Effect of Dodecane and Oleic Acid on the Attachment between Oxidized Coal and Bubbles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Flotation Experiments
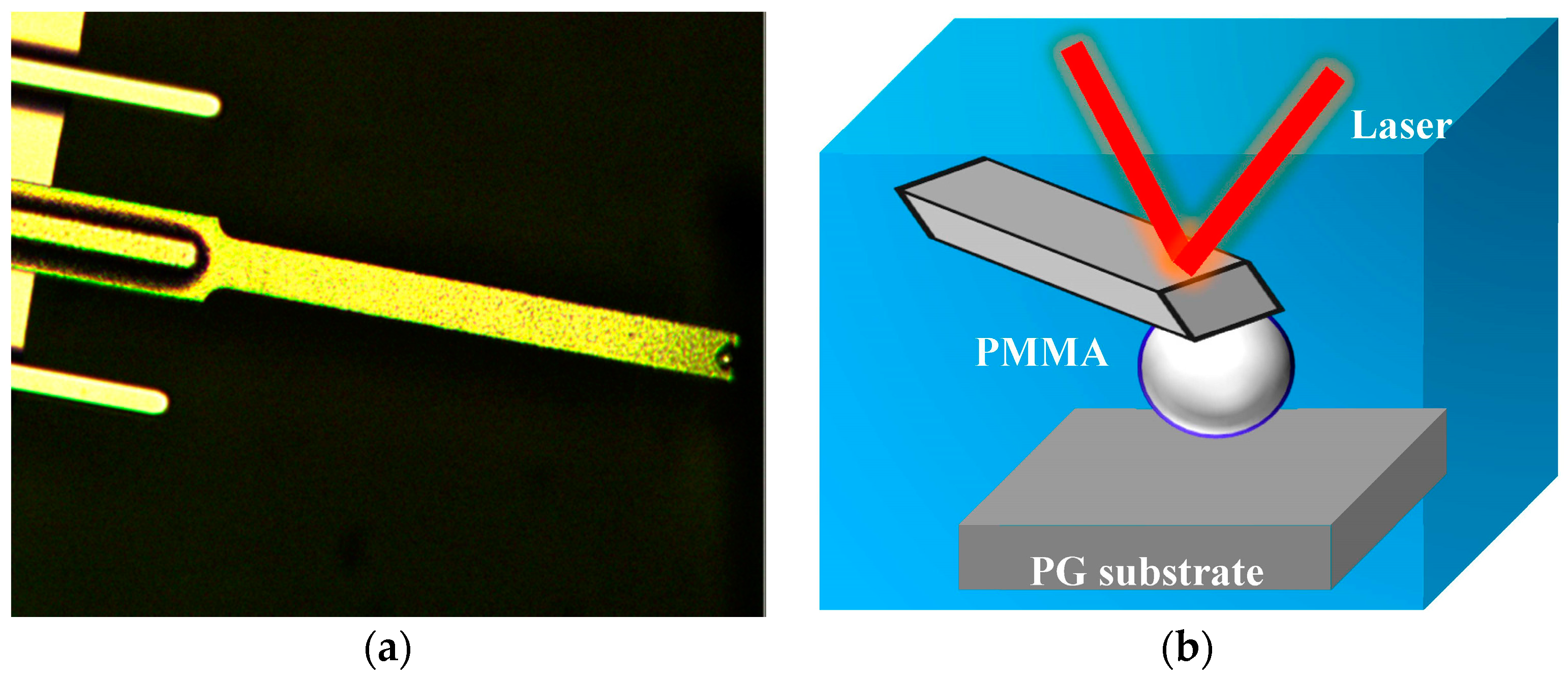
2.2.2. AFM Force Measurements
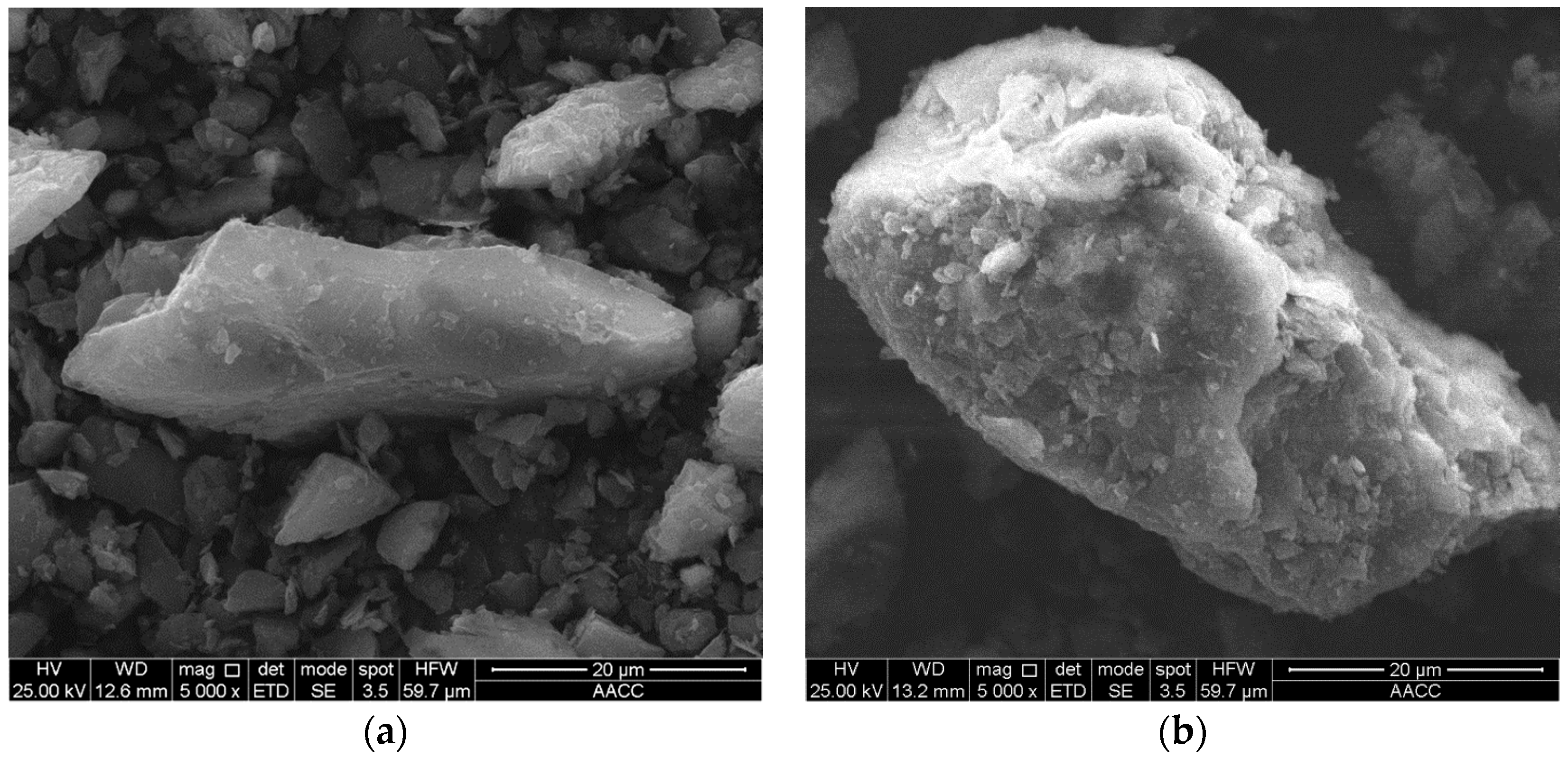
2.2.3. High Speed Visualization for Bubble–Oxidized Coal Attachment
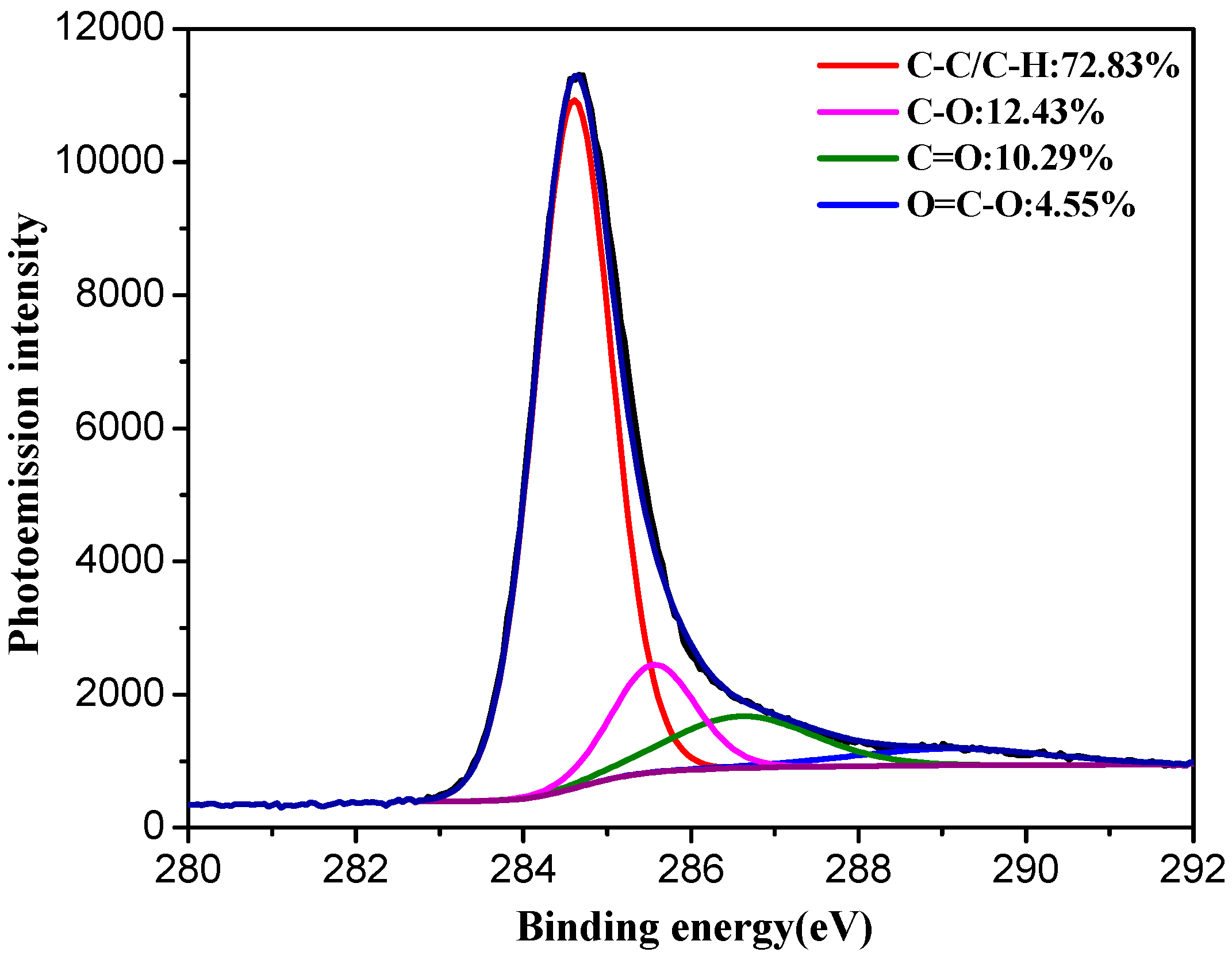
3. Results and Discussion
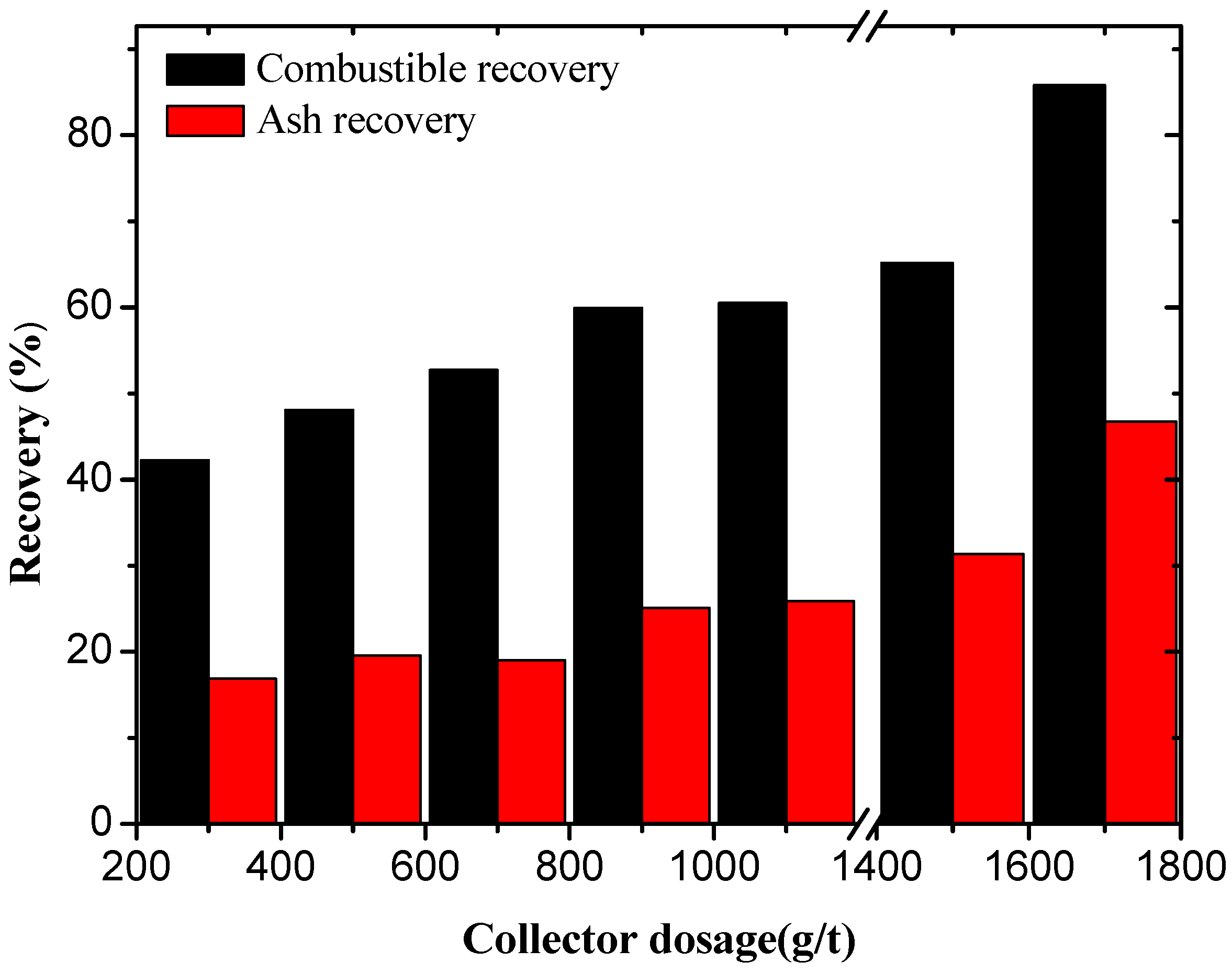
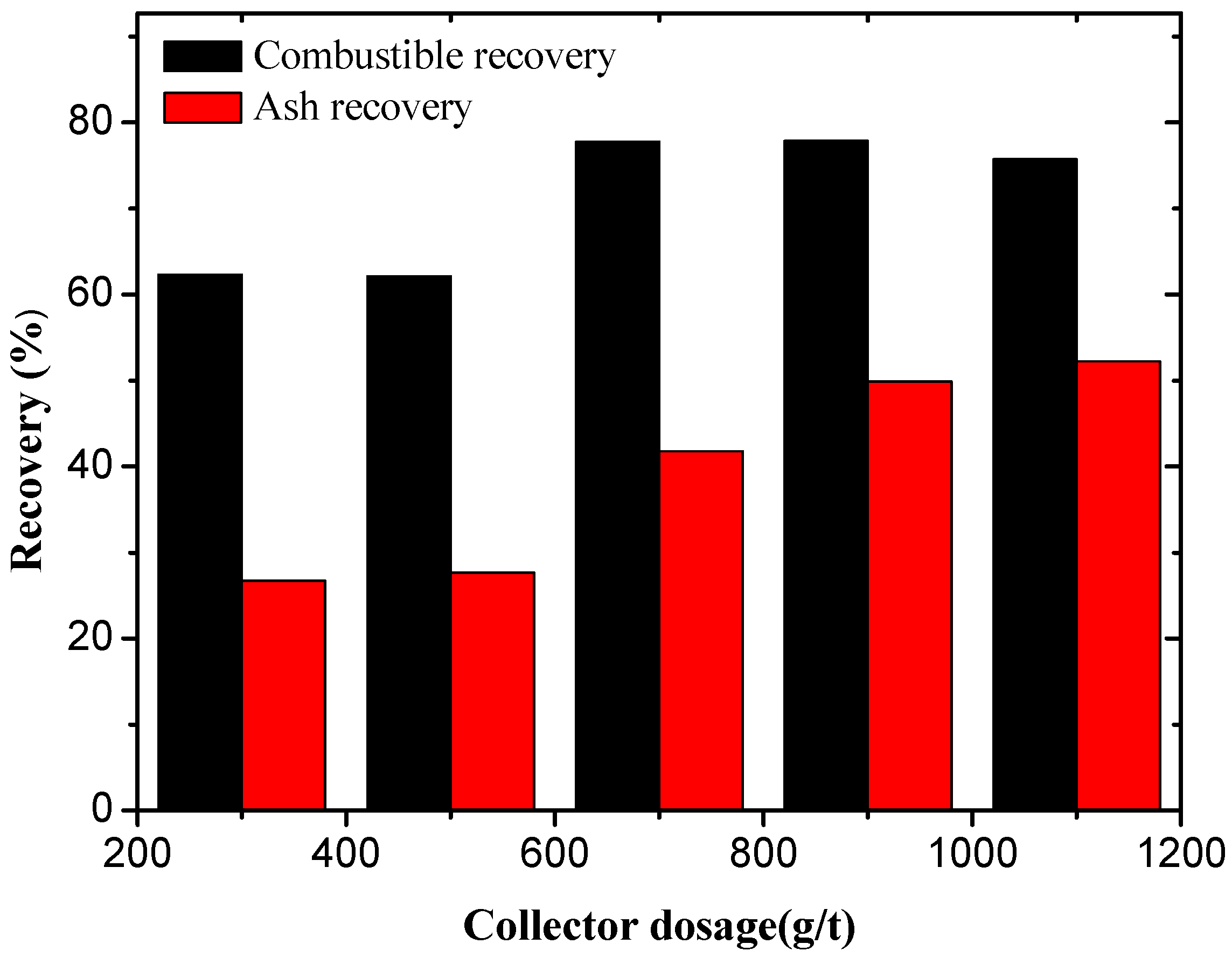
3.1. Oxidized Coal Flotation Using Dodecane and Oleic Acid
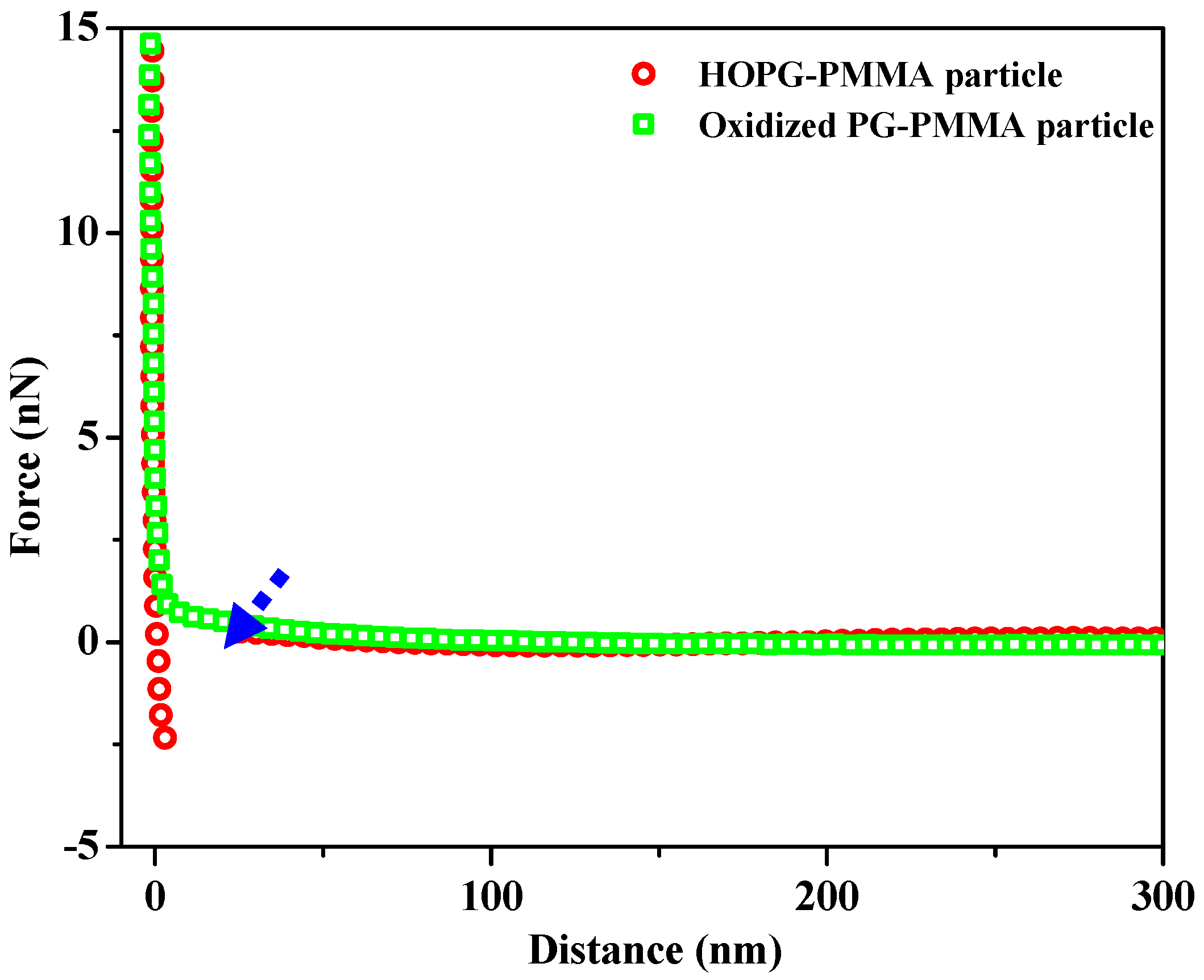
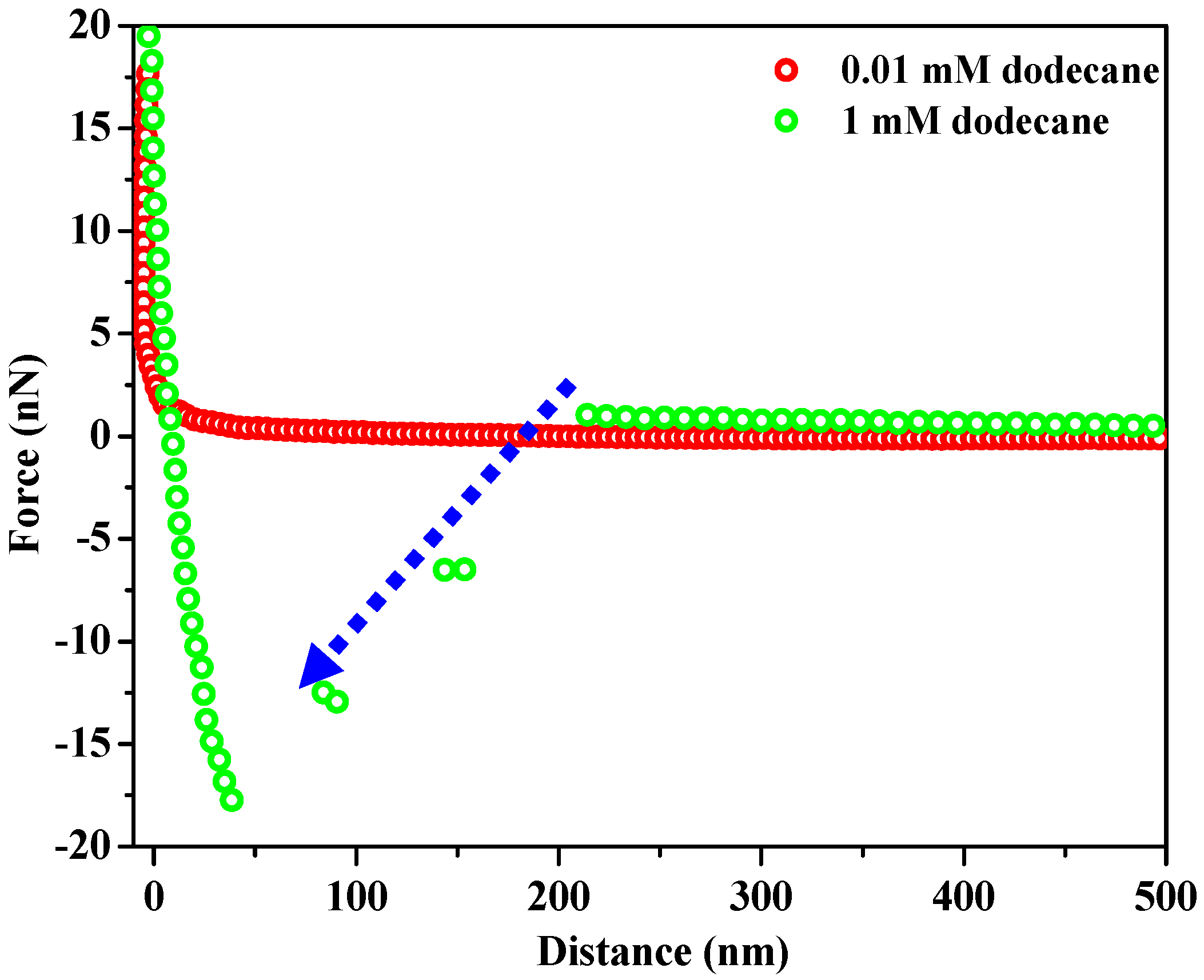
3.2. Forces between Oxidized PG Surface and PMMA Colloidal Probe in the Presence of Dodecane and Oleic Acid
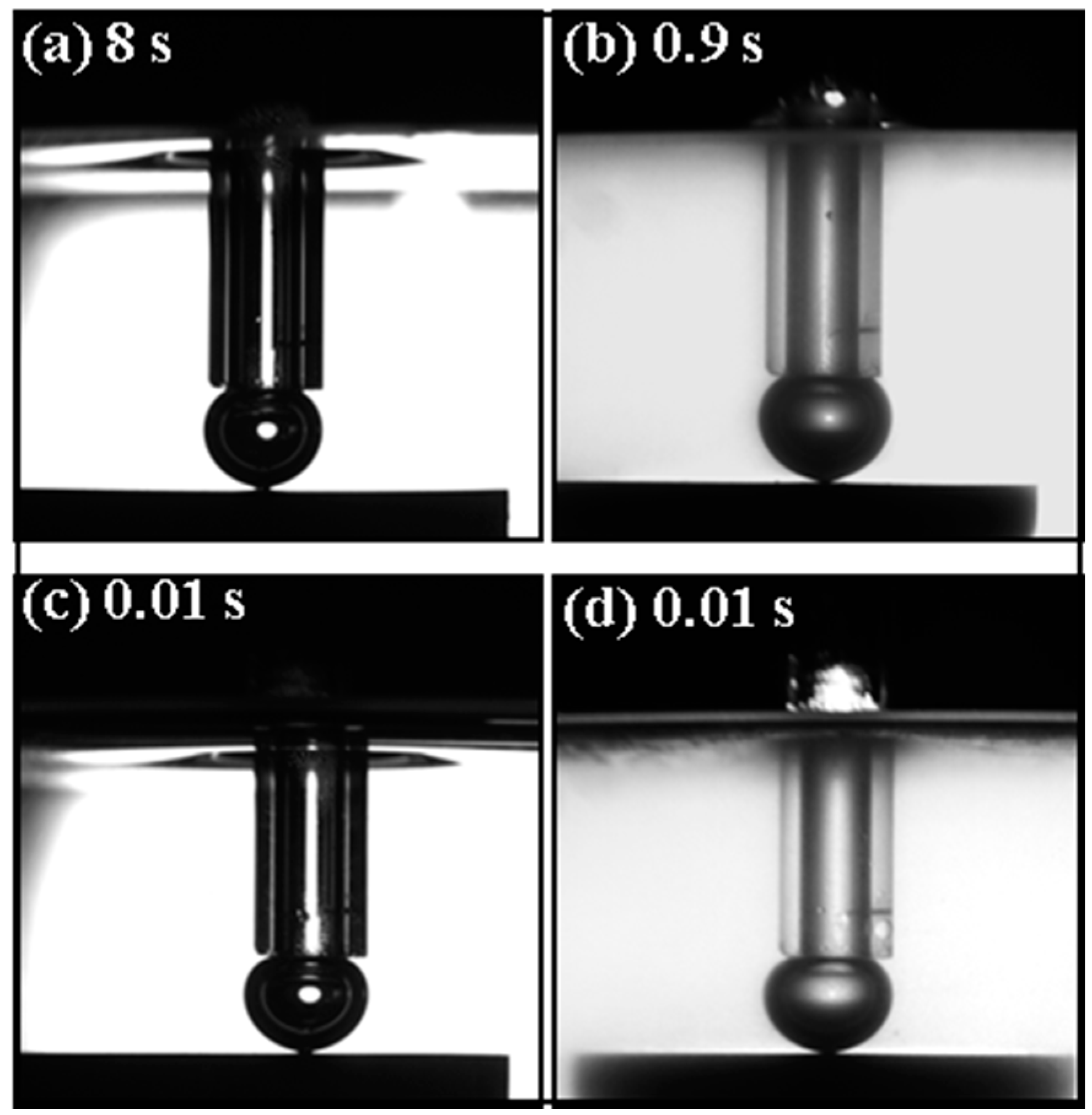
3.3. High Speed Visualization for Bubble–Oxidized Coal Attachment in the Presence of Dodecane and Oleic Acid
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xing, Y.; Gui, X.; Cao, Y.; Wang, Y.; Xu, M.; Wang, D.; Li, C. Effect of compound collector and blending frother on froth stability and flotation performance of oxidized coal. Powder Technol. 2017, 305, 166–173. [Google Scholar] [CrossRef]
- Xing, Y.; Li, C.; Gui, X.; Cao, Y. Interaction forces between paraffin/stearic acid and fresh/oxidized coal particles measured by atomic force microscopy. Energy Fuels 2017, 31, 3305–3312. [Google Scholar] [CrossRef]
- Xia, W.; Yang, J.; Liang, C. A short review of improvement in flotation of low rank/oxidized coals by pretreatments. Powder Technol. 2013, 237, 1–8. [Google Scholar] [CrossRef]
- Gui, X.; Xing, Y.; Wang, T.; Cao, Y.; Miao, Z.; Xu, M. Intensification mechanism of oxidized coal flotation by using oxygen-containing collector α-furanacrylic acid. Powder Technol. 2017, 305, 109–116. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.; Fuerstenau, D. An improved class of universal collectors for the flotation of oxidized and/or low-rank coal. Int. J. Miner. Process. 2000, 58, 99–118. [Google Scholar] [CrossRef]
- Jia, R.; Harris, G.; Fuerstenau, D. Chemical reagents for enhanced coal flotation. Int. J. Coal Prep. Util. 2002, 22, 123–149. [Google Scholar] [CrossRef]
- Chang, Z.; Chen, X.; Peng, Y. Understanding and improving the flotation of coals with different degrees of surface oxidation. Powder Technol. 2017, 321, 190–196. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930–933. [Google Scholar] [CrossRef] [PubMed]
- Drake, B.; Prater, C.B.; Weisenhorn, A.L.; Gould, S.A.; Albrecht, T.R.; Quate, C.F.; Cannell, D.S.; Hansma, H.G.; Hansma, P.K. Imaging Crystals, Polymers, and Processes in Water with the Atomic Force Microscope. Science 1989, 243, 1586–1589. [Google Scholar] [CrossRef] [PubMed]
- Butt, H.J.; Berger, R.; Bonaccurso, E.; Chen, Y.; Wang, J. Impact of atomic force microscopy on interface and colloid science. Adv. Colloid Interface Sci. 2007, 133, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Rugar, D.; Hansma, P. Atomic force microscopy. Phys. Today 1990, 43, 23–30. [Google Scholar] [CrossRef]
- Meyer, E. Atomic force microscopy. Prog. Surf. Sci. 1992, 41, 3–49. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Direct measurement of colloidal forces using an atomic force microscope. Nature 1991, 353, 239–241. [Google Scholar] [CrossRef]
- Butt, H.-J. Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope. Biophys. J. 1991, 60, 1438–1444. [Google Scholar] [CrossRef]
- Butt, H.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Gillies, G.; Kappl, M.; Butt, H.J. Direct measurements of particle-bubble interactions. Adv. Colloid Interface Sci. 2005, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Ducker, W.; Xu, Z.; Israelachvili, J.N. Measurements of hydrophobic and DLVO forces in bubble-surface interactions in aqueous solutions. Langmuir 1994, 10, 3279–3289. [Google Scholar] [CrossRef]
- Butt, H.J. A technique for measuring the force between a colloidal particle in water and a bubble. J. Colloid Interface Sci. 1994, 166, 109–117. [Google Scholar] [CrossRef]
- Assemi, S.; Nguyen, A.; Miller, J. Direct measurement of particle-bubble interaction forces using atomic force microscopy. Int. J. Miner. Process. 2008, 89, 65–70. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–142. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Nalaskowski, J.; Paruchuri, V.; Miller, J. Interaction forces between a coal surface and a polystyrene sphere in the presence of cationic and anionic surfactants as measured using atomic force microscopy. Coal Perp. 2000, 21, 337–353. [Google Scholar] [CrossRef]
- Claesson, P.M.; Ederth, T.; Bergeron, V.; Rutland, M.W. Techniques for measuring surface forces. Adv. Colloid Interface Sci. 1996, 67, 119–183. [Google Scholar] [CrossRef]
- Meyer, E.E.; Rosenberg, K.J.; Israelachvili, J. Recent progress in understanding hydrophobic interactions. Proc. Natl. Acad. Sci. USA 2006, 103, 15739–15746. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Gui, X.; Cao, Y. The hydrophobic force for bubble-particle attachment in flotation-a brief review. Phys. Chem. Chem. Phys. 2017, 19, 24421–24435. [Google Scholar] [CrossRef] [PubMed]
- Ralston, J.; Fornasiero, D.; Mishchuk, N. The hydrophobic force in flotation-a critique. Colloids Surf. A 2001, 192, 39–51. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Xing, Y.; Cao, Y.; Gui, X. Effect of Dodecane and Oleic Acid on the Attachment between Oxidized Coal and Bubbles. Minerals 2018, 8, 29. https://doi.org/10.3390/min8020029
Xu M, Xing Y, Cao Y, Gui X. Effect of Dodecane and Oleic Acid on the Attachment between Oxidized Coal and Bubbles. Minerals. 2018; 8(2):29. https://doi.org/10.3390/min8020029
Chicago/Turabian StyleXu, Mengdi, Yaowen Xing, Yijun Cao, and Xiahui Gui. 2018. "Effect of Dodecane and Oleic Acid on the Attachment between Oxidized Coal and Bubbles" Minerals 8, no. 2: 29. https://doi.org/10.3390/min8020029



