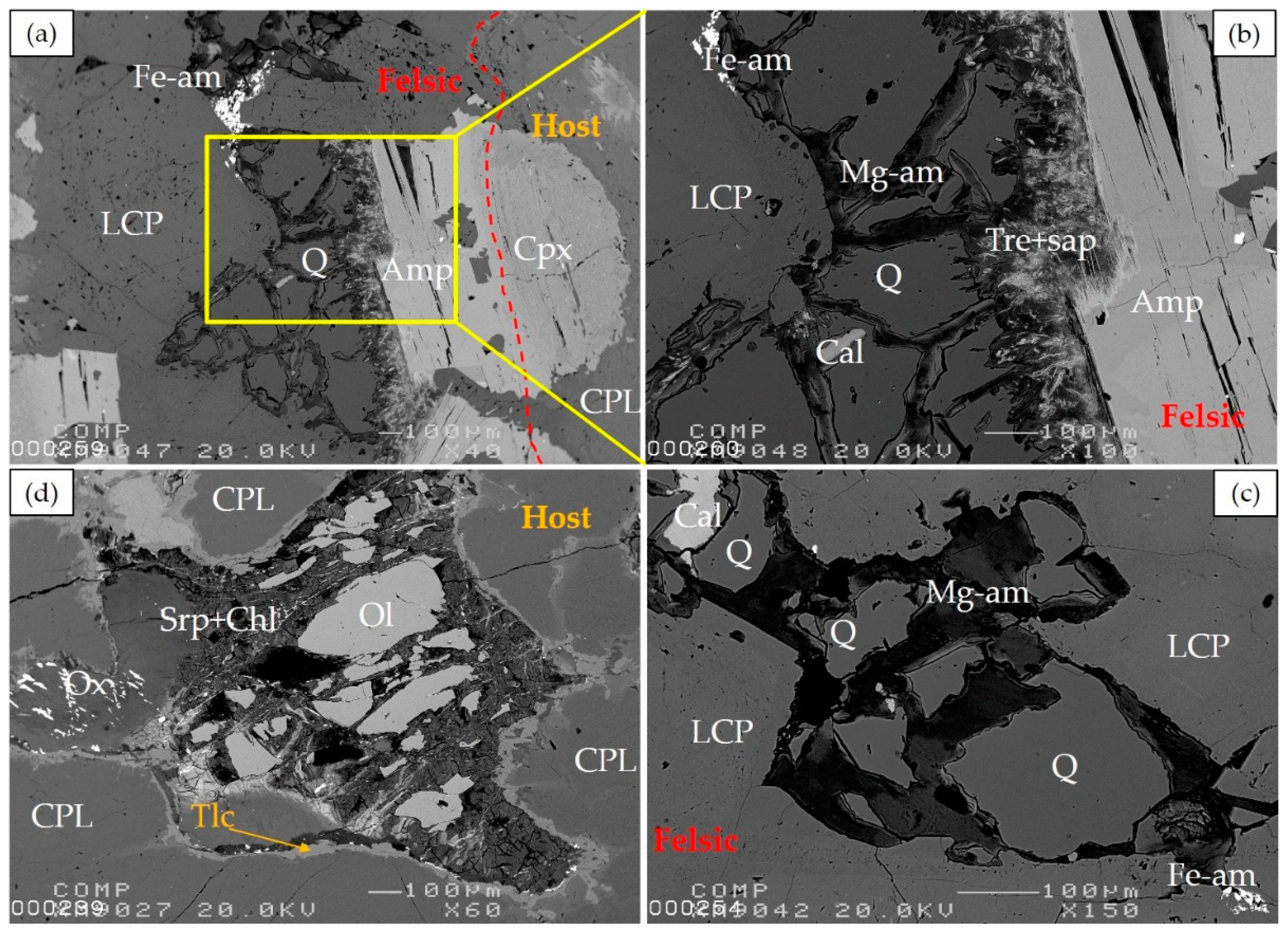
b. General Description of Vein Mineralogy
2.3. Analytical Methods
3. Mineral Major and Trace Composition
3.1. Olivine
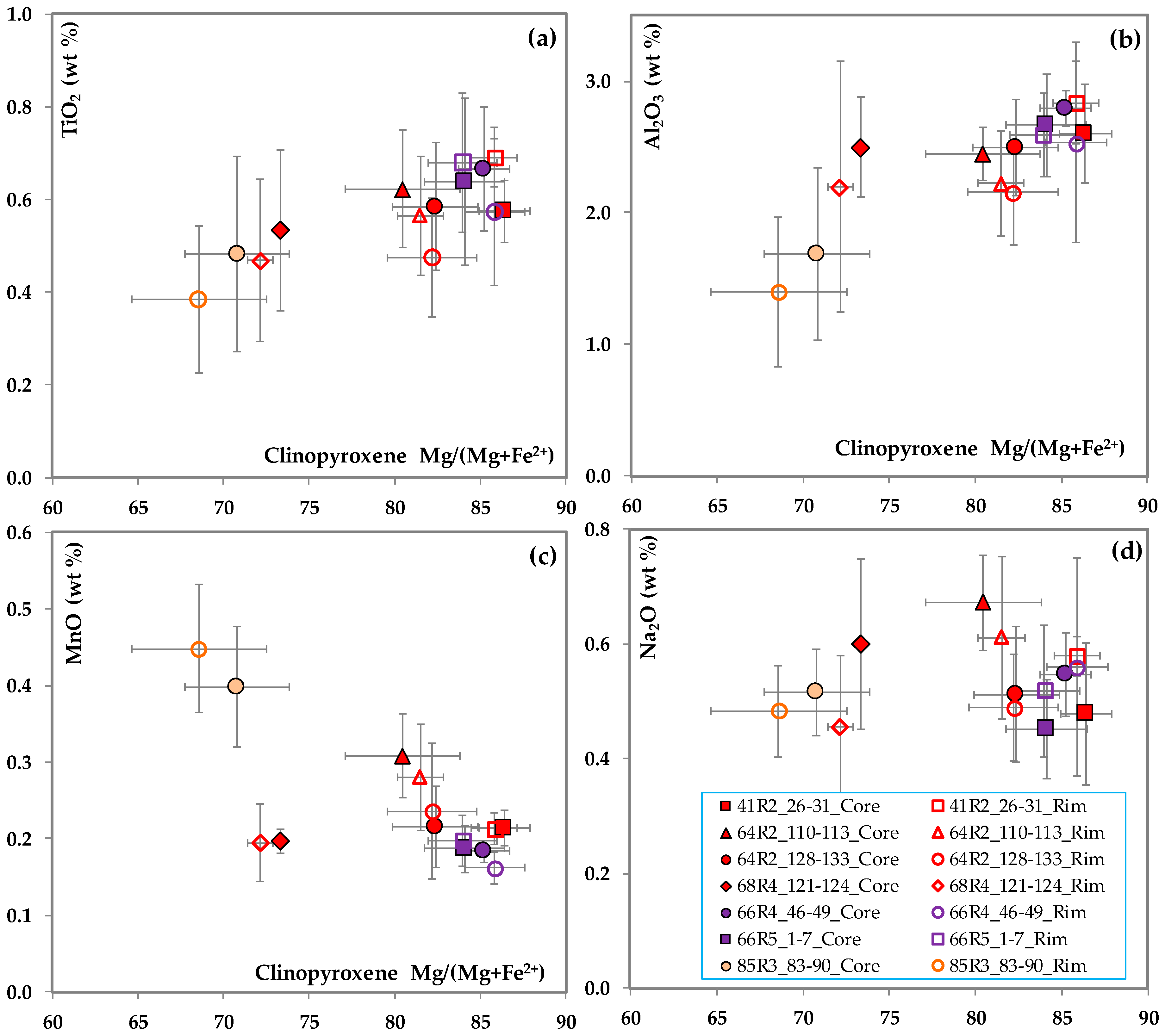
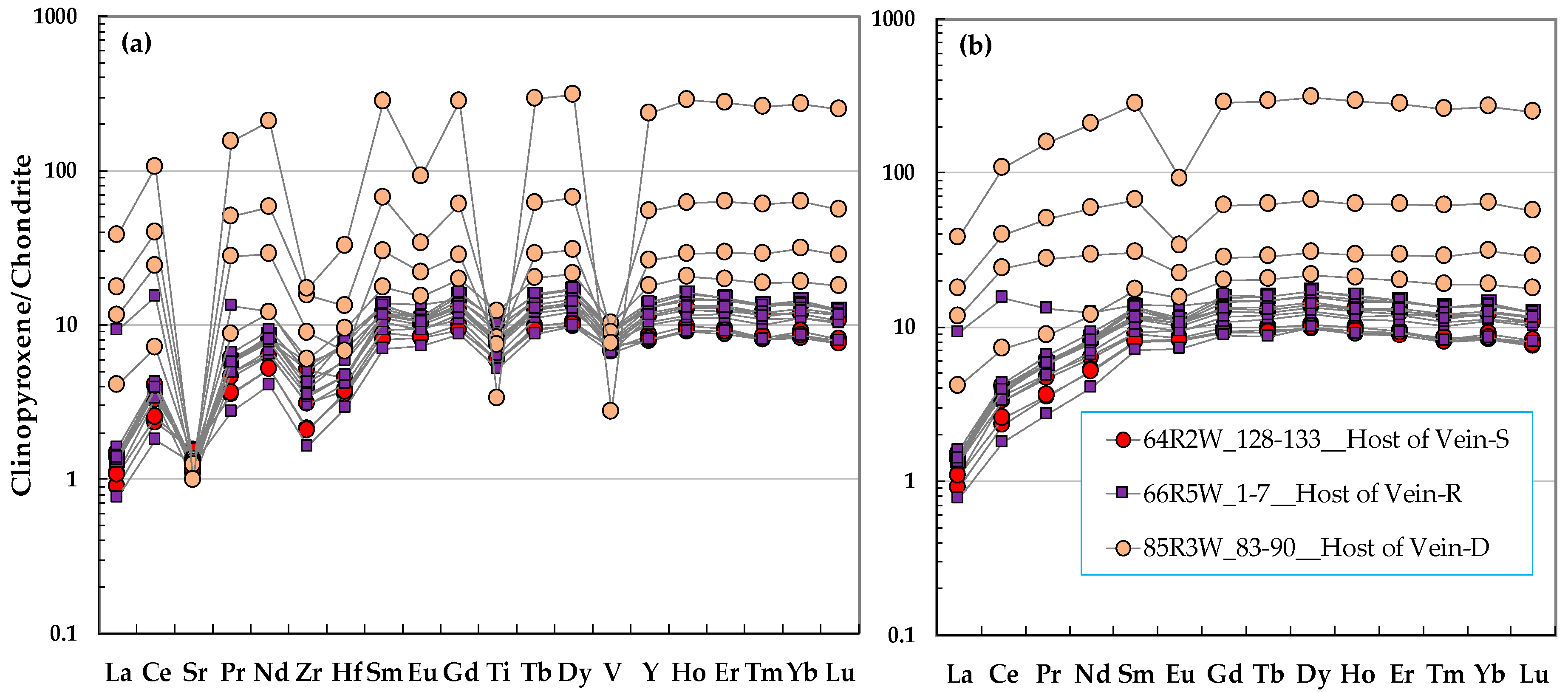
3.2. Clinopyroxene
3.3. Plagioclase
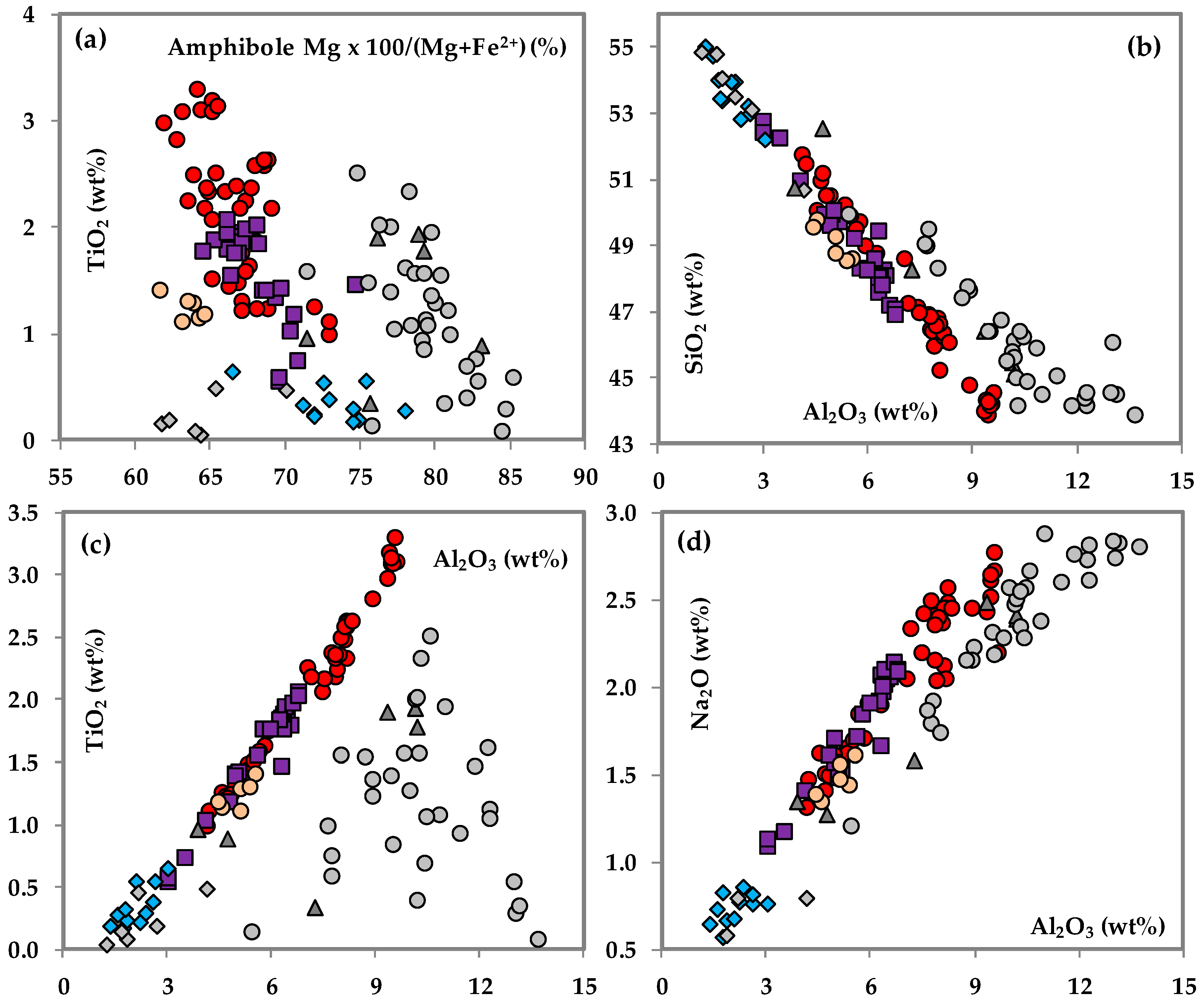
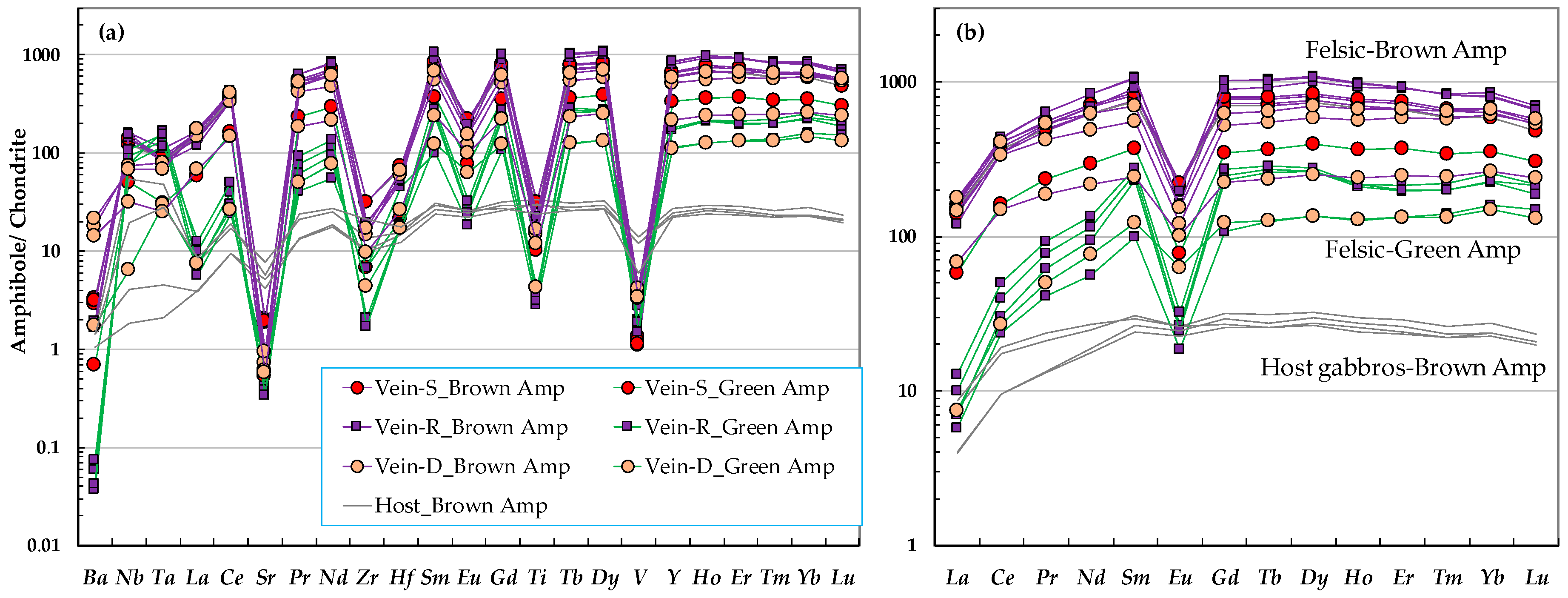
3.4. Amphibole
3.5. Zircon
4. Bulk Rock Estimation
5. Discussion
5.1. Preliminary Geochemical Comparison between ODP Hole 735B and IODP Hole U1473A
5.2. Effect of Later Hydrothermal Alteration on the Studied Felsic Rocks
5.3. Proposed Model for the Origin of Studied Felsic Veins
5.4. The Interaction between Gabbros and Highly Evolved Melts That Formed Felsic Veins in Hole U1473A
6. Summary
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maeda, J.; Naslund, H.R.; Jang, Y.D.; Kikawa, E.; Tajima, T.; Blackburn, W.H. High-temperature fluid migration within oceanic Layer 3 gabbros, Hole 735B, Southwest Indian Ridge: implications for the magmatic-hydrothermal transition at slow-spreading mid-ocean ridges. Proc. ODP Sci. Results 2002, 176, 56. [Google Scholar] [CrossRef]
- Lissenberg, C.J.; MacLeod, C.J.; Howard, K.A.; Godard, M. Pervasive reactive melt migration through fast-spreading lower oceanic crust (Hess Deep, equatorial Pacific Ocean). Earth Planet Sci. Lett. 2013, 361, 436–447. [Google Scholar] [CrossRef]
- Lissenberg, C.J.; Dick, H.J.B. Melt–rock reaction in the lower oceanic crust and its implications for the genesis of mid-ocean ridge basalt. Earth Planet Sci. Lett. 2008, 271, 311–325. [Google Scholar] [CrossRef]
- Lissenberg, C.J.; MacLeod, C.J. A Reactive Porous Flow Control on Mid-ocean Ridge Magmatic Evolution. J. Petrol. 2016, 57, 2195–2220. [Google Scholar] [CrossRef]
- MacLeod, C.J.; Dick, H.J.B.; Blum, P.; Abe, N.; Blackman, D.K.; Bowles, J.A.; Cheadle, M.J.; Cho, K.; Ciazela, J.; Deans, J.R.; et al. Site U1473. Proc. IODP Sci. Results 2017, 360, 136. [Google Scholar] [CrossRef]
- Robinson, P.T.; Erzinger, J.; Emmermann, R. The composition and origin of igneous and hydrothermal veins in the lower ocean crust; ODP Hole 735B, Southwest Indian Ridge. Proc. ODP Sci. Results 2002, 176, 66. [Google Scholar] [CrossRef]
- Niu, Y.; Gilmore, T.; Mackie, S.M.; Bach, W. Mineral chemistry, whole-rock compositions, and petrogenesis of Leg 176 gabbros; data and discussion. Proc. ODP Sci. Results 2002, 176, 60. [Google Scholar] [CrossRef]
- France, L.; Koepke, J.; MacLeod, C.J.; Ildefonse, B.; Godard, M.; Deloule, E. Contamination of MORB by anatexis of magma chamber roof rocks: Constraints from a geochemical study of experimental melts and associated residues. Lithos 2014, 202–203, 120–137. [Google Scholar] [CrossRef]
- Zhang, C.; Koepke, J.; France, L.; Godard, M. Felsic Plutonic Rocks from IODP Hole 1256D, Eastern Pacific: Implications for the Nature of the Axial Melt Lens at Fast-Spreading Mid-Ocean Ridges. J. Petrol. 2017, 58, 1535–1565. [Google Scholar] [CrossRef]
- Koepke, J.; Feig, S.; Snow, J. Late stage magmatic evolution of oceanic gabbros as a result of hydrous partial melting: Evidence from the Ocean Drilling Program (ODP) Leg 153 drilling at the Mid-Atlantic Ridge. Geochem. Geophys. Geosyst. 2005, 6, 27. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Natland, J.H.; Alt, J.C.; Bach, W.; Bideau, D.; Gee, J.S.; Haggas, S.; Hertogen, J.G.H.; Hirth, G.; Holm, P.M.; et al. A long in situ section of the lower ocean crust: results of ODP Leg 176 drilling at the Southwest Indian Ridge. Earth Planet Sci. Lett. 2000, 179, 31–51. [Google Scholar] [CrossRef] [Green Version]
- Pietranik, A.; Storey, C.; Koepke, J.; Lasalle, S. Zircon record of fractionation, hydrous partial melting and thermal gradients at different depths in oceanic crust (ODP Site 735B, South-West Indian Ocean). Contrib. Mineral. Petrol. 2017, 172, 10. [Google Scholar] [CrossRef]
- Koepke, J.; Feig, S.T.; Snow, J.; Freise, M. Petrogenesis of oceanic plagiogranites by partial melting of gabbros: an experimental study. Contrib. Mineral. Petrol. 2004, 146, 414–432. [Google Scholar] [CrossRef]
- Koepke, J.; Berndt, J.; Feig, S.T.; Holtz, F. The formation of SiO2-rich melts within the deep oceanic crust by hydrous partial melting of gabbros. Contrib. Mineral. Petrol. 2007, 153, 67–84. [Google Scholar] [CrossRef]
- France, L.; Koepke, J.; Ildefonse, B.; Cichy, S.B.; Deschamps, F. Hydrous partial melting in the sheeted dike complex at fast spreading ridges: experimental and natural observations. Contrib. Mineral. Petrol. 2010, 160, 683–704. [Google Scholar] [CrossRef]
- Erdmann, M.; Fischer, L.A.; France, L.; Zhang, C.; Godard, M.; Koepke, J. Anatexis at the roof of an oceanic magma chamber at IODP Site 1256 (equatorial Pacific): An experimental study. Contrib. Mineral. Petrol. 2015, 169, 39. [Google Scholar] [CrossRef]
- Erdmann, M.; France, L.; Fischer, L.A.; Deloule, E.; Koepke, J. Trace elements in anatectic products at the roof of mid-ocean ridge magma chambers: An experimental study. Chem. Geol. 2017, 456, 43–57. [Google Scholar] [CrossRef]
- Natland, J.H. Magnetic susceptibility as an index of the lithology and composition of gabbros, ODP Leg 176, Hole 735B, Southwest Indian Ridge. Proc. ODP Sci. Results 2002, 176. [Google Scholar] [CrossRef]
- Charlier, B.; Namur, O.; Grove, T.L. Compositional and kinetic controls on liquid immiscibility in ferrobasalt–rhyolite volcanic and plutonic series. Geochim. Cosmochim. Acta 2013, 113, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.; Rutherford, M.J. Plagiogranites as late-stage immiscible liquids in ophiolite and mid-ocean ridge suites: An experimental study. Earth Planet Sci. Lett. 1979, 45, 45–60. [Google Scholar] [CrossRef]
- Charlier, B.; Grove, T.L. Experiments on liquid immiscibility along tholeiitic liquid lines of descent. Contrib. Mineral. Petrol. 2012, 164, 27–44. [Google Scholar] [CrossRef] [Green Version]
- Sauter, D.; Cannat, M. The Ultraslow Spreading Southwest Indian Ridge. In Diversity Of Hydrothermal Systems On Slow Spreading Ocean Ridges, American; Geophysical Union: Washington, DC, USA, 2013; Volume 188, pp. 153–173. ISBN 978-0-87590-478-8. [Google Scholar]
- Dick, H.J.B.; Natland, J.H.; Miller, D.J.; Alt, J.C.; Bach, W.; Bideau, D.; Gee, J.S.; Haggas, S.; Hertogen, J.G.H.; Hirth, G.; et al. Leg 176 summary. Proc. ODP Sci. Results 1999, 176, 70. [Google Scholar] [CrossRef]
- Casey, J.F.; Banerji, D.; Zarian, P. Leg 179 Synthesis: Geochemistry, Stratigraphy, and Structure of Gabbroic Rocks Drilled in ODP Hole 1105A, Southwest Indian Ridge. Proc. ODP Sci. Results 2007, 179. [Google Scholar] [CrossRef]
- Dick, H.J.B.; Meyer, P.S.; Bloomer, S.; Kirby, S.; Stakes, D.; Mawer, C. Lithostratigraphic evolution of an in-situ section of oceanic layer 3. Proc. ODP Sci. Results 1991, 118. [Google Scholar] [CrossRef]
- MacLeod, C.J.; Dick, H.J.B.; Blum, P.; Abe, N.; Blackman, D.K.; Bowles, J.A.; Cheadle, M.J.; Cho, K.; Ciazela, J.; Deans, J.R.; et al. Hole U1473A remediation operations, Expedition 362T. Proc. IODP Sci. Results 2017, 360, 11. [Google Scholar] [CrossRef]
- Le Maitre, R.; Streckeisen, A.; Zanettin, B.; Le Bas, M.; Bonin, B.; Bateman, P. Igneous Rocks: A Classification and Glossary of Terms, 2nd ed.; Cambridge University Press: New York, NY, USA, 2002; p. 236. [Google Scholar]
- Putnis, A. Mineral Replacement Reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Putnis, A.; Austrheim, H. Mechanisms of Metasomatism and Metamorphism on the Local Mineral Scale: The Role of Dissolution-Reprecipitation During Mineral Re-equilibration. In Metasomatism and the Chemical Transformation of Rock: The Role of Fluids in Terrestrial and Extraterrestrial Processes; Harlov, D.E., Austrheim, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 141–170. [Google Scholar]
- Putnis, A.; Putnis, C.V. The mechanism of reequilibration of solids in the presence of a fluid phase. J. Solid State Chem. 2007, 180, 1783–1786. [Google Scholar] [CrossRef]
- Putnis, A.; John, T. Replacement Processes in the Earth’s Crust. Elements 2010, 6, 159–164. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Putnis, C.V.; Putnis, A. Coupled dissolution and precipitation at mineral–fluid interfaces. Chem. Geol. 2014, 383, 132–146. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Droop, G.T.R. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral Mag 1987, 51, 431–435. [Google Scholar] [CrossRef]
- Morishita, T.; Ishida, Y.; Arai, S. Simultaneous determination of multiple trace element compositions in thin (<30 μm) layers of BCR-2G by 193 nm ArF excimer laser ablation-ICP-MS: Implications for matrix effect and elemental fractionation on quantitative analysis. Geochem. J. 2005, 39, 327–340. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.s. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Ridolfi, F.; Zanetti, A.; Renzulli, A.; Perugini, D.; Holtz, F.; Oberti, R. AMFORM, a new mass-based model for the calculation of the unit formula of amphiboles from electron microprobe analyses. Am. Mineral. 2018, 103, 1112–1125. [Google Scholar] [CrossRef]
- Locock, A.J. An Excel spreadsheet to classify chemical analyses of amphiboles following the IMA 2012 recommendations. Comput. Geosci. 2014, 62, 1–11. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Oberti, R.; Harlow, G.E.; Maresch, W.V.; Martin, R.F.; Schumacher, J.C.; Welch, M.D. Nomenclature of the amphibole supergroup. Am. Mineral. 2012, 97, 2031. [Google Scholar] [CrossRef]
- Winter, J.D. Principles of Igneous and Metamorphic Petrology, 2nd ed.; Pearson Education Limited: Edinburgh Gate, UK, 2013; p. 737. [Google Scholar]
- Dick, H.J.B.; Ozawa, K.; Meyer, P.S.; Niu, Y.; Robinson, P.T.; Constantin, M.; Herbert, R.; Natland, J.H.; Hirth, J.G.; Mackie, S.M. Primary silicate mineral chemistry of a 1.5-km section of very slow spreading lower ocean crust; ODP Hole 735B, Southwest Indian Ridge. Proc. ODP Sci. Results 2002, 176, 60. [Google Scholar] [CrossRef]
- Hoskin, P.W.O. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochim. Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Fu, B.; Mernagh, T.P.; Kita, N.T.; Kemp, A.I.S.; Valley, J.W. Distinguishing magmatic zircon from hydrothermal zircon: A case study from the Gidginbung high-sulphidation Au–Ag–(Cu) deposit, SE Australia. Chem. Geol. 2009, 259, 131–142. [Google Scholar] [CrossRef]
- Coogan, L.A.; Wilson, R.N.; Gillis, K.M.; MacLeod, C.J. Near-solidus evolution of oceanic gabbros: Insights from amphibole geochemistry. Geochim. Cosmochim. Acta 2001, 65, 4339–4357. [Google Scholar] [CrossRef]
- Klein, M.; Stosch, H.G.; Seck, H.A. Partitioning of high field-strength and rare-earth elements between amphibole and quartz-dioritic to tonalitic melts: an experimental study. Chem. Geol. 1997, 138, 257–271. [Google Scholar] [CrossRef]
- Mysen, B.O. The Structure of Silicate Melts. Annu. Rev. Earth Planet Sci. 1983, 11, 75–97. [Google Scholar] [CrossRef]
- Gresens, R.L. Composition-volume relationships of metasomatism. Chem. Geol. 1967, 2, 47–65. [Google Scholar] [CrossRef]
















| No | Leg 360—Hole U1473A-Sample | Depth (mbsf) | Vol (%) | Domain Lithology | General Comments | Host Gabbros—Mineral Mode (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Plagioclase Modal; An Mean (Range); N | Clinopyroxene Modal; Mg# Mean (Range); N | Olivine Modal; Fo Mean (Range); N | Ortho-pyroxene | Fe–Ti Oxide | ||||||
| 1 | 41R2, 26–31 cm | 373.11 | 20 | Olivine gabbro | Coarse grained; strongly altered; Host of felsic Vein-S | 43; Core: 53 (52–54); 4 Rim: 53 (52–53); 4 | 50; Core: 86 (83–90); 5 Rim: 86 (85–88); 4 | 7; completely altered | - | <1 |
| 2 | 64R2, 110–113 cm | 578.78 | 20 | Gabbro | Medium grained; moderately altered; Host of felsic Vein-S | 60; Core: 55 (54–56); 4 | 40; Core: 80 (76–84); 6 Rim: 81 (80–83); 5 | - | <1 | T |
| 3 | 64R2, 128–133 cm | 578.96 | 70 | Olivine gabbro | Medium grained; strongly altered; Host of felsic Vein-S | 47; Core: 57 (53–59); 20 Rim: 57 (53–59); 20 | 35; Core: 82 (76–87); 33 Rim: 82 (76–85); 19 | 18; Core: 74 (72–75); 16 Rim: 74 (73–75); 13 | <1 | <1 |
| 4 | 66R4, 46–49 cm | 601.82 | 55 | Gabbro | Pegmatitic grained; strongly altered; Host of felsic Vein-R | 70; Core: 57 (54–62); 25 Rim: 57 (54–61); 18 | 30; Core: 85 (84–88); 7 Rim: 86 (84–88); 6 | - | - | T |
| 5 | 66R5, 1–7 cm | 602.83 | 80 | Olivine gabbro | Medium grained; strongly altered; Host of felsic Vein-R | 46; Core: 58 (54–60); 32 Rim: 58 (52–63); 28 | 40; Core: 84 (81–92); 41 Rim: 84 (79–88); 32 | 14; Core: 76 (74–79); 8 Rim: 77 (75–78); 7 | <1 | T |
| 6 | 68R4, 121–124 cm | 621.73 | 40 | Olivine gabbro | Medium grained; strongly altered; Host of felsic Vein-S | 50; Core: 54 (52–56); 5 Rim: 54 (53–55); 4 | 40; Core: 73 (73–74); 3 Rim: 72 (72–73); 2 | 10; Core: 76 (75–76); 3 | <1 | T |
| 7 | 85R3, 83–90 cm | 754.08 | 35 | Oxide bearing gabbro | Medium grained; strongly altered; Host of felsic Vein-D | 36; Core: 41 (36–45); 15 Rim: 30 (22–44); 15 | 60; Core: 71 (67–76); 12 Rim: 69 (65–74); 6 | - | <1 | 4 |
| No | Leg 360—Hole U1473A-Sample | Depth (mbsf) | Vol (%) | Domain Lithology | General Comments | Felsic Rocks—Mineral Mode (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plagioclase Modal; An Mean (Range); N | Orthopyroxene Modal; Mg# Mean (Range); N | Brown Amphibole | Fe–Ti Oxide | Quartz | Apatite | Zircon | Biotite | ||||||
| 1 | 41R2, 26–31 cm | 373.11 | 80 | Felsic vein_ Diorite | Planar felsic Vein-S | 60; Core: 32 (23–35); 12 Rim: 24 (15–33); 11 | - | 15 | 13 | - | 7 | 3 | 2 |
| 2 | 64R2, 110–113 cm | 578.78 | 80 | Felsic vein_ Tonalite | Comb-like planar felsic Vein-S | 60; Core1: 38 (38–39); 2 Rim1: 37 (36–37); 2 Core2: 6 (2–11); 6 Rim2: 8 (8–9); 3 | 1; Core: 66; 1 Rim: 66; 1 | 6 | 7 | 25 | 1 | T | - |
| 3 | 64R2, 128–133 cm | 578.96 | 30 | Felsic vein_ Diorite | Planar felsic Vein-S | 60; Core: 27 (15–38); 17 Rim: 21 (15–28); 12 | - | 25 | 8 | 3 | 1 | 3 | <1 |
| 4 | 66R4, 46–49 cm | 601.82 | 45 | Felsic vein_ Tonalite | Network felsic Vein-R | 65; Core: 20 (10–28); 23 Rim: 15 (6–26); 23 | - | 10 | 2 | 22 | <1 | 1 | <1 |
| 5 | 66R5, 1–7 cm | 602.83 | 20 | Felsic vein_ Quartz diorite | Branched felsic Vein-R | 50; Core: 16 (6–21); 19 Rim: 10 (4–21); 18 | - | 36 | 1 | 13 | - | <1 | - |
| 6 | 68R4, 121–124 cm | 621.73 | 60 | Felsic vein_ Diorite | Lower part of large planar felsic Vein-S | 70; Core1: 31 (25–34); 8 Rim1: 21 (13–33); 7 Core2: 10 (5–15); 6 Rim2: 13 (3–17); 5 | 7; Core: 63 (62–64); 3 | 10 | 8 | - | 5 | - | <1 |
| 7 | 85R3, 83–90 cm | 754.08 | 65 | Felsic vein_ Diorite | Network felsic Vein-D | 67; Core: 31 (27–35); 16 Rim: 25 (19–31); 16 | 5; Core: 57 (54–63); 10 Rim: 56 (53–64); 7 | 3 | 15 | - | 10 | <1 | <1 |
| Mineral | Felsic Vein Types | ||
|---|---|---|---|
| Vein-S | Vein-D | Vein-R | |
| Plagioclase | Fine–coarse grained, subhedral–euhedral, weak-strong zonation, An (mol.%): 3–40 | Fine–medium grained, same size with host plagioclase, sub-euhedral, An (mol.%): 20–35 | Continuous twinning from plagioclase in the host gabbros, An (mol.%): 4–25 |
| Brown amphibole | Fine–coarse grained, subhedral–euhedral, brownish-dark brown; TiO2 (wt.%): 1–3.5 SiO2 (wt.%): 43–52 | Fine grained, sub-euhedral, brownish; TiO2 (wt.%): 1–1.5 SiO2 (wt.%): 48–50 | Continuity from the clinopyroxene in the host gabbro, anhedral–subhedral; TiO2 (wt.%): 1–2 SiO2 (wt.%): 47–53 |
| Orthopyroxene | Few grains appear along the boundaries of some felsic veins | Relative abundance along boundaries of the vein, subhedral–euhedral | - |
| Quartz | Myrmekite or interstitial fine grains aggregate | - | Same size, shape, and modal% as those of the olivine in the host olivine gabbro |
| Zircon | Up to 3 (modal%) total REEs (ppm): 900–2200 | Rare, very few grains can be observed | Few grains are observed, total REEs (ppm): 6200–11200 |
| Apatite | Up to >10 (modal%) in both 2 felsic vein types; subhedral–euhedral, rounded fine grains, or anhedral exsolved in plagioclase; intimately associated with Fe–Ti oxides | - | |
| Fe-Ti oxides | Up to 15 (modal%) in both 2 felsic vein types, including ilmenite and Ti–magnetite relatively pure, homogeneous compositions | Lesser abundances, 1–2 (modal%) | |
| Core | 41R2, 26–31 cm | 64R2, 110–113 cm | 64R2, 128–133 cm | 66R4, 46–49 cm | 66R5, 1–7 cm | 68R4, 121–124 cm | 85R3, 83–90 cm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxide | Host | Vein-S | Host | Vein-S | Host | Vein-S | Host | Vein-R | Host | Vein-R | Host | Vein-S | Host | Vein-D |
| SiO2 | 52.0 | 40.9 | 53.3 | 62.4 | 50.3 | 50.4 | 53.4 | 70.5 | 50.8 | 63.7 | 53.3 | 49.4 | 51.3 | 40.1 |
| Al2O3 | 12.3 | 14.1 | 17.3 | 13.6 | 13.1 | 14.8 | 20.0 | 15.2 | 13.1 | 12.6 | 14.4 | 15.9 | 8.9 | 14.4 |
| TiO2 | 0.3 | 4.1 | 0.3 | 2.5 | 0.2 | 5.1 | 0.2 | 0.2 | 0.3 | 0.6 | 0.2 | 2.8 | 1.9 | 7.3 |
| FeO* | 5.7 | 19.2 | 3.4 | 10.4 | 7.5 | 11.4 | 2.3 | 1.9 | 6.3 | 5.8 | 5.2 | 13.6 | 11.4 | 17.6 |
| MgO | 11.8 | 2.1 | 6.8 | 1.3 | 13.6 | 3.6 | 5.6 | 1.9 | 13.3 | 5.8 | 11.8 | 3.2 | 8.4 | 1.4 |
| MnO | 0.1 | 0.2 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.3 | 0.2 |
| CaO | 15.5 | 8.4 | 15.7 | 3.9 | 12.9 | 5.6 | 15.1 | 3.7 | 13.9 | 5.5 | 12.5 | 7.1 | 15.2 | 9.4 |
| Na2O | 2.3 | 4.5 | 3.1 | 5.2 | 2.2 | 5.2 | 3.4 | 6.4 | 2.2 | 5.5 | 2.4 | 5.6 | 2.6 | 4.9 |
| K2O | - | 0.4 | - | 0.1 | - | 0.3 | - | 0.2 | - | 0.3 | - | 0.1 | 0.1 | 0.2 |
| P2O5 | - | 2.9 | - | 0.5 | - | - | - | - | - | - | - | 2.2 | - | 4.2 |
| V2O3 | - | 0.2 | - | - | - | 0.3 | - | - | - | - | - | - | - | 0.4 |
| ZrO2 | - | 2.9 | - | - | - | 2.9 | - | - | - | - | - | - | - | - |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| ρ | 3.05 | 3.13 | 2.94 | 2.84 | 3.04 | 2.99 | 2.88 | 2.69 | 3.04 | 2.80 | 3.01 | 2.94 | 3.14 | 3.08 |
| ASI | 0.39 | 0.61 | 0.51 | 0.86 | 0.48 | 0.78 | 0.61 | 0.87 | 0.46 | 0.65 | 0.54 | 0.72 | 0.28 | 0.57 |
| NBO/T | 1.03 | 0.47 | 0.68 | 0.28 | 0.62 | 0.77 | 0.99 | |||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, D.K.; Morishita, T.; Soda, Y.; Tamura, A.; Ghosh, B.; Harigane, Y.; France, L.; Liu, C.; Natland, J.H.; Sanfilippo, A.; et al. Occurrence of Felsic Rocks in Oceanic Gabbros from IODP Hole U1473A: Implications for Evolved Melt Migration in the Lower Oceanic Crust. Minerals 2018, 8, 583. https://doi.org/10.3390/min8120583
Nguyen DK, Morishita T, Soda Y, Tamura A, Ghosh B, Harigane Y, France L, Liu C, Natland JH, Sanfilippo A, et al. Occurrence of Felsic Rocks in Oceanic Gabbros from IODP Hole U1473A: Implications for Evolved Melt Migration in the Lower Oceanic Crust. Minerals. 2018; 8(12):583. https://doi.org/10.3390/min8120583
Chicago/Turabian StyleNguyen, Du Khac, Tomoaki Morishita, Yusuke Soda, Akihiro Tamura, Biswajit Ghosh, Yumiko Harigane, Lydéric France, Chuanzhou Liu, James H. Natland, Alessio Sanfilippo, and et al. 2018. "Occurrence of Felsic Rocks in Oceanic Gabbros from IODP Hole U1473A: Implications for Evolved Melt Migration in the Lower Oceanic Crust" Minerals 8, no. 12: 583. https://doi.org/10.3390/min8120583