Coal-Mining Tailings as a Pozzolanic Material in Cements Industry
Abstract
:1. Introduction
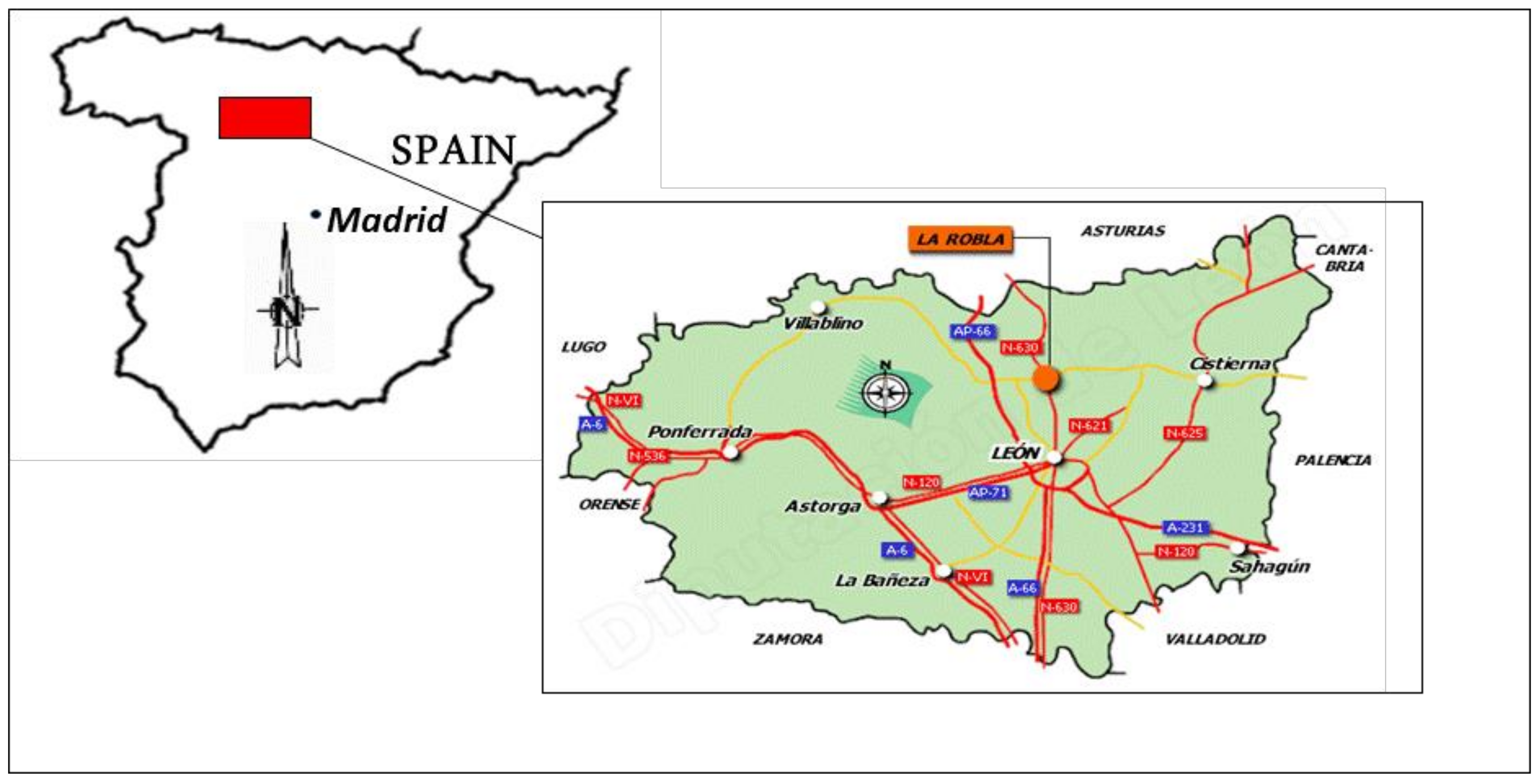
2. Materials and Methods
2.1. Materials
2.2. Methods
- (1)
- The chemical characterization was performed with Inductively Coupled Plasma Mass Spectrometry (ICP/MS) (Perkin Elmer, Waltham, MA, USA) prior to having immersed the sample in an acidic solution (ICP-MS Elan 6000 Perkin Elmer Sciex with an AS91 autosampler, Perkin Elmer, Waltham, MA, USA). The content of organic coal in the solid samples was determined by the difference between the total content of coal and inorganic coal, using a total organic carbon analyzer, the Shimadzu (Total Organic Carbon Analyzer) TOC-5000 A (Shimadzu, Kyoto, Japan), with a module for solid samples (Solid Sample Module) SSM-5000 A (Shimadzu, Kyoto, Japan).
- (2)
- The mineralogical composition was determined by X-ray diffraction (XRD, Siemens, Munich, Germany) with the powder method and the <2 µm fraction with the oriented aggregate method; in both cases completing the diffractograms in a Siemens diffractometer D-5000 fitted with a Cu anode [34]. The characterization of the bulk samples was performed with the Rietveld method [35].
- (3)
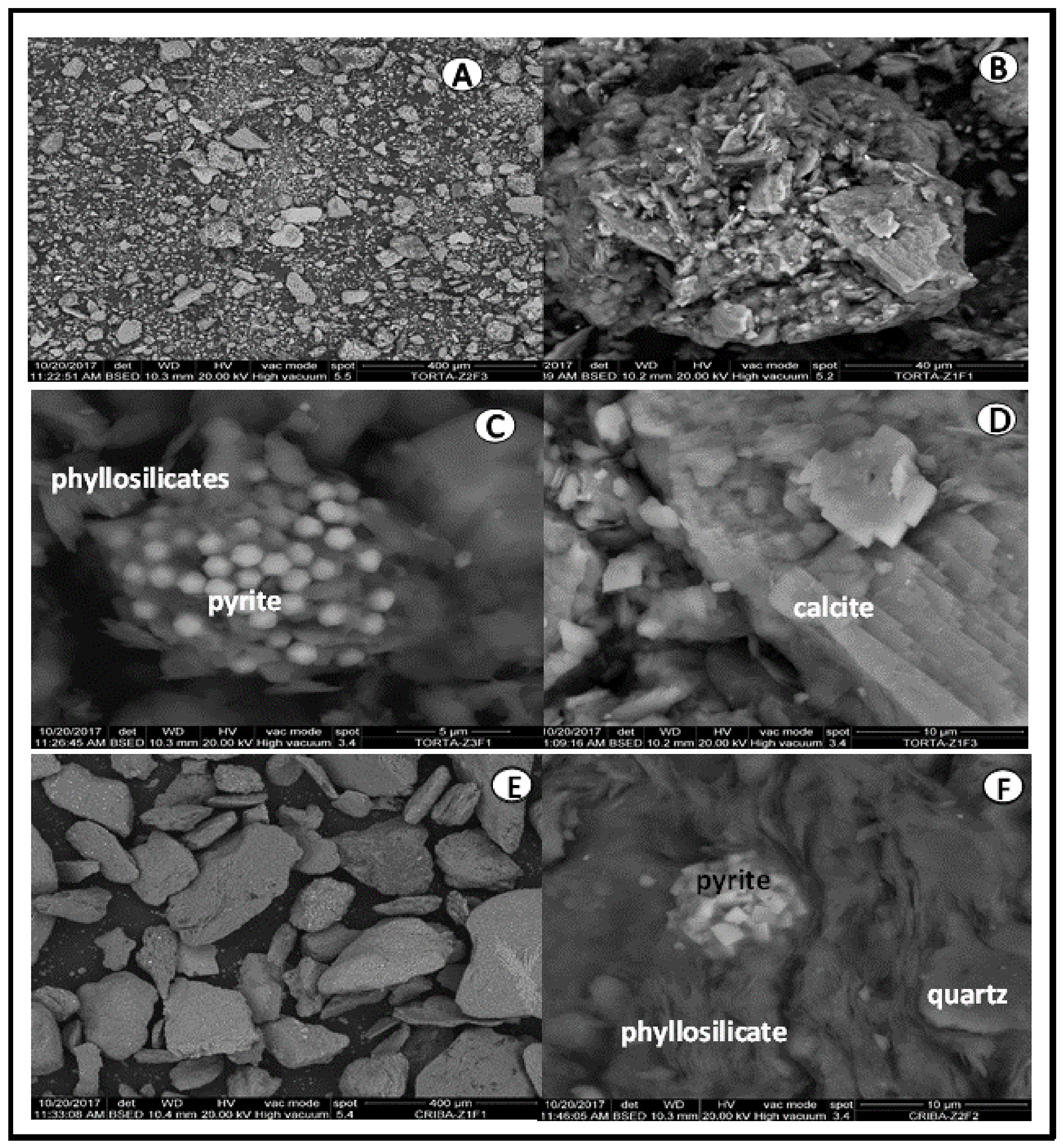
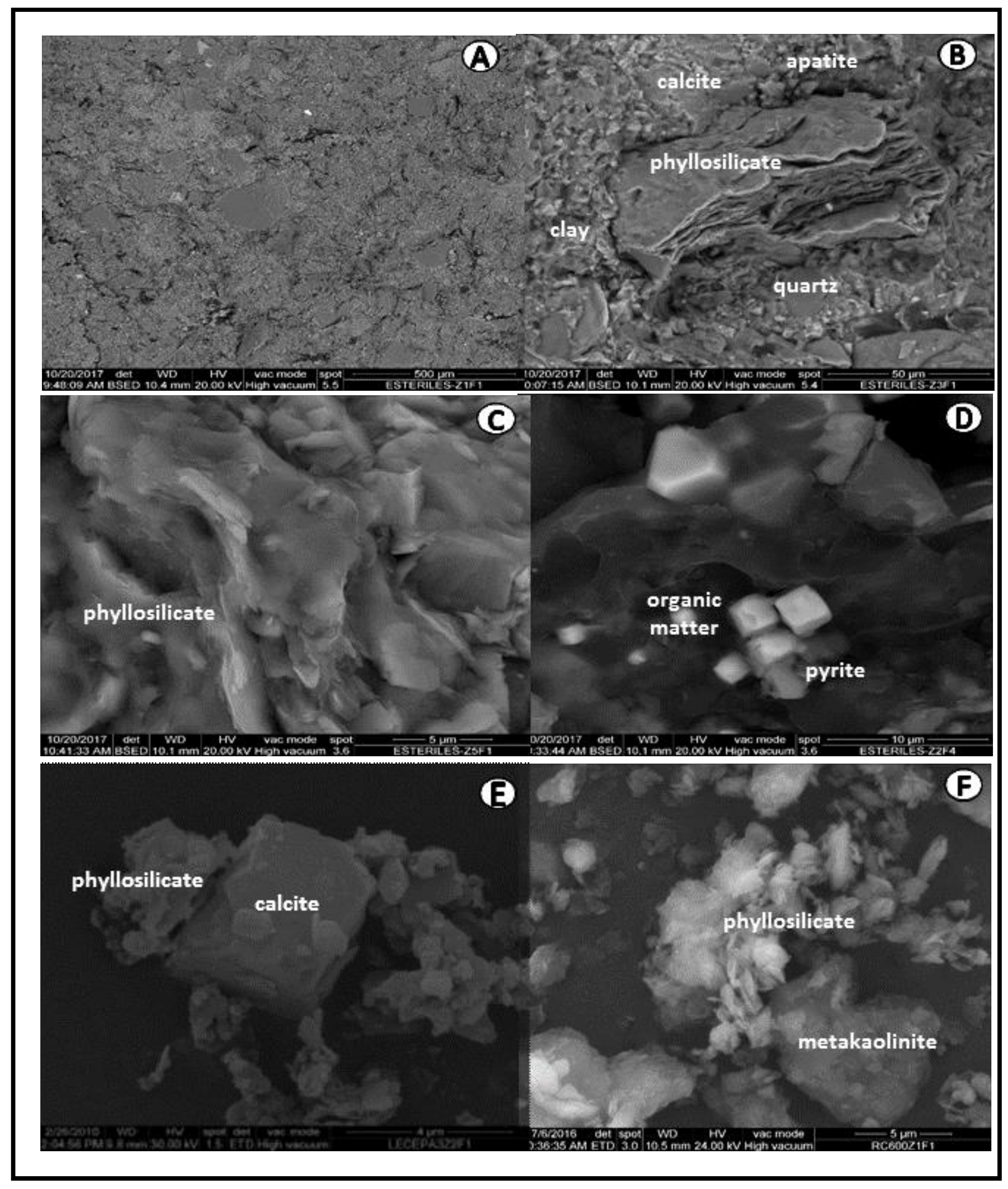
- The scanning electron microscopy (SEM) morphological observations and the energy dispersive X-ray (EDX) microanalysis were performed with an FEI electronic microscope equipped with an energy dispersive X-ray spectrometer (source of W, DX4i analyzer and Si/Li detector).
3. Results and Discussion
3.1. Raw Materials
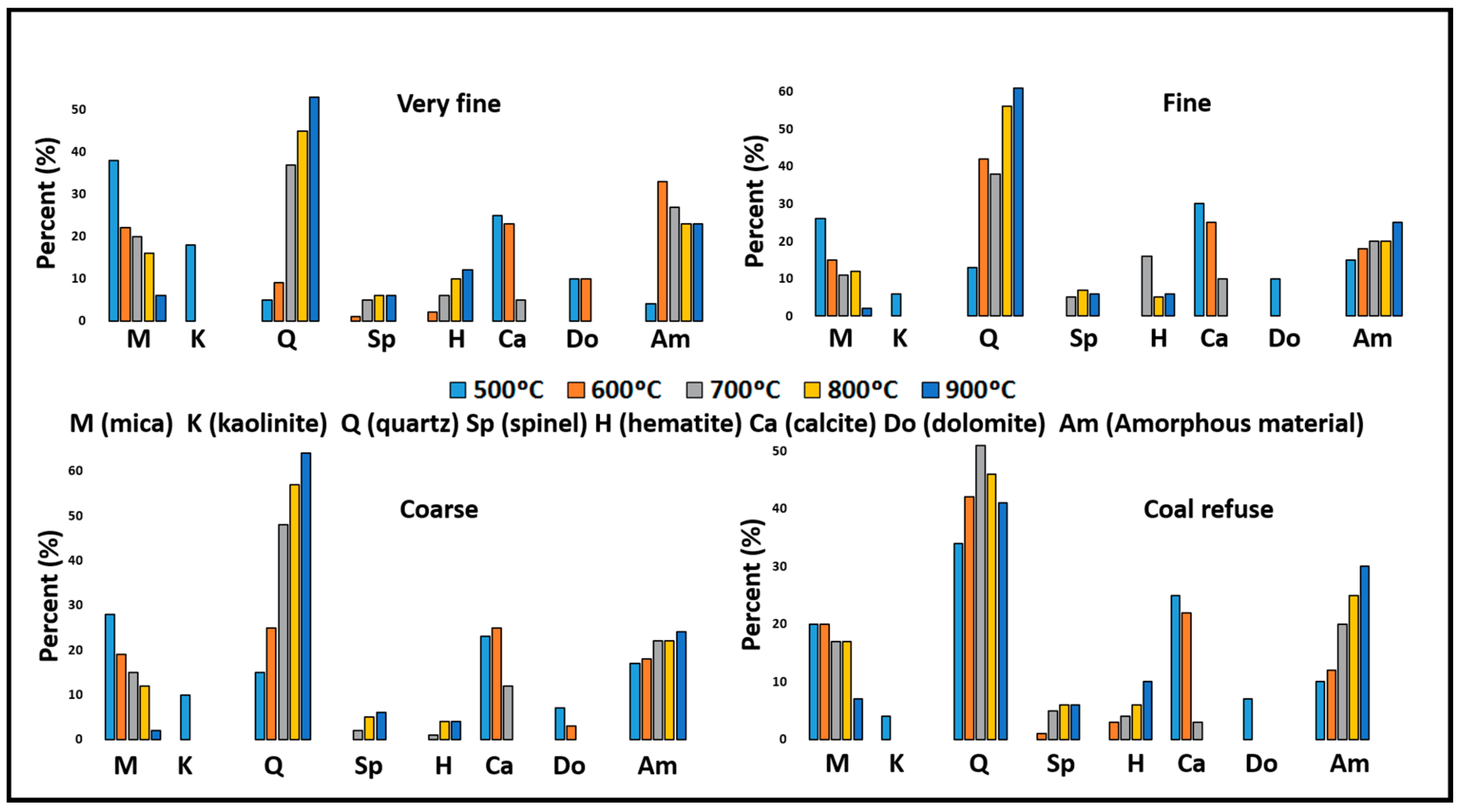
3.2. Thermally Activated Materials
4. Conclusions
- Four types of coal-mining waste—very fine-grade, fine-grade, and coarse-grade coal tailings, and coal refuse—extracted from the slag heaps of abandoned coal mines near the locality of Santa Lucía, in the Spanish province of León, have been studied. From among them all, one was selected due to the presence of high volumes of waste and the facility of its use as an additional pozzolanic cement additive.
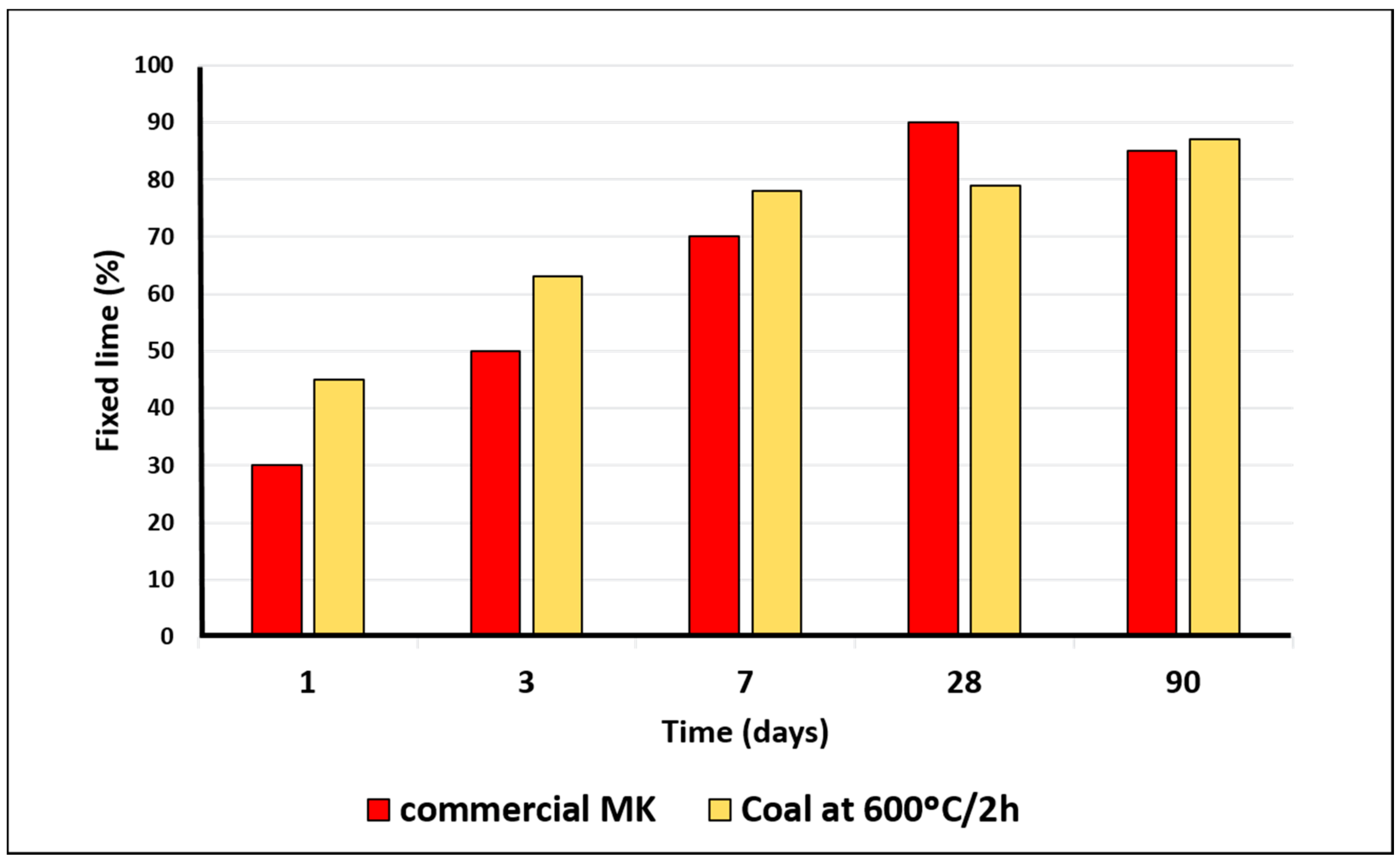
- The four samples of coal waste were thermally activated at 600 °C, over two hours in an electric furnace to improve the pozzolanic properties. The selected temperature has advantages from both an economic and an energetic point of view.
- The results of XRD analysis have confirmed that all K is transformed into MK (a pozzolan specified in commercial cement manufacturing standards) over a heating time of 2 hours at that temperature (600 °C/2 h).
- The results show that after the activation process, the coal refuse presented sufficiently good pozzolanic activity for it to be used as a pozzolanic addition in industrial cements, emphasizing the potential of this process for the removal of contaminated waste from the environment.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frías, M.; Sánchez de Rojas, M.I.; Santamaría, J.; Rodríguez, C. Recycling of silicomanganese slag as a pozzolanic material in Portland cements: Basic and engineering properties. Cem. Concr. Res. 2006, 36, 487–491. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Savastano, H. Brazilian sugar bagasse ashes from the cogeneration industry as active pozzolans for cement manufacture. Cem. Concr. Comp. 2011, 33, 490–496. [Google Scholar] [CrossRef]
- Vegas, I.; Urreta, J.; Frías, M.; García, R. Freeze-thaw resistance of blended cements containing calcined paper sludge. Constr. Build. Mater. 2009, 23, 2862–2868. [Google Scholar] [CrossRef]
- Pereira, M.L.; Jane, S.; Tomazini, F.; Petrisin, C.; Kunihiko, P.; Vieira, A.; Leslie, R. Red mud from Brazil: Thermal behaviour and physical properties. Ind. Eng. Chem. Res. 2012, 51, 775–786. [Google Scholar]
- Loh, Y.R.; Sujan, D.; Rahman, M.E.; Das, C.A. Sugarcane bagasse—The future composite material: A literature review. Res. Concr. Rec. 2013, 75, 14–22. [Google Scholar]
- Ekosse, G. Provenance of the Kgwakgwe kaolin deposit in Southeastern Botswana and its possible utilization. Appl. Clay Sci. 2001, 20, 137–152. [Google Scholar] [CrossRef]
- Frías, M. Study of Hydrated Phases Present in a MK-Lime System Cured at 60 °C and 60 Months of Reaction. Cem. Concr. Res. 2006, 36, 827–831. [Google Scholar] [CrossRef]
- Snelson, D.; Wild, S.; O’Farrell, M. Heat of hydration of Portland cement-MK-FA blends. Cem. Concr. Res. 2008, 38, 832–840. [Google Scholar] [CrossRef]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Ríos, C.; Williams, C.; Fullen, M. Hydrothermal synthesis of hydrogarnet and tobermorite at 175 °C from kaolinite and metakaolinite in the CaO-Al2O3-SiO2-H2O system: A comparative study. Appl. Clay Sci. 2009, 43, 228–237. [Google Scholar] [CrossRef]
- Frías, M.; Vigil de la Villa, R.; García, R.; Sánchez de Rojas, M.I.; Juan, A. The influence of slate waste activation conditions on mineralogical changes and pozzolanic behavior. J. Am. Cer. Soc. 2013, 96, 2276–2282. [Google Scholar] [CrossRef]
- García-Giménez, R.; Vigil de la Villa Mencía, R.; Rubio, V.; Frías, M. The transformation of coal-mining waste minerals in pozzolanic reactions of cements. Minerals 2016, 6, 64. [Google Scholar] [CrossRef]
- Finkelman, R.B. Potential health impacts of burning coal beds and waste banks. Int. J. Coal Geol. 2004, 51, 19–24. [Google Scholar] [CrossRef]
- Suárez-Ruiz, I.; Crelling, J.C. Applied Coal Petrology: The Role of Petrology in Coal Utilization; Elsevier: Amsterdam, The Netherlands, 2008; p. 388. [Google Scholar]
- Suárez-Ruiz, I.; Flores, D.; Mendonça Filho, J.G.; Hackley, P.C. Review and update of the applications of organic petrology: Part 2, geological and multidisciplinary applications. Int. J. Coal Geol. 2012, 98, 73–94. [Google Scholar] [CrossRef]
- Ribeiro, J.; Suárez-Ruiz, I.; Flores, D. Geochemistry of self-burning coal mining residues from El Bierzo Coalfield (NW Spain): Environmental implications. Int. J. Coal Geol. 2016, 159, 155–168. [Google Scholar] [CrossRef]
- Nádudvari, A.; Fabiańska, M.J. The impact of water-washing, biodegradation and self-heating processes on coal waste dumps in the Rybnik Industrial Region (Poland). Int. J. Coal Geol. 2016, 154–155, 286–299. [Google Scholar] [CrossRef]
- Ribeiro, J.; Ferreira da Silva, E.; Flores, D. Burning of coal waste piles from Douro Coalfield (Portugal): Petrological, geochemical and mineralogical characterization. Int. J. Coal Geol. 2010, 81, 359–372. [Google Scholar] [CrossRef]
- Bell, F.G.; Bullock, S.E.T.; Hälbich, T.F.J.; Lindsay, P. Environmental impacts associated with an abandoned mine in the Witbank Coalfield, South Africa. Int. J. Coal Geol. 2001, 45, 195–216. [Google Scholar] [CrossRef]
- Younger, P.L. Environmental impacts of coal mining and associated wastes: A geochemical perspective. In Energy, Waste and the Environment: A Geochemical Perspective; Gieré, R., Stille, P., Eds.; Geological Society, Special Publications: London, UK, 2004; Volume 236, pp. 169–209. [Google Scholar]
- Bailey, M.T.; Gandy, C.J.; Watson, I.A.; Wyatt, L.M.; Jarvis, A.P. Heat recovery potential of mine water treatment systems in Great Britain. Int. J. Coal Geol. 2016, 164, 77–84. [Google Scholar] [CrossRef]
- Gorakhki, M.H.; Bareither, C.A. Unconfined Compressive Strength of Synthetic and Natural Mine Tailings Amended with Fly Ash and Cement. J. Geotech. Geoenviron. Eng. 2017, 143, 1–14. [Google Scholar] [CrossRef]
- Kesimal, A.; Yilmaz, E.; Ercikdi, B.; Alp, I.; Deveci, H. Effect of properties of tailings and binder on the short-and long-term strength and stability of cemented paste backfill. Mater. Lett. 2005, 59, 3703–3709. [Google Scholar] [CrossRef]
- Qian, G.; Huang, T.; Bai, S. Use of cement-stabilized granite mill tailings as pavement subbase. J. Mater. Civ. Eng. 2011, 23, 1575–1578. [Google Scholar] [CrossRef]
- Bian, Z.; Dong, J. The impact of disposal and treatment of coal mining wastes on environment and farmland. Environ. Geol. 2009, 58, 625–634. [Google Scholar] [CrossRef]
- CEDEX. Monografía sobre Catálogo de Residuos Utilizables en la Construcción; Ficha Técnica; CEDEX: Madrid, Spain, 2007. [Google Scholar]
- Hernández-Mendoza, H.; Mejuto, M.; Cardona, A.I.; García-Álvarez, A.; Millán, R.; Yllera, A. Optimization and validation of a method for heavy metals quantification in soil samples by inductively coupled plasma sector field mass spectrometry (ICP-SFMS). Am. J. Anal. Chem. 2013, 4, 9–15. [Google Scholar] [CrossRef]
- Chen, X.D. On the fundamentals of diffusive self-heating in water containing combustible materials. Chem. Eng. Process. 1998, 37, 367–378. [Google Scholar] [CrossRef]
- Garcia, P.; Hall, P.J.; Mondragon, F. The use of differential scanning calorimetry to identify coals susceptible to spontaneous combustion. Thermochim. Acta 1999, 336, 41–46. [Google Scholar] [CrossRef]
- Lyman, R.; Volkmer, J. Pyrophoricity (Spontaneous Combustion) of Powder River Basin Coals—Considerations for Coalbed Methane Development; Coal Report CR01; Wyoming State Geological Survey: Laramie, WY, USA, 2001.
- Kaymakci, E.; Didari, V. Relations between coal properties and spontaneous combustion parameters. Turk. J. Eng. Environ. Sci. 2002, 26, 59–64. [Google Scholar]
- Lohrer, C.; Schmidt, M.; Krause, U. Influence of environmental parameters on the self-ignition behavior of coal. In Proceedings of the International Conference on Coal Fire Research, Beijing, China, 29 November–1 December 2005. [Google Scholar]
- Ribeiro, J.; Suárez-Ruiz, I.; Ward, C.R.; Flores, D. Petrography and mineralogy of self-burning coal wastes from anthracite mining in the El Bierzo Coalfield (NW Spain). Int. J. Coal Geol. 2016, 154, 92–106. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-ray Diffraction and the Identification and Analysis of Clay Minerals, 2nd ed.; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Renaudin, G.; Russias, J.; Leroux, F.; Frizon, F. Structural characterization of C–S–H and C–A–S–H samples—Part I: Long-range order investigated by Rietveld analyses. J. Solid State Chem. 2009, 182, 3312–3319. [Google Scholar] [CrossRef]
- Stephan, D.; Maleki, H.; Knofel, D.; Eber, B.; Hardtl, R. Influence of Cr, Ni, Zn on the properties of pure clinker phases. Part I. C3S. Cem. Concr. Res. 1999, 29, 545–552. [Google Scholar] [CrossRef]
- Ambroise, J.; Murat, M.; Pera, J. Investigations on Synthetic Binders Obtained by Middle-Temperature Thermal Dissociation of Clay Minerals. Silic. Ind. 1986, 7, 99–107. [Google Scholar]
- Vigil de la Villa, R.; Frías, M.; García-Giménez, R.; Martínez Ramírez, S.; Fernández-Carrasco, L. Chemical and mineral transformations that occur in mine waste and washery rejects during pre-utilization calcination. Int. J. Coal Geol. 2014, 132, 123–130. [Google Scholar] [CrossRef]
- Frías, M.; Cabrera, J. Influence of MK on the reaction kinetics in MK/lime and MK blended cement systems at 20 °C. Cem. Concr. Res. 2001, 31, 519–527. [Google Scholar] [CrossRef]
- Sayanam, R.A.; Kalsotra, A.K.; Mehta, S.K.; Singh, R.S.; Mandal, G. Studies on thermal transformations and pozzolanic activities of clay from Jammu region (India). J. Therm. Anal. 1989, 35, 99–106. [Google Scholar] [CrossRef]

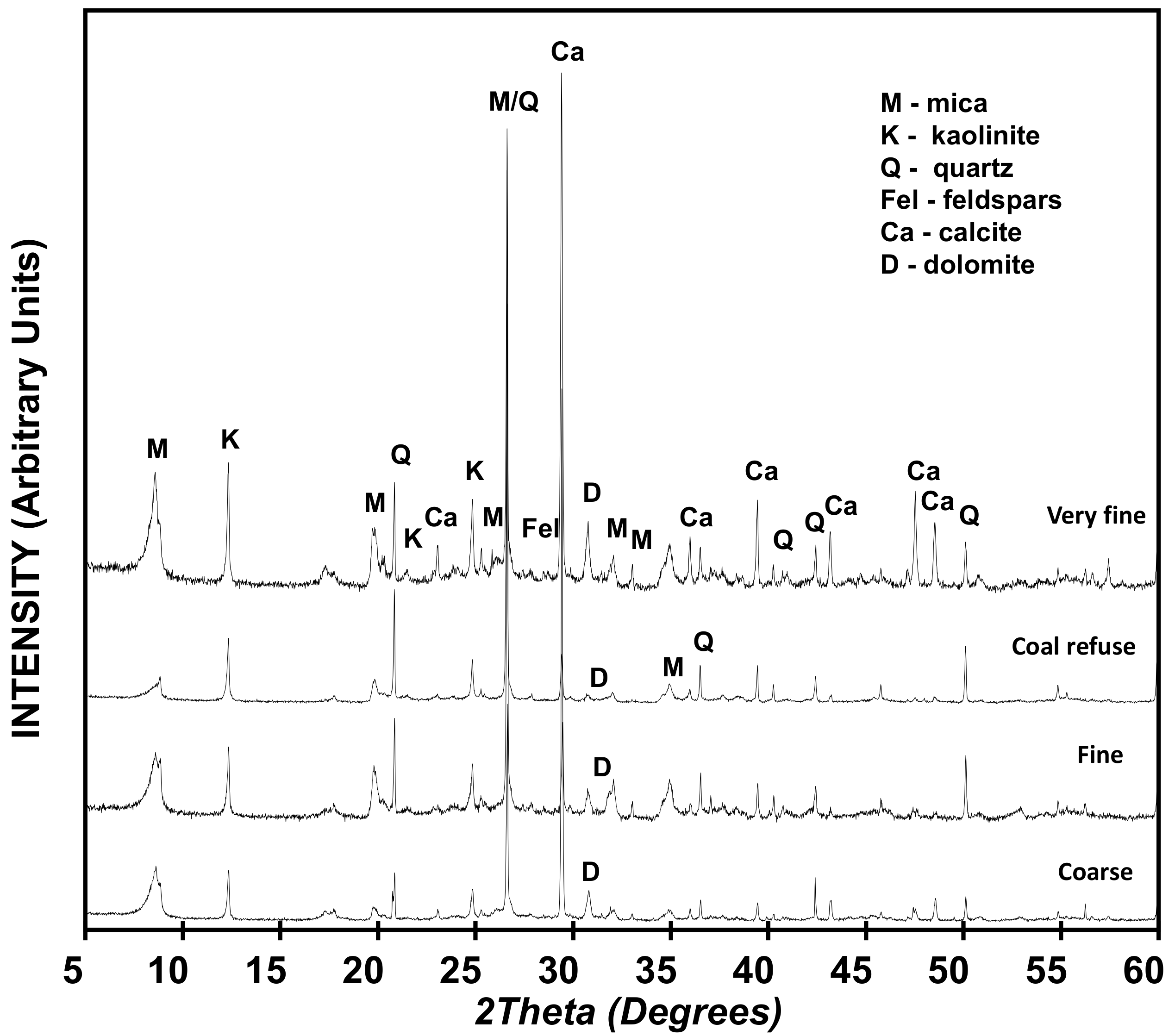
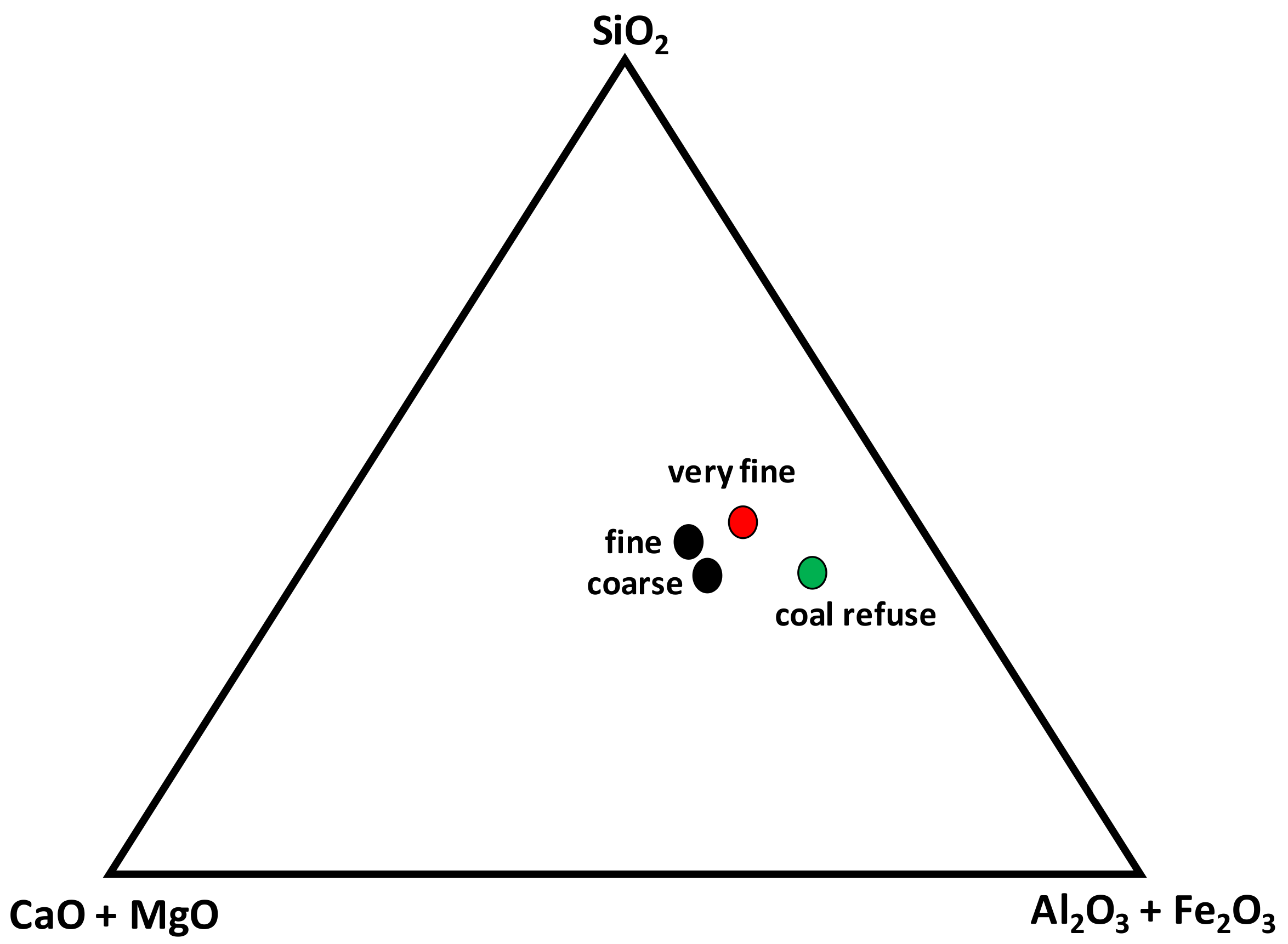
| Mineral (%) | Tailings | Coal Refuse | ||
|---|---|---|---|---|
| Very fine | Fine | Coarse | ||
| Mica | 30 | 26 | 28 | 20 |
| Kaolinite | 18 | 6 | 10 | 4 |
| Quartz | 5 | 13 | 10 | 34 |
| Feldspars | Traces | Traces | Traces | Traces |
| Calcite | 25 | 30 | 30 | 23 |
| Dolomite | 15 | 10 | 10 | 7 |
| Amorphous material | 7 | 15 | 12 | 12 |
| R | 26.0 | 16.4 | 19.6 | 17.3 |
| Global chi2 | 8.6 | 5.4 | 8.3 | 7.0 |
| Percent (%) | Tailings | Coal Refuse | ||
|---|---|---|---|---|
| Very Fine | Fine | Coarse | ||
| Sand | 13 | 23 | 43 | 13 |
| Silt | 15 | 39 | 36 | 26 |
| Clay | 72 | 38 | 21 | 61 |
| Oxides (%) | Phyllosilicate | Pyrite | Quartz | Calcite |
|---|---|---|---|---|
| Na2O | 0.83 ± 0.22 | n.d. | n.d. | n.d. |
| MgO | 0.98 ± 0.43 | n.d. | n.d. | n.d. |
| Al2O3 | 36.31 ± 1.39 | n.d. | n.d. | n.d. |
| SiO2 | 54.45 ± 1.63 | n.d. | 100 | n.d. |
| S2− | n.d. | 72.03 | n.d. | n.d. |
| Fe2+ | n.d. | 27.97 | n.d. | n.d. |
| K2O | 4.43 ± 1.28 | n.d. | n.d. | n.d. |
| CaO | 0.33 ± 0.12 | n.d. | n.d. | 100 |
| TiO2 | 1.43 ± 0.98 | n.d. | n.d. | n.d. |
| Fe2O3 | 1.24 ± 0.85 | n.d. | n.d. | n.d. |
| Oxides (%) | Phyllosilicate | Clay | Apatite | Pyrite | Quartz | Calcite |
|---|---|---|---|---|---|---|
| Na2O | 0.76 ± 0.35 | n.d. | n.d. | n.d. | n.d. | n.d. |
| MgO | 1.27 ± 0.63 | 22.12 | n.d. | n.d. | n.d. | n.d. |
| Al2O3 | 32.53 ± 1.58 | 3.27 | n.d. | n.d. | n.d. | n.d |
| SiO2 | 55.96 ± 2.41 | 4.83 | n.d. | n.d. | 100 | n.d. |
| S2− | n.d. | n.d. | n.d. | 72.03 | n.d. | n.d. |
| Fe2+ | n.d. | n.d. | n.d. | 27.97 | n.d. | n.d. |
| K2O | 5.32 ± 1.93 | 0.71 | n.d. | n.d. | n.d. | n.d. |
| CaO | 1.20 ± 0.74 | 44.61 | 53.67 | n.d. | n.d. | 100 |
| TiO2 | 0.54 ± 0.46 | n.d. | n.d. | n.d. | n.d. | n.d. |
| Fe2O3 | 2.39 ± 1.49 | 24.46 | n.d. | n.d. | n.d. | n.d. |
| P2O5 | n.d. | n.d. | 43.33 | n.d. | n.d. | n.d. |
| Oxides (%) | Tailings | Coal Refuse | ||
|---|---|---|---|---|
| Very Fine | Fine | Coarse | ||
| SiO2 | 32.99 | 41.35 | 43.12 | 49.79 |
| Al2O3 | 19.90 | 9.37 | 12.16 | 21.77 |
| Fe2O3 | 4.09 | 4.13 | 4.21 | 4.07 |
| MnO | 0.13 | 0.11 | 0.10 | 0.08 |
| MgO | 0.87 | 0.65 | 0.59 | 0.64 |
| CaO | 11.44 | 15.21 | 16.20 | 3.84 |
| Na2O | 0.35 | 0.32 | 0.25 | 0.13 |
| SO3 | 0.83 | 0.92 | 0.77 | 0.27 |
| K2O | 2.11 | 2.18 | 2.27 | 2.74 |
| TiO2 | 0.57 | 0.46 | 0.65 | 1.07 |
| P2O5 | 0.26 | 0.16 | 0.22 | 0.13 |
| Loss on ignition | 26.47 | 25.14 | 19.46 | 15.18 |
| TOTAL | 100.01 | 100.00 | 100.00 | 99.71 |
| Organic carbon | 24.27 | 21.54 | 18.14 | 16.04 |
| Element (mg/L) | Tailings | Coal Refuse | ||
|---|---|---|---|---|
| Very fine | Fine | Coarse | ||
| Cu | n.d. | n.d. | n.d. | n.d. |
| Ni | 50 | 63 | 77 | 26 |
| Cr | 100 | 150 | 176 | 98 |
| V | 88 | 103 | 133 | 112 |
| Zn | 10 | 18 | 30 | 32 |
| Pb | 3 | 4 | 3 | 5 |
| Cd | n.d. | n.d. | n.d. | n.d. |
| As | 2 | 2 | 2 | 2 |
| Co | 11 | 15 | 12 | 8 |
| Mo | 18 | 15 | 17 | 21 |
| Oxides (%) | Phyllosilicate | Metakaolinite |
|---|---|---|
| MgO | 0.97 | n.d. |
| Al2O3 | 26.55 | 35.62 |
| SiO2 | 61.69 | 58.58 |
| SO3 | 3.22 | 1.00 |
| K2O | 3.10 | 2.92 |
| CaO | 1.41 | 1.89 |
| TiO2 | 0.88 | n.d. |
| Fe2O3 | 2.17 | n.d. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagüe, S.; Sánchez, I.; Vigil de la Villa, R.; García-Giménez, R.; Zapardiel, A.; Frías, M. Coal-Mining Tailings as a Pozzolanic Material in Cements Industry. Minerals 2018, 8, 46. https://doi.org/10.3390/min8020046
Yagüe S, Sánchez I, Vigil de la Villa R, García-Giménez R, Zapardiel A, Frías M. Coal-Mining Tailings as a Pozzolanic Material in Cements Industry. Minerals. 2018; 8(2):46. https://doi.org/10.3390/min8020046
Chicago/Turabian StyleYagüe, Santiago, Isabel Sánchez, Raquel Vigil de la Villa, Rosario García-Giménez, Antonio Zapardiel, and Moisés Frías. 2018. "Coal-Mining Tailings as a Pozzolanic Material in Cements Industry" Minerals 8, no. 2: 46. https://doi.org/10.3390/min8020046
APA StyleYagüe, S., Sánchez, I., Vigil de la Villa, R., García-Giménez, R., Zapardiel, A., & Frías, M. (2018). Coal-Mining Tailings as a Pozzolanic Material in Cements Industry. Minerals, 8(2), 46. https://doi.org/10.3390/min8020046





