Mineralogy and Genesis of the Polymetallic and Polyphased Low Grade Fe-Mn-Cu Ore of Jbel Rhals Deposit (Eastern High Atlas, Morocco)
Abstract
:1. Introduction
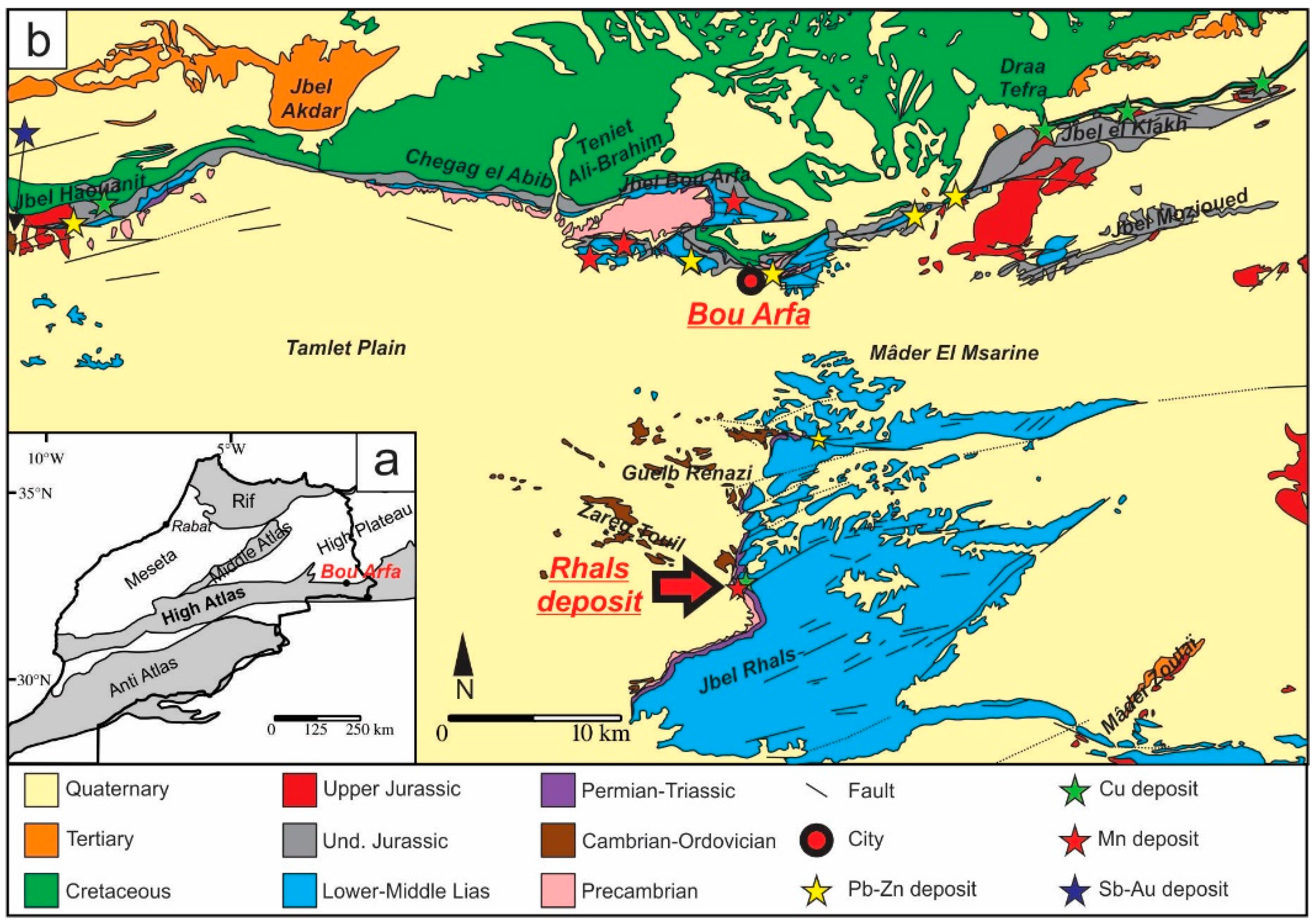
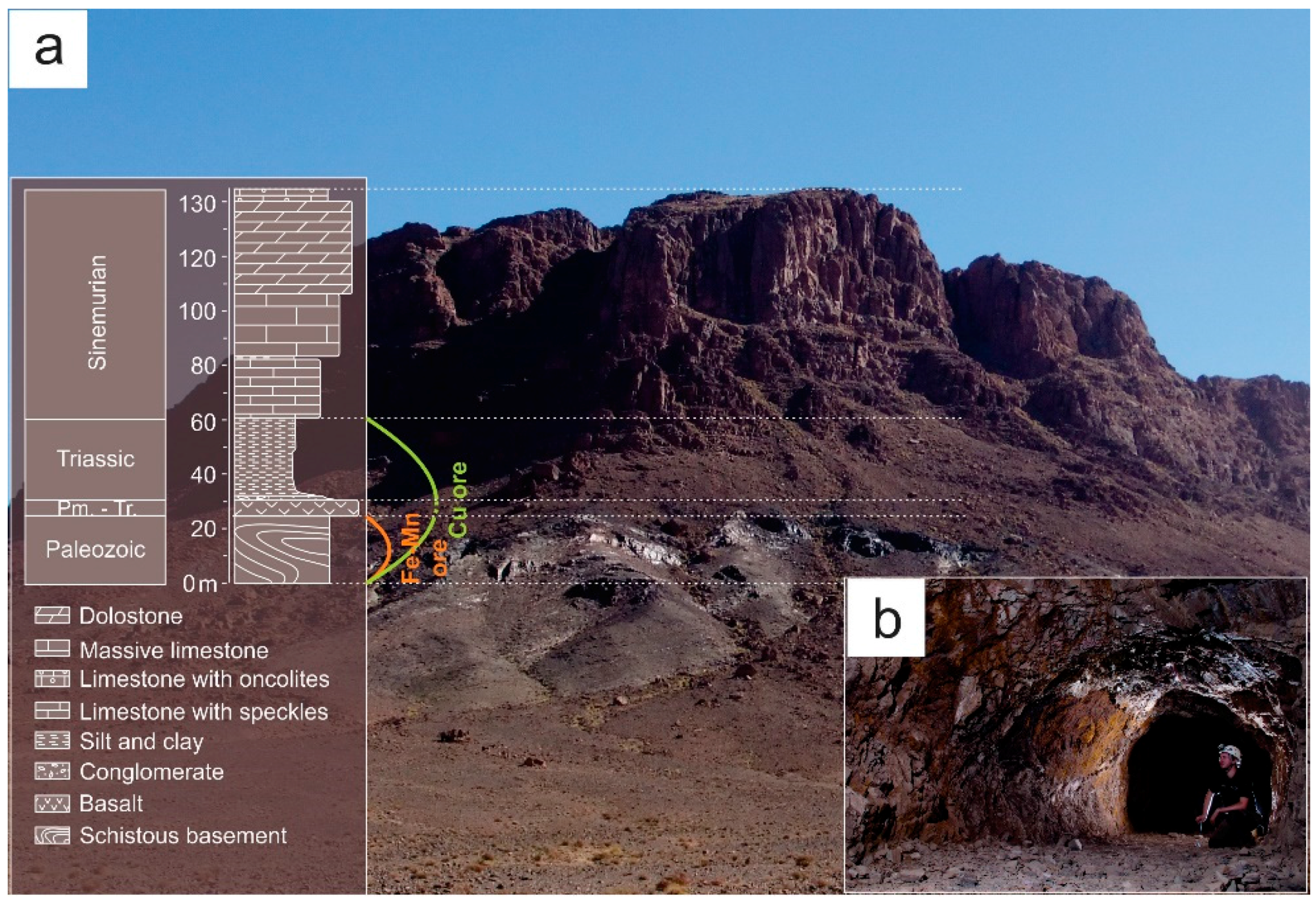
Geological Setting
2. Materials and Methods
3. Results
3.1. Petrographic Characterization
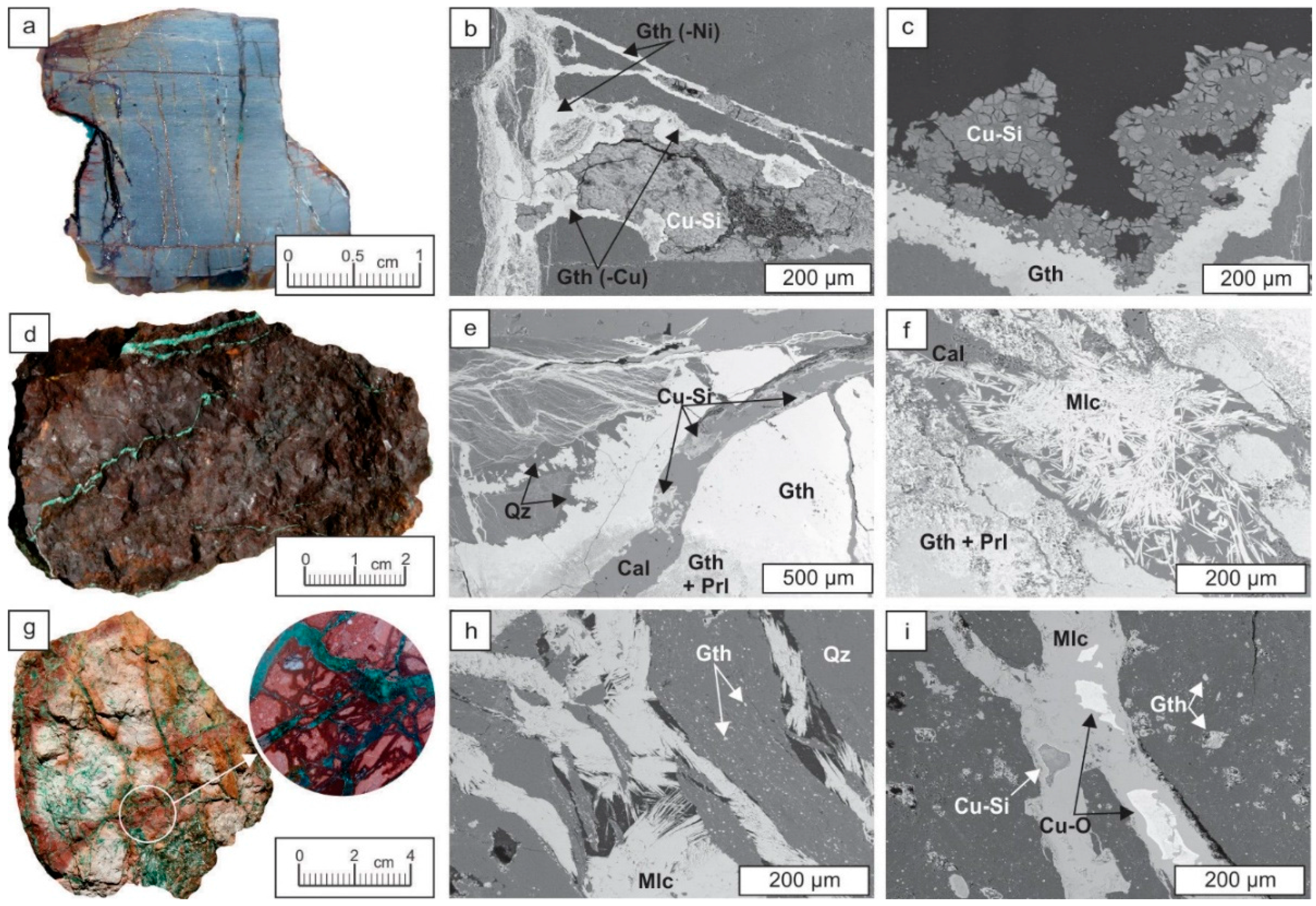
3.1.1. Fe-Mn Mineralization
3.1.2. Cu Mineralization
3.2. Geochemical Characterization
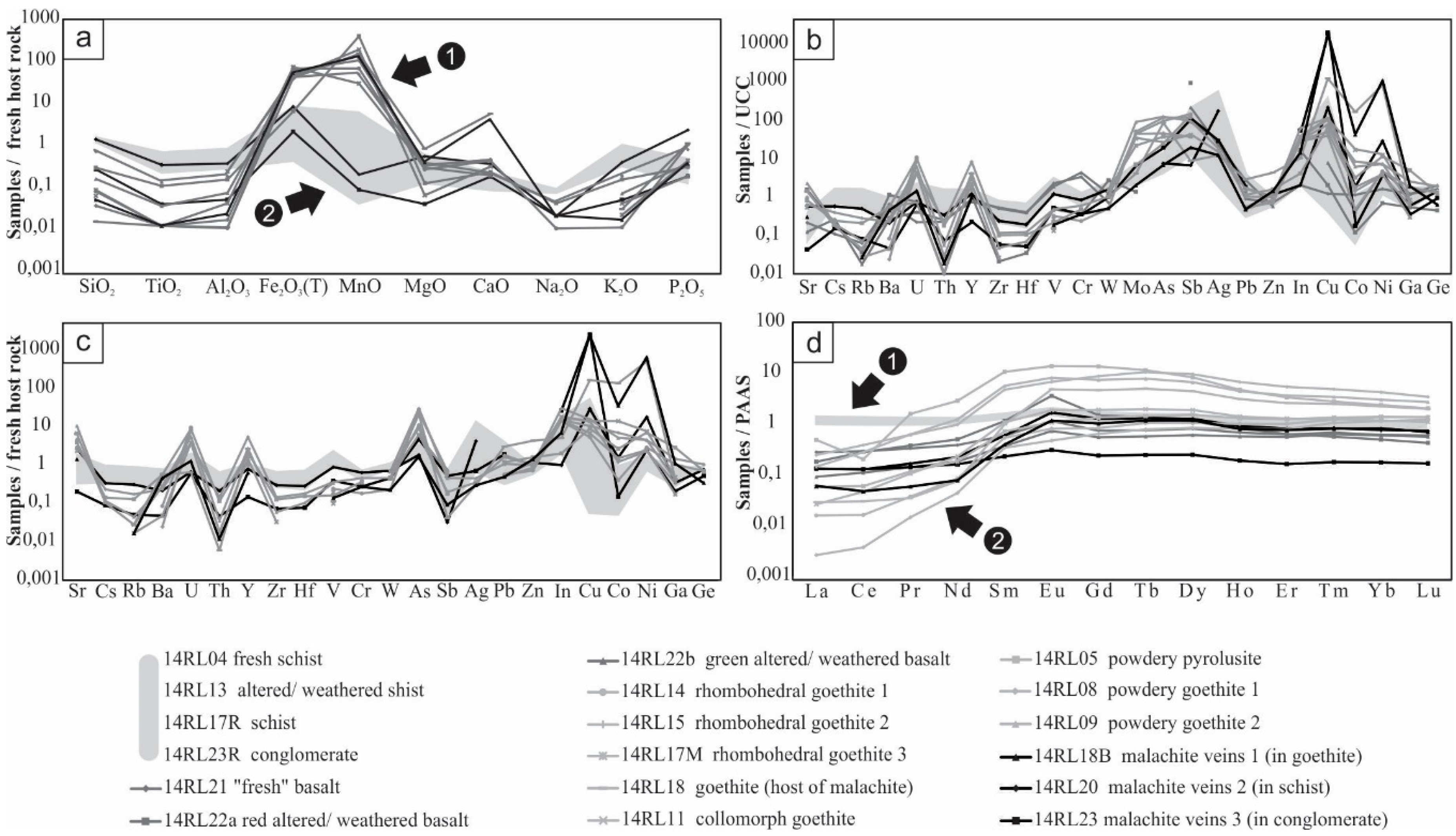
3.2.1. Major Element Patterns
3.2.2. Minor and Trace Elements Patterns
3.2.3. Rare Earth Elements Patterns
4. Discussion
4.1. Hydrothermal Alteration and/or Weathering of Basaltic Rocks
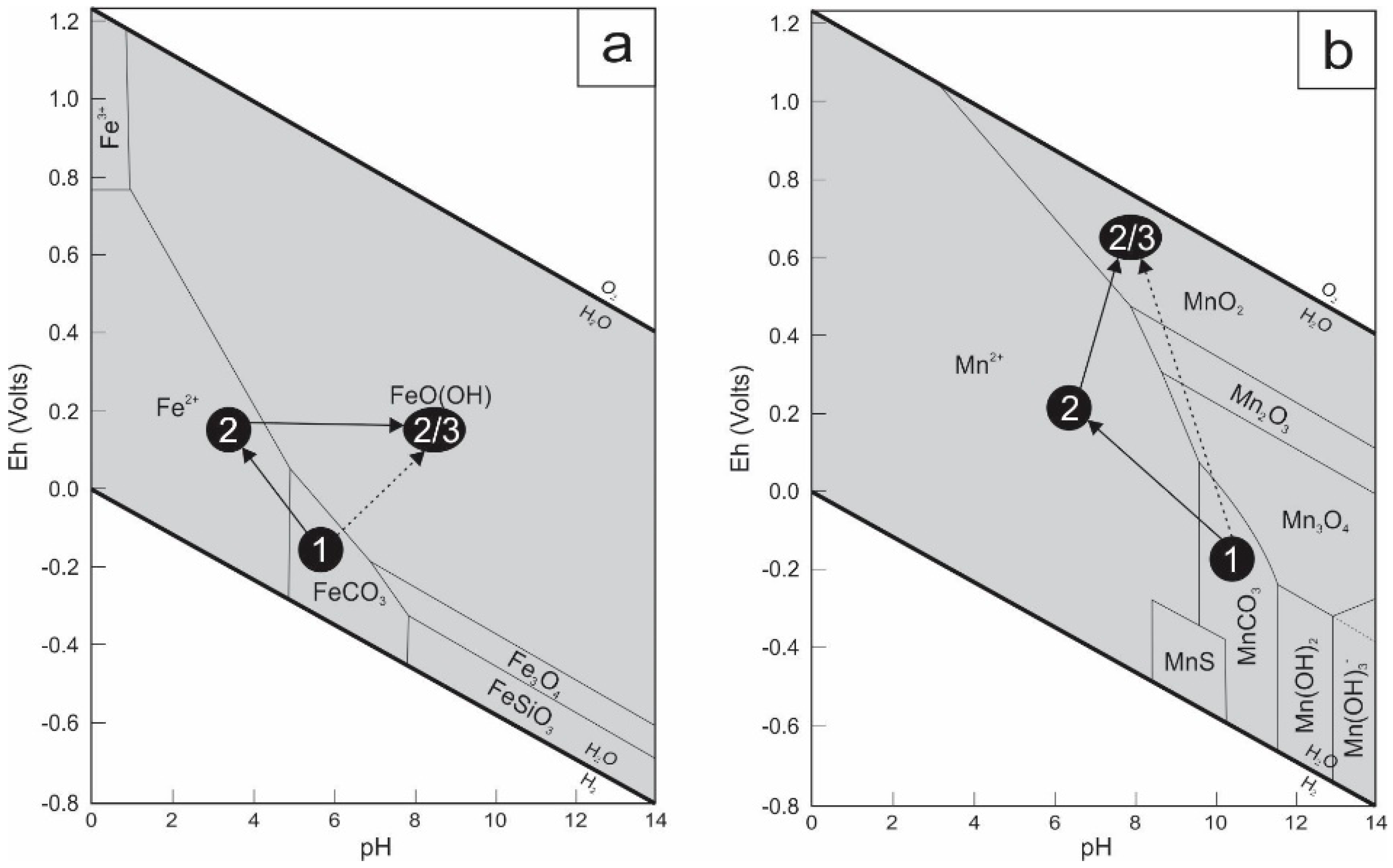
4.2. Fe-Mn Mineralization
4.3. Cu Mineralization
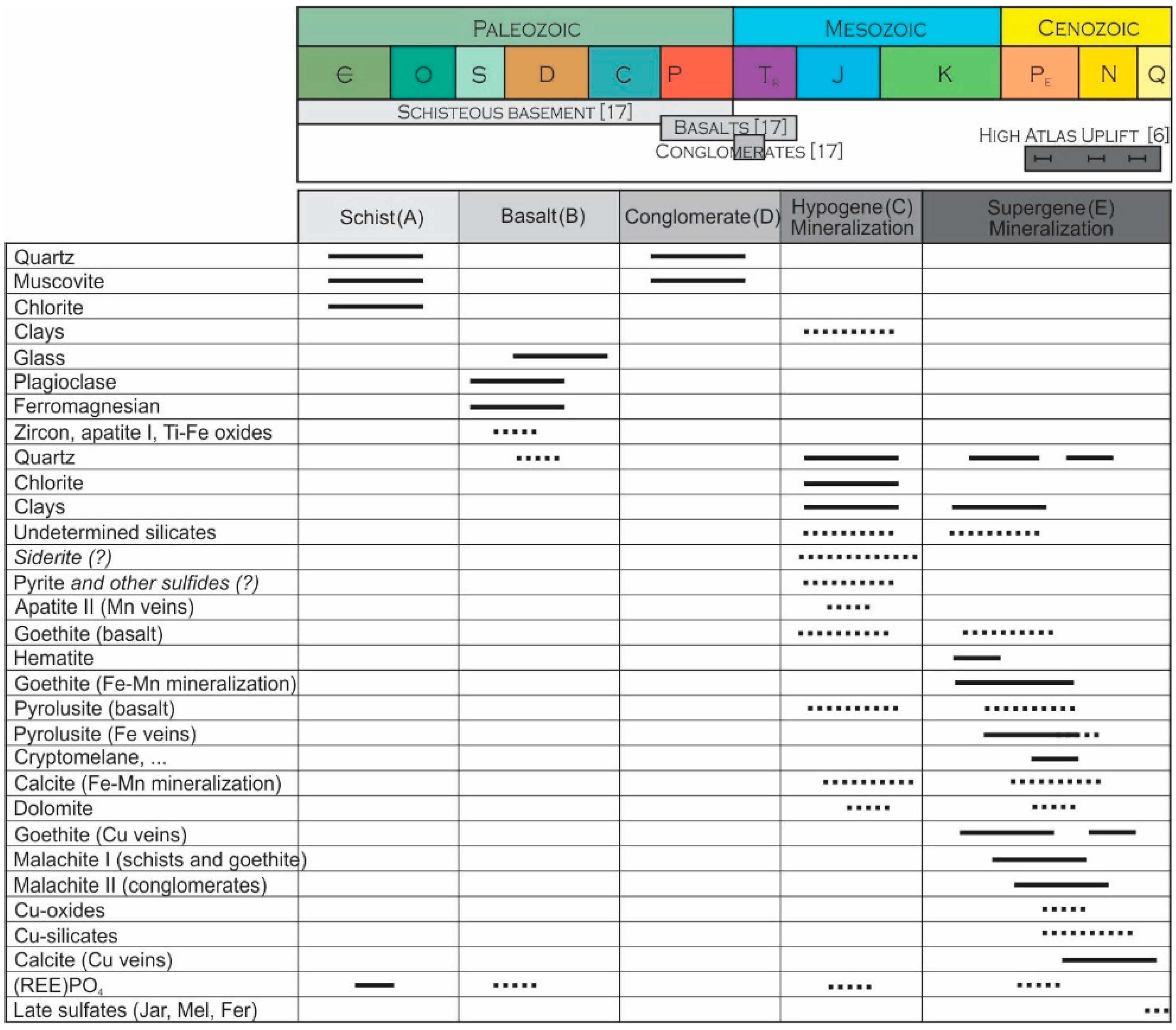
4.4. Late Sulfates
4.5. Metallogenic Model of Formation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Verhaert, M.; Bernard, A.; Dekoninck, A.; Yans, J. Characterization and genesis of Cu-Pb-Zn-V-Fe-Mn supergene ore deposits in the area of Bou Arfa (Oriental High Atlas, Morocco). Geol. Belg. 2016, 19, 303–304. [Google Scholar]
- Verhaert, M.; Bernard, A.; Dekoninck, A.; Lafforgue, L.; Saddiqi, O.; Yans, J. Mineralogical and geochemical characterization of supergene Cu–Pb–Zn–V ores in the Oriental High Atlas, Morocco. Miner. Depos. 2017, 52, 1049–1068. [Google Scholar] [CrossRef]
- Lafforgue, L. Place de la Minéralisation de Manganèse de Bou Arfa Dans L’évolution Méso-Cénozoïque de L’oriental Marocain. Ph.D. Thesis, University Paris-Saclay, Orsay, France, 2016. [Google Scholar]
- Chefchaouni, Y.C.; Diouri, M.; Choubert, G. Carte géologique du Haut Atlas oriental, feuilles Bou Arfa, Iche, Talzaga et Figuig. Notes Mémoires Serv Géol. Maroc 1963, 158. [Google Scholar]
- Frizon de Lamotte, D.; Zizi, M.; Missenard, Y.; Hafid, M.; El Azzouzi, M.; Maury, R.; Charriere, A.; Taki, Z.; Benammi, M.; Michard, A. The Atlas System. In Continental Evolution: The Geology of Morocco: Structure, Stratigraphy, and Tectonics of the Africa-Atlantic-Mediterranean Triple Junction; Michard, A., Ed.; Springer: Heidelberg, Germany, 2008; pp. 133–202. ISBN 3540770755. [Google Scholar]
- Leprêtre, R.; Missenard, Y.; Saint-Bezar, B.; Barbarand, J.; Delpech, G.; Yans, J.; Dekoninck, A.; Saddiqi, O. The three main steps of the Marrakech High Atlas building in Morocco: Structural evidences from the southern foreland, Imini area. J. Afr. Earth Sci. 2015, 109, 177–194. [Google Scholar] [CrossRef]
- Teixell, A.; Arboleya, M.L.; Julivert, M.; Charroud, M. Tectonic shortening and topography in the central High Atlas (Morocco). Tectonics 2003, 22, 1051–1064. [Google Scholar] [CrossRef]
- Laville, E.; Pique, A.; Amrhar, M.; Charroud, M. A restatement of the Mesozoic Atlasic Rifting (Morocco). J. Afr. Earth Sci. 2004, 38, 145–153. [Google Scholar] [CrossRef]
- El Kochri, A.; Chorowicz, J. Oblique extension in the Jurassic trough of the central and eastern High Atlas (Morocco). Can. J. Earth Sci. 1995, 33, 84–92. [Google Scholar] [CrossRef]
- Sarih, S. Géodynamique et Transferts Sédimentaires des Bassins Liasiques Du Haut-Atlas Central (Maroc). Ph.D. Thesis, University of Bourgogne, Dijon, France, 2008. [Google Scholar]
- Mattauer, M.; Tapponnier, P.; Proust, F. Sur les mécanismes de formations des chaines intracontinentales. L’exemple des chaines atlasiques du Maroc. Bull. Soc. Geol. Fr. 1977, 7, 521–526. [Google Scholar] [CrossRef]
- Giese, P.; Jacobshagen, V. Inversion tectonics of intracontinental ranges: High and Middle Atlas, Morocco. Geol. Rundsch. 1992, 81, 249–259. [Google Scholar] [CrossRef]
- Knight, K.B.; Nomade, S.; Renne, P.R.; Marzoli, A.; Bertrand, H.; Youbi, N. The Central Atlantic Magmatic Province at the Triassic-Jurassic boundary: Paleomagnetic and 40Ar/39Ar evidence from Morocco for brief, episodic volcanism. Earth Planet. Sci. Lett. 2004, 228, 143–160. [Google Scholar] [CrossRef]
- Youbi, N.; Martins, L.T.; Munhá, J.M.; Ibouh, H.; Madeira, J.; Aït Chayeb, E.H.; El Boukhari, A. The Late Triassic-Early Jurassic Volcanism of Morocco and Portugal in the Framework of the Central Atlantic Magmatic Province: An Overview. In The Central Atlantic Province; Insights from Fragments of Pangea; Hames, W.E., McHone, J.G., Renne, P.R., Ruppel, C., Eds.; American Geophysical Union: Washington, DC, USA, 2003; pp. 179–207. ISBN 9781118668771. [Google Scholar]
- Laville, E.; Piqué, A. La distension crustale atlantique et atlasique au Maroc au début du Mésozoïque: Le rejeu des structures hercyniennes. Bull. Soc. Geol. Fr. 1991, 162, 1161–1171. [Google Scholar]
- Frizon de Lamotte, D.; Saint Bezar, B.; Bracène, R.; Mercier, E. The two main steps of the Atlas building and geodynamics of the Western Mediterranean. Tectonics 2000, 19, 740–761. [Google Scholar] [CrossRef]
- Missenard, Y.; Zeyen, H.; de Lamotte, D.F.; Leturmy, P.; Petit, C.; Sébrier, M.; Saddiqi, O. Crustal versus asthenospheric origin of relief of the Atlas mountains of Morocco. J. Geophys. Res. 2006, 111, 1–13. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; an Examination of the Geochemical Record Preserved in Sedimentary Rocks; Blackwell Scientific Publications: Oxford, UK, 1985; ISBN 0632011483. [Google Scholar]
- Dekoninck, A.; Bernard, A.; Barbarand, J.; Saint-Bezar, B.; Missenard, Y.; Lepretre, R.; Saddiqi, O.; Yans, J. Detailed mineralogy and petrology of manganese oxyhydroxide deposits of the Imini district (Morocco). Miner. Depos. 2016, 52, 1049–1064. [Google Scholar] [CrossRef]
- Ramdohr, P. The Ore Minerals and Their Intergrowths; Pergamon Press, Ed.; Pergamon Press: Oxford, UK, 1980; ISBN 9780080116358. [Google Scholar]
- Dekayir, A.; El-Maataoui, M. Mineralogy and geochemistry of supergene alteration of an alkali basalt from the Middle Atlas, Morocco. J. Afr. Earth Sci. 2001, 32, 619–633. [Google Scholar] [CrossRef]
- Macaire, J.; Perruchot, A. Transformations Géochimiques au Cours de l’Altération Météorique d’une Basanite Pliocène du Massif Central Français. Geoderma 1988, 41, 287–314. [Google Scholar] [CrossRef]
- Karrat, L.; Perruchot, A.; Macaire, J.J. Weathering of a quaternary glass-rich basalt in Bakrit, Middle Atlas Mountains, Morocco. Geodin. Acta 1998, 11, 205–215. [Google Scholar] [CrossRef]
- Ludden, J.N.; Thompson, G. An evaluation of the behavior of the rare earth elements during the weathering of sea-floor basalt. Earth Planet. Sci. Lett. 1979, 43, 85–92. [Google Scholar] [CrossRef]
- Nesbitt, H.W.; Wilson, R.E. Recent chemical weathering of basalts. Am. J. Sci. 1992, 292, 740–777. [Google Scholar] [CrossRef]
- Maulana, A.; Yonezu, K.; Watanabe, K. Geochemistry of rare earth elements (REE) in the weathered crusts from the granitic rocks in Sulawesi Island, Indonesia. J. Earth Sci. 2014, 25, 460–472. [Google Scholar] [CrossRef]
- Sanematsu, K.; Moriyama, T.; Sotouky, L.; Watanabe, Y. Mobility of Rare Earth Elements in Basalt-Derived Laterite at the Bolaven Plateau, Southern Laos. Resour. Geol. 2011, 61, 140–158. [Google Scholar] [CrossRef]
- Daoudi, L.; Pot de Vin, J.L. Effets thermique et hydrothermal de la coulée de basalte triasico-liasique sur les argiles du bassin d’Argana (Maroc). C. R. Geosci. 2002, 334, 463–468. [Google Scholar] [CrossRef]
- Kamel, S.; Bouabid, R.; Boulangé, B.; Colin, F. Paléoaltérations hydrothermale et supergène dans un basalte triasique du Moyen Atlas, Maroc. J. Afr. Earth Sci. 1996, 23, 225–235. [Google Scholar] [CrossRef]
- Torres-Alvarado, I.S.; Pandarinath, K.; Verma, S.P.; Dulski, P. Mineralogical and geochemical effects due to hydrothermal alteration in the Los Azufres geothermal field, Mexico. Rev. Mex. Cienc. Geol. 2007, 24, 15–24. [Google Scholar]
- Hamidi, E.M.; Boulangé, B.; Colin, F. Altération d’un basalte triasique de la région d’Elhajeb, Moyen Atlas, Maroc. J. Afr. Earth Sci. 1997, 24, 141–151. [Google Scholar] [CrossRef]
- Morey, G.B.; Setterholm, D.R. Rare earth elements in weathering profiles and sediments of Minnesota: Implicatinos for provenance studies. J. Sediment. Res. 1997, 67, 105–115. [Google Scholar] [CrossRef]
- Lewis, A.J.; Palmer, M.R.; Sturchio, N.C.; Kemp, A.J. The rare earth element geochemistry of acid-sulphate and acid-sulphate-chloride geothermal systems from Yellowstone National Park, Wyoming, USA. Geochim. Cosmochim. Acta 1997, 61, 695–706. [Google Scholar] [CrossRef]
- Sverjensky, D.A. Europium redox equilibria in aqueous solution. Earth Planet. Sci. Lett. 1984, 67, 70–78. [Google Scholar] [CrossRef]
- Alderton, D.H.M.; Pearce, J.A.; Potts, P.J. Rare earth element mobility during granite alteration: Evidence from southwest England. Earth Planet. Sci. Lett. 1980, 49, 149–165. [Google Scholar] [CrossRef]
- Dawood, Y.H.; Abd El-Naby, H.H.; Sharafeldin, A.A. Influence of the alteration processes on the origin of uranium and europium anomalies in trachyte, central Eastern Desert, Egypt. J. Geochem. Explor. 2004, 88, 15–27. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Peter, J.M.; Layton-Matthews, D.; Volesky, J.; Boyle, D.R. Mobility and fractionation of rare earth elements during supergene weathering and gossan formation and chemical modification of massive sulfide gossan. Geochim. Cosmochim. Acta 2006, 70, 1091–1112. [Google Scholar] [CrossRef]
- Michard, A. Rare earth element systematics in hydrothermal fluids. Geochim. Cosmochim. Acta 1989, 53, 745–750. [Google Scholar] [CrossRef]
- Kholodov, V.N.; Butuzova, G.Y. Siderite formation and evolution of sedimentary iron ore deposition in the Earth’s history. Geol. Ore Depos. 2008, 50, 299–319. [Google Scholar] [CrossRef]
- Skala, G.; Hollabaugh, C.L. Goethite after siderite: The presence of goethite after siderite pseudomorphs in a pegmatic matrix from Lake George, Colorado. Geol. Soc. Am. 2012, 44, 6. [Google Scholar]
- Makkoudi, D. Minéralisations Pb-Ba de M’fiss: Etude Géologique et Contribution à la Gîtologie des Gisements du Tafilalet. Ph.D. Thesis, University Mohammed V, Rabat, Morocco, 1995. [Google Scholar]
- Garasic, V.; Jurkovic, I. Geochemical characteristics of different iron ore types from the Southern Tomašica deposit, Ljubija, NW Bosnia. Geol. Croat. 2012, 65, 255–270. [Google Scholar] [CrossRef]
- Markl, G.; von Blanckenburg, F.; Wagner, T. Iron isotope fractionation during hydrothermal ore deposition and alteration. Geochim. Cosmochim. Acta 2006, 70, 3011–3030. [Google Scholar] [CrossRef]
- Torres Ruiz, J. Genesis and evolution of the Marquesado and adjacent ore deposits, Granada, Spain. Econ. Geol. 1983, 78, 1657–1673. [Google Scholar] [CrossRef]
- Decrée, S.; De Putter, T.; Yans, J.; Moussi, B.; Recourt, P.; Jamoussi, F.; Bruyère, D.; Dupuis, C. Iron mineralisation in Mio-Pliocene sediments of the Tamra iron mine (Nefza mining district, Tunisia): Mixed influence of pedogenesis and hydrothermal alteration. Ore Geol. Rev. 2008, 33, 397–410. [Google Scholar] [CrossRef]
- Palinkaš, L.; Damyanov, Z.K.; Borojević Šoštarić, S.; Strmić Palinkaš, S.; Marinova, I. Divergent drift of Adriatic-Dinaridic and Moesian carbonate platforms during the rifting phase witnessed by triassic MVT Pb-Zn and SEDEX deposits; a metallogenic approach. Geol. Croat. 2016, 69, 75–78. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berlin, Heidelberg, 1988; ISBN 3540184856, 0387184856, 9780387184852, 9783540184850. [Google Scholar]
- Pracejus, B.; Bolton, B.R.; Frakes, L.A.; Abbott, M. Rare-earth element geochemistry of supergene manganese deposits from Groote Eylandt, Northern Territory, Australia. Ore Geol. Rev. 1990, 5, 293–314. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Bruyère, D.; De Putter, T.; Decrée, S.; Dupuis, C.; Fuchs, Y.; Jamoussi, F.; Perruchot, A.; Arbey, F. Miocene karsts and associated Fe–Zn-rich minerals in Aïn Khamouda (Central Tunisia). J. Afr. Earth Sci. 2010, 57, 70–78. [Google Scholar] [CrossRef]
- Koppi, A.J.; Edis, R.; Field, D.J.; Geering, H.R.; Klessa, D.A.; Cockayne, D.J.H. Rare earth element trends and cerium-uranium-manganese associations in weathered rock from Koongarra, Northern Territory, Australia. Geochim. Cosmochim. Acta 1996, 60, 1695–1707. [Google Scholar] [CrossRef]
- Pagel, M.; Braun, J.; Muller, J. Mécanismes de fractionnement géochimique des terres rares, de l’uranium et du thorium lors des altérations supergènes. In Séminaire ORSTOM 90: Organisation et Fonctionnement des Altérites et des Sols; Wackermann, J.M., Ed.; ORSTOM: Bondy, France, 1990; pp. 219–226. [Google Scholar]
- De Putter, T.; André, L.; Bernard, A.; Dupuis, C.; Jedwab, J.; Nicaise, D.; Perruchot, A. Trace element (Th, U, Pb, REE) behaviour in a cryptokarstic halloysite and kaolinite deposit from Southern Belgium: Importance of “accessory” mineral formation for radioactive pollutant trapping. Appl. Geochem. 2002, 17, 1313–1328. [Google Scholar] [CrossRef]
- Nicaise, D.; André, L.; Jedwab, J.; Dupuis, C.; De Putter, T. Neoformed LREE phosphates at the nanometer scale, in acid low temperature weathering: Consequences in rare earth elements, uranium and thorium trapping. C. R. Acad. Sci. Paris 1996, 323, 113–120. [Google Scholar]
- Braun, J.J.; Pagel, M.; Muller, J.P.; Bilong, P.; Michard, A.; Guillet, B. Cerium anomalies in lateritic profiles. Geochim. Cosmochim. Acta 1990, 54, 781–795. [Google Scholar] [CrossRef]
- Decrée, S.; Deloule, É.; Ruffet, G.; Dewaele, S.; Mees, F.; Marignac, C.; Yans, J.; De Putter, T. Geodynamic and climate controls in the formation of Mio–Pliocene world-class oxidized cobalt and manganese ores in the Katanga province, DR Congo. Miner. Depos. 2010, 45, 621–629. [Google Scholar] [CrossRef]
- Kamineni, D.C.; Chung, C.F.; Dugal, J.J.B.; Ejeckam, R.B. Distribution of uranium and thorium in core samples from the Underground Research Laboratory lease area, southeastern Manitoba, Canada. Chem. Geol. 1986, 54, 97–111. [Google Scholar] [CrossRef]
- Decrée, S.; Marignac, C.; De Putter, T.; Yans, J.; Clauer, N.; Dermech, M.; Aloui, K.; Baele, J.M. The Oued Belif hematite-rich breccia: A Miocene iron oxide Cu-Au-(U-REE) deposit in the Nefza mining district, Tunisia. Econ. Geol. 2013, 108, 1425–1457. [Google Scholar] [CrossRef]
- Farmer, J.G.; Lovell, M.A. Natural enrichment of arsenic in Loch Lomond sediments. Geochim. Cosmochim. Acta 1986, 50, 2059–2067. [Google Scholar] [CrossRef]
- Bau, M.; Dulski, P. Distribution of yttrium and rare-earth elements in the Penge and Kuruman iron-formations, Transvaal Supergroup, South Africa. Precambrian Res. 1996, 79, 37–55. [Google Scholar] [CrossRef]
- Cotten, J.; Le Dez, A.; Bau, M.; Caroff, M.; Maury, R.C.; Dulski, P.; Fourcade, S.; Bohn, M.; Brousse, R. Origin of anomalous rare-earth element and yttrium enrichments in subaerially exposed basalts: Evidence from French Polynesia. Chem. Geol. 1995, 119, 115–138. [Google Scholar] [CrossRef]
- Castor, S.B.; Hendrick, J.B. Rare Earth Elements. In Industrial Minerals and Rocks; Kogel, J.E., Trivedi, N.C., Barker, J.M., Eds.; Society for Mining, Metallurgy and Exploration: Littleton, CO, USA, 2006; pp. 769–792. ISBN 4186530777. [Google Scholar]
- Fodor, R.V.; Frey, F.A.; Bauer, G.R.; Clague, D.A. Ages, rare-earth element enrichment, and petrogenesis of tholeiitic and alkalic basalts from Kahoolawe Island, Hawaii. Contrib. Mineral. Petrol. 1992, 110, 442–462. [Google Scholar] [CrossRef]
- Cocker, M.D. Lateritic, supergene rare earth element (REE) deposits. In 48th Annual Forum on the Geology of Industrial Minerals; Arizona Geological Survey: Scottsdale, AZ, USA, 2012; pp. 1–20. [Google Scholar]
- Leybourne, M.I.; Johannesson, K.H. Rare earth elements (REE) and yttrium in stream waters, stream sediments, and Fe-Mn oxyhydroxides: Fractionation, speciation, and controls over REE + Y patterns in the surface environment. Geochim. Cosmochim. Acta 2008, 72, 5962–5983. [Google Scholar] [CrossRef]
- Ayora, C.; Macías, F.; Torres, E.; Nieto, J.M. Rare Earth Elements in Acid Mine Drainage. In XXXV Reunión de la Sociedad Española de Mineralogía; Sociedad Espanola de Mineralogia: Huelva, Spain, 2015; pp. 1–22. [Google Scholar]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- Banfield, J.F. Apatite Replacement and Rare Earth Mobilization, Fractionation, and Fixation During Weathering. Clays Clay Miner. 1989, 37, 113–127. [Google Scholar] [CrossRef]
- Hannigan, R.E.; Sholkovitz, E.R. The development of middle rare earth element enrichments in freshwaters: Weathering of phosphate minerals. Chem. Geol. 2001, 175, 495–508. [Google Scholar] [CrossRef]
- Köhler, S.J.; Harouiya, N.; Chaïrat, C.; Oelkers, E.H. Experimental studies of REE fractionation during water–mineral interactions: REE release rates during apatite dissolution from pH 2.8 to 9.2. Chem. Geol. 2005, 222, 168–182. [Google Scholar] [CrossRef]
- Göb, S.; Wenzel, T.; Bau, M.; Jacob, D.E.; Loges, A.; Markl, G. The redistribution of rare-earth elements in secondary minerals of hydrothermal veins, Schwarzwald, Southwestern Germany. Can. Mineral. 2011, 49, 1305–1333. [Google Scholar] [CrossRef]
- Lottermoser, B.G. Rare-earth element mineralisation within the Mt. Weld carbonatite laterite, Western Australia. Lithos 1990, 24, 151–167. [Google Scholar] [CrossRef]
- De Putter, T.; Charlet, J.-M.M.; Quinif, Y. REE, Y and U concentration at the fluid-iron oxide interface in late cenozoic cryptodolines from Southern Belgium. Chem. Geol. 1999, 153, 139–150. [Google Scholar] [CrossRef]
- Bouabdellah, M.; Margoum, D. Geology, Fluid Inclusions, and Geochemistry of the Aouli Sulphide-Fluorite-Barite Vein Deposit (Upper Moulouya District, Morocco) and Its Relationships to Pangean Rifting and Opening of the Tethys and Central Atlantic Oceans. In Mineral Deposits of North Africa, Mineral Resource Reviews; Bouabdellah, M., Slack, J.F., Eds.; Springer: Cham, Switzerland, 2016; pp. 291–305. [Google Scholar]
- Bouabdellah, M.; Zemri, O.; Jébrak, M.; Klügel, A.; Levresse, G.; Maacha, L.; Gaouzi, A.; Souiah, M. Geology and Mineralogy of the El Hammam REE-Rich Fluorite Deposit (Central Morocco): A Product of Transtensional Pangean Rifting and Central Atlantic Opening. In Mineral Deposits of North Africa, Mineral Resource Reviews; Bouabdellah, M., Slack, J.F., Eds.; Springer: Cham, Switzerland, 2016; pp. 307–324. [Google Scholar]
- Cheilletz, A.; Gasquet, D.; Filali, F.; Archibald, D.A.; Nespolo, M. A Late Triassic 40Ar/39Ar age for the El Hammam high-REE fluorite deposit (Morocco): Mineralization related to the Central Atlantic Magmatic Province. Miner. Depos. 2010, 45, 323–329. [Google Scholar] [CrossRef]
- Margoum, D.; Bouabdellah, M.; Klügel, A.; Banks, D.A.; Castorina, F.; Cuney, M.; Jébrak, M.; Bozkaya, G. Pangea rifting and onward pre-Central Atlantic opening as the main ore-forming processes for the genesis of the Aouli REE-rich fluorite–barite vein system, upper Moulouya district, Morocco. J. Afr. Earth Sci. 2015, 108, 22–39. [Google Scholar] [CrossRef]


| Oxides/Elements | Detection Limit | Host Rocks | Basalts | Fe-Mn Mineralization | Cu-Mineralization | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14RL04 (Fresh Schist) | 14RL13 (W/A Schist) | 14RL17R (Host Schist of Goethite) | 14RL23R (Conglomerate Host of Malachite) | 14RL21 (Fresh Basalt) | 14RL22A (Red W/A Basalt) | 14RL22B (Green W/A Basalt) | 14RL05 (Powdery Pyrolusite) | 14RL08 (Powdery Goethite) | 14RL09 (Powdery Goethite) | 14RL11 (Collomorph Goethite) | 14RL14 (Rhombohedral Goethite) | 14RL15 (Rhombohedral Goethite) | 14RL17M (Rhombohedral Goethite) | 14RL18 (Goethite Host of Malachite) | 14RL18B (Malachite in Goethite) | 14RL20 (Malachite in Schist) | 14RL23 (Malachite in Conglomerate) | ||
| Al2O3 (%) | 0.01 | 18.84 | 8.79 | 15.23 | 5.00 | 13.14 | 0.70 | 11.86 | 2.77 | 3.31 | 1.32 | 4.31 | 0.19 | 0.30 | 0.75 | 0.20 | 0.41 | 6.63 | 0.92 |
| CaO (%) | 0.01 | 0.87 | 0.07 | 0.19 | 0.20 | 5.57 | 26.45 | 2.54 | 0.19 | 0.17 | 0.38 | 0.26 | 0.36 | 0.38 | 0.16 | 4.72 | 3.44 | 0.30 | 0.15 |
| Fe2O3 (%) | 0.01 | 1.06 | 0.43 | 9.08 | 1.68 | 4.80 | 15.76 | 2.99 | 6.63 | 39.15 | 49.13 | 44.81 | 69.85 | 62.97 | 75.38 | 41.79 | 54.59 | 8.62 | 2.16 |
| K2O (%) | 0.01 | 3.81 | 2.45 | 3.82 | 1.14 | 0.22 | 0.05 | 0.05 | 0.56 | 0.68 | 0.26 | 0.15 | 0.04 | <0.01 | 0.08 | 0.11 | 0.06 | 1.44 | 0.18 |
| MgO (%) | 0.01 | 2.11 | 0.34 | 1.22 | 0.25 | 5.73 | 5.37 | 4.68 | 0.25 | 0.71 | 0.66 | 0.13 | 0.54 | 0.69 | 0.60 | 1.67 | 0.80 | 1.11 | 0.08 |
| MnO (%) | 0.001 | 0.156 | 0.028 | 0.935 | 0.006 | 0.921 | 4.441 | 0.279 | 60.940 | 7.391 | 21.220 | 29.150 | 10.330 | 16.150 | 4.502 | 23.020 | 19.840 | 0.030 | 0.013 |
| Na2O (%) | 0.01 | 1.01 | 0.07 | 0.09 | 0.07 | 2.98 | 0.05 | 0.74 | 0.04 | 0.04 | <0.01 | <0.01 | 0.01 | <0.01 | <0.01 | <0.01 | 0.02 | 0.02 | 0.02 |
| P2O5 (%) | 0.01 | 0.17 | 0.02 | 0.08 | 0.03 | 0.14 | <0.01 | 0.19 | 0.05 | 0.13 | 0.06 | 0.03 | 0.17 | 0.05 | 0.07 | 0.18 | 0.06 | 0.38 | 0.03 |
| SiO2 (%) | 0.01 | 58.13 | 85.67 | 65.44 | 88.22 | 44.42 | 16.73 | 54.21 | 16.28 | 39.58 | 8.86 | 3.45 | 4.44 | 2.02 | 4.78 | 0.84 | 2.77 | 77.56 | 14.93 |
| TiO2 (%) | 0.01 | 0.88 | 0.53 | 0.59 | 0.18 | 1.07 | 0.03 | 0.95 | 0.09 | 0.11 | 0.03 | < 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.29 | 0.03 |
| LOI (%) | 4.67 | 1.59 | 4.29 | 2.03 | 9.79 | 28.72 | 6.88 | 10.45 | 8.17 | 12.27 | 11.66 | 12.41 | 12.91 | 11.36 | 15.90 | 11.94 | 3.14 | 25.09 | |
| Total (%) | 0.01 | 91.70 | 99.99 | 100.96 | 98.81 | 88.78 | 98.30 | 85.37 | 98.25 | 99.44 | 94.19 | 93.95 | 98.35 | 95.48 | 97.69 | 88.44 | 93.94 | 99.52 | 43.61 |
| FeO (%) | 0.1 | 6.0 | 0.1 | 1.7 | <0.1 | 8.7 | <0.1 | 12.4 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 2.6 | <0.1 |
| V (ppm) | 5 | 142 | 195 | 140 | 333 | 237 | 51 | 197 | 23 | 51 | 48 | 14 | 34 | 18 | 28 | 16 | 20 | 125 | 55 |
| Cr (ppm) | 20 | 110 | 60 | 80 | 50 | 280 | 30 | 360 | 40 | 50 | <20 | <20 | 20 | <20 | <20 | <20 | 30 | 70 | 30 |
| Co (ppm/%) | 1 | 20 | 1 | 19 | 1 | 19 | 2 | 7 | 53 | 138 | 102 | 277 | 8 | 24 | 30 | 0.268 * | 702 | 31 | 3 |
| Ni (ppm/%) | 20 | 70 | <20 | 120 | 50 | 70 | 30 | 200 | 490 | 310 | 310 | 530 | 200 | 160 | 170 | 3.48 * | 4.54 * | 1230 | 170 |
| Cu (ppm/%) | 10 | 180 | 10 | 150 | 9480 | 30 | 50 | 190 | 1600 | 1860 | 2790 | 2530 | 950 | 2480 | 1200 | 2.92 * | 43.9 * | 5160 | 44.5 * |
| Zn (ppm) | 30 | 70 | <30 | 40 | <30 | 50 | <30 | 40 | 110 | 70 | 70 | 70 | 50 | 80 | 60 | 300 | 100 | 80 | <30 |
| Ga (ppm) | 1 | 29 | 17 | 24 | 8 | 20 | 9 | 20 | 82 | 42 | 12 | 14 | 30 | 10 | 5 | 12 | 32 | 10 | 6 |
| Ge (ppm) | 1 | 3 | 3 | 3 | 2 | 3 | 1 | 3 | 2 | 3 | <1 | <1 | 2 | <1 | <1 | <1 | 1 | 2 | 2 |
| As (ppm) | 5 | 6 | <5 | 9 | 21 | <5 | <5 | <5 | 36 | 138 | 180 | 37 | 168 | 66 | 54 | 124 | 10 | 11 | 28 |
| Rb (ppm) | 2 | 179 | 117 | 162 | 57 | 7 | 4 | 2 | 23 | 31 | 8 | 5 | <2 | <2 | 3 | 5 | 3 | 55 | 9 |
| Sr (ppm) | 2 | 74 | 24 | 138 | 57 | 74 | 537 | 43 | 283 | 220 | 776 | 496 | 205 | 339 | 176 | 476 | 105 | 196 | 15 |
| Y (ppm) | 1/0.5 | 33 | 27 | 35 | 26 | 19 | 24 | 16 | 69 | 179 | 90 | 38 | 61 | 32 | 48 | 88 | 21 | 26 | 5 |
| Zr (ppm) | 5 | 152 | 84 | 101 | 40 | 94 | 4 | 100 | 19 | 22 | 9 | <5 | <5 | <5 | 5 | <5 | <5 | 45 | 11 |
| Nb (ppm) | 1 | 17 | 8 | 9 | 3 | 9 | 5 | 7 | <1 | 2 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | 3 | 1 |
| Mo (ppm/%) | 2 | <2 | 11 | 3 | 7 | <2 | 2 | <2 | 10 | 64 | 0.013 * | 48 | 74 | 64 | 32 | 6 | 3 | <2 | 10 |
| Ag (ppm) | 0.5 | 2.0 | 3.9 | 1.3 | 27.6 | 1.5 | <0.5 | 1.1 | <0.5 | <0.5 | 0.9 | 0.7 | <0.5 | <0.5 | <0.5 | 0.6 | 0.6 | 8.4 | 1.4 |
| In (ppm) | 0.1 | 0.1 | <0.1 | 0.2 | 1.9 | 0.1 | 0.8 | <0.1 | 0.2 | 1.5 | 3.0 | 1.8 | 1.4 | 1.7 | 1.5 | 0.5 | 0.7 | 0.1 | 2.6 |
| Sb (ppm) | 0.2 | 40.7 | 30.7 | 2.3 | 2.7 | 17.3 | 179.0 | 41.4 | 26.2 | 28.2 | 18.5 | 8.0 | 22.7 | 7.2 | 7.4 | 1.7 | 3.8 | 1.3 | 21.1 |
| Cs (ppm) | 0.5 | 7.8 | 3.1 | 7.6 | 3.7 | 0.5 | 0.9 | 1.2 | 1.1 | 1.8 | 0.9 | <0.5 | <0.5 | <0.5 | <0.5 | 0.8 | <0.5 | 2.6 | 0.7 |
| Ba (ppm) | 1 | 527 | 353 | 437 | 151 | 353 | 615 | 142 | 297 | 123 | 148 | 146 | 45 | 13 | 25 | 302 | 243 | 114 | 25 |
| Hf (ppm) | 0.2 | 3.9 | 2.1 | 2.8 | 0.9 | 2.2 | 0.2 | 2.3 | 0.6 | 0.7 | 0.4 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | 1.1 | 0.3 |
| Ta (ppm) | 0.1 | 1.4 | 1.0 | 1.1 | 0.6 | 0.6 | 0.2 | 0.5 | <0.1 | <0.1 | 0.3 | 0.3 | <0.1 | 0.3 | 0.3 | 0.3 | <0.1 | 0.6 | 0.1 |
| W (ppm) | 1 | 4 | 5 | 4 | 4 | 1 | 5 | 2 | <1 | 2 | 3 | 2 | 1 | 2 | 3 | 2 | 1 | 3 | 2 |
| Tl (ppm) | 0.1 | 0.9 | 0.6 | 0.7 | 0.5 | 0.1 | <0.1 | <0.1 | <0.1 | 0.8 | 0.9 | 1.6 | <0.1 | 0.1 | <0.1 | 0.5 | 0.7 | 0.3 | <0.1 |
| Pb (ppm) | 5 | 16 | 65 | 5 | 28 | 15 | 21 | 46 | 19 | 29 | 17 | 26 | 9 | 9 | 8 | 48 | 8 | <5 | 30 |
| Bi (ppm) | 0.1 | 0.1 | 11.8 | <0.1 | 2.1 | <0.1 | <0.1 | <0.1 | <0.1 | 1.0 | 0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | <0.1 | 1.1 |
| Th (ppm) | 0.1 | 16.8 | 8.8 | 11.0 | 3.6 | 2.4 | 0.1 | 2.7 | 1.9 | 2.4 | 0.6 | <0.1 | <0.1 | <0.1 | 0.3 | 0.1 | 0.2 | 3.6 | 0.8 |
| U (ppm) | 0.1 | 3.1 | 6.4 | 2.3 | 5.3 | 0.6 | 2.1 | 1.1 | 27.0 | 11.8 | 17.2 | 11.2 | 29.3 | 24.8 | 11.8 | 2.0 | 4.0 | 2.1 | 1.9 |
| La (ppm) | 0.1 | 54.7 | 37.2 | 41.1 | 35.8 | 10.6 | 6.9 | 3.5 | 18.5 | 9.3 | 5.5 | 1.0 | 0.6 | 0.1 | 2.2 | 1.1 | 2.3 | 4.9 | 5.0 |
| Ce (ppm) | 0.1 | 111.0 | 77.6 | 103.0 | 73.1 | 23.3 | 22.4 | 8.9 | 16.2 | 30.7 | 22.4 | 3.7 | 1.3 | 0.3 | 4.8 | 2.4 | 3.8 | 10.2 | 10.3 |
| Pr (ppm) | 0.01 | 12.10 | 9.37 | 10.00 | 8.17 | 2.95 | 3.35 | 1.25 | 14.10 | 5.46 | 5.56 | 0.94 | 0.33 | 0.13 | 1.04 | 0.31 | 0.52 | 1.48 | 1.26 |
| Nd (ppm) | 0.1 | 42.3 | 38.0 | 39.2 | 31.7 | 12.3 | 15.9 | 5.8 | 91.4 | 31.0 | 39.3 | 7.3 | 2.7 | 1.4 | 5.9 | 2.4 | 2.5 | 7.14 | 5.14 |
| Sm (ppm) | 0.1 | 8.2 | 8.4 | 9.1 | 6.8 | 3.3 | 6.4 | 2.1 | 59.4 | 26.5 | 31.5 | 6.0 | 3.0 | 1.9 | 4.0 | 5.5 | 2.2 | 3.3 | 1.3 |
| Eu (ppm) | 0.05 | 1.75 | 1.77 | 2.21 | 1.68 | 1.26 | 3.93 | 0.80 | 15.10 | 7.41 | 8.92 | 2.05 | 0.89 | 0.52 | 1.18 | 5.29 | 1.28 | 1.85 | 0.34 |
| Gd (ppm) | 0.1 | 6.6 | 5.5 | 8.2 | 5.0 | 3.5 | 7.0 | 2.6 | 63.4 | 41.0 | 34.8 | 8.9 | 3.9 | 3.0 | 6.3 | 21.7 | 4.8 | 6.0 | 1.1 |
| Tb (ppm) | 0.1 | 1.0 | 0.9 | 1.3 | 0.8 | 0.6 | 1.0 | 0.4 | 8.8 | 8.1 | 6.0 | 1.5 | 0.8 | 0.6 | 1.1 | 3.8 | 0.9 | 1.0 | 0.2 |
| Dy (ppm) | 0.1 | 6.0 | 5.1 | 6.8 | 4.7 | 3.6 | 4.9 | 2.7 | 36.4 | 42.0 | 29.3 | 8.3 | 4.6 | 3.7 | 6.7 | 19.5 | 5.2 | 5.6 | 1.1 |
| Ho (ppm) | 0.1 | 1.1 | 0.9 | 1.2 | 0.8 | 0.7 | 0.8 | 0.6 | 4.7 | 6.7 | 4.4 | 1.4 | 1.0 | 0.8 | 1.3 | 3.1 | 0.8 | 0.9 | 0.2 |
| Er (ppm) | 0.1 | 3.3 | 2.6 | 3.4 | 2.2 | 1.8 | 1.8 | 1.6 | 9.7 | 15.6 | 10.5 | 3.7 | 2.9 | 2.3 | 3.6 | 7.5 | 2.2 | 2.4 | 0.5 |
| Tm (ppm) | 0.05 | 0.48 | 0.39 | 0.46 | 0.33 | 0.28 | 0.23 | 0.26 | 1.13 | 1.98 | 1.40 | 0.51 | 0.50 | 0.43 | 0.55 | 1.02 | 0.33 | 0.34 | 0.07 |
| Yb (ppm) | 0.1 | 3.2 | 2.6 | 2.9 | 2.1 | 1.7 | 1.4 | 1.7 | 6.6 | 11.8 | 8.4 | 3.3 | 3.8 | 3.2 | 3.9 | 6.2 | 2.3 | 2.1 | 0.5 |
| Lu (ppm) | 0.01 | 0.49 | 0.38 | 0.43 | 0.30 | 0.26 | 0.18 | 0.24 | 0.87 | 1.47 | 1.16 | 0.47 | 0.59 | 0.52 | 0.58 | 0.85 | 0.30 | 0.31 | 0.07 |
| La/Lu | 111.4 | 99.2 | 95.1 | 118.2 | 41.4 | 37.6 | 14.4 | 21.3 | 6.3 | 4.7 | 2.1 | 1.0 | 0.2 | 3.8 | 1.3 | 7.7 | 15.8 | 70.0 | |
| ΣREE (ppm) | 255.2 | 193.2 | 232.5 | 176.2 | 67.5 | 80.0 | 33.1 | 360.2 | 246.1 | 217.5 | 51.0 | 27.7 | 19.4 | 44.3 | 85.5 | 30.6 | 49.3 | 27.5 | |
| Eu/Eu* | 1.12 | 1.21 | 1.19 | 1.34 | 1.74 | 2.75 | 1.62 | 1.15 | 1.05 | 1.26 | 1.31 | 1.21 | 1.02 | 1.10 | 2.26 | 1.84 | 1.95 | 1.30 | |
| Ce/Ce* | 0.99 | 0.96 | 1.17 | 0.98 | 0.96 | 1.07 | 0.98 | 0.23 | 0.99 | 0.93 | 0.88 | 0.67 | 0.60 | 0.73 | 0.94 | 0.80 | 0.87 | 0.94 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhaert, M.; Bernard, A.; Saddiqi, O.; Dekoninck, A.; Essalhi, M.; Yans, J. Mineralogy and Genesis of the Polymetallic and Polyphased Low Grade Fe-Mn-Cu Ore of Jbel Rhals Deposit (Eastern High Atlas, Morocco). Minerals 2018, 8, 39. https://doi.org/10.3390/min8020039
Verhaert M, Bernard A, Saddiqi O, Dekoninck A, Essalhi M, Yans J. Mineralogy and Genesis of the Polymetallic and Polyphased Low Grade Fe-Mn-Cu Ore of Jbel Rhals Deposit (Eastern High Atlas, Morocco). Minerals. 2018; 8(2):39. https://doi.org/10.3390/min8020039
Chicago/Turabian StyleVerhaert, Michèle, Alain Bernard, Omar Saddiqi, Augustin Dekoninck, Mourad Essalhi, and Johan Yans. 2018. "Mineralogy and Genesis of the Polymetallic and Polyphased Low Grade Fe-Mn-Cu Ore of Jbel Rhals Deposit (Eastern High Atlas, Morocco)" Minerals 8, no. 2: 39. https://doi.org/10.3390/min8020039





