Nanoscale Study of Clausthalite-Bearing Symplectites in Cu-Au-(U) Ores: Implications for Ore Genesis
Abstract
:1. Introduction
2. Background
3. Sampling and Analytical Methodology
4. Results
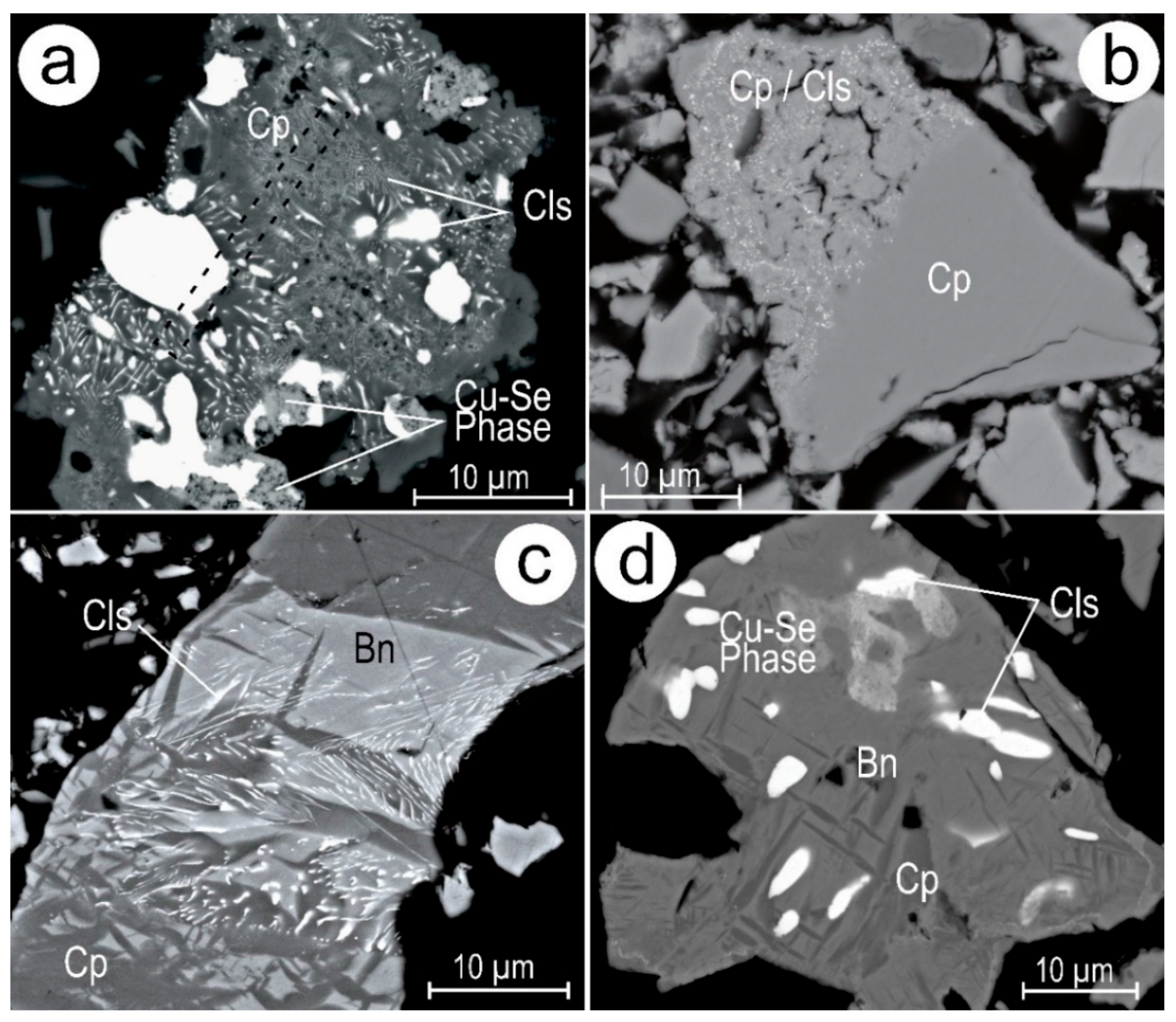
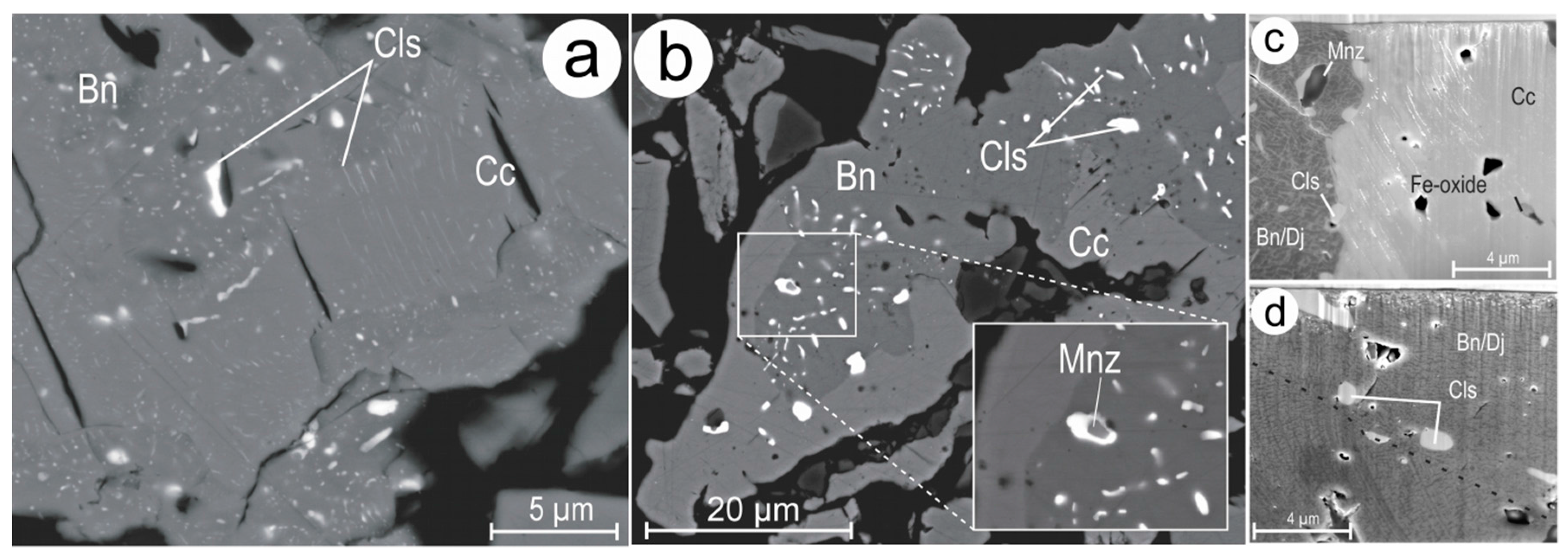
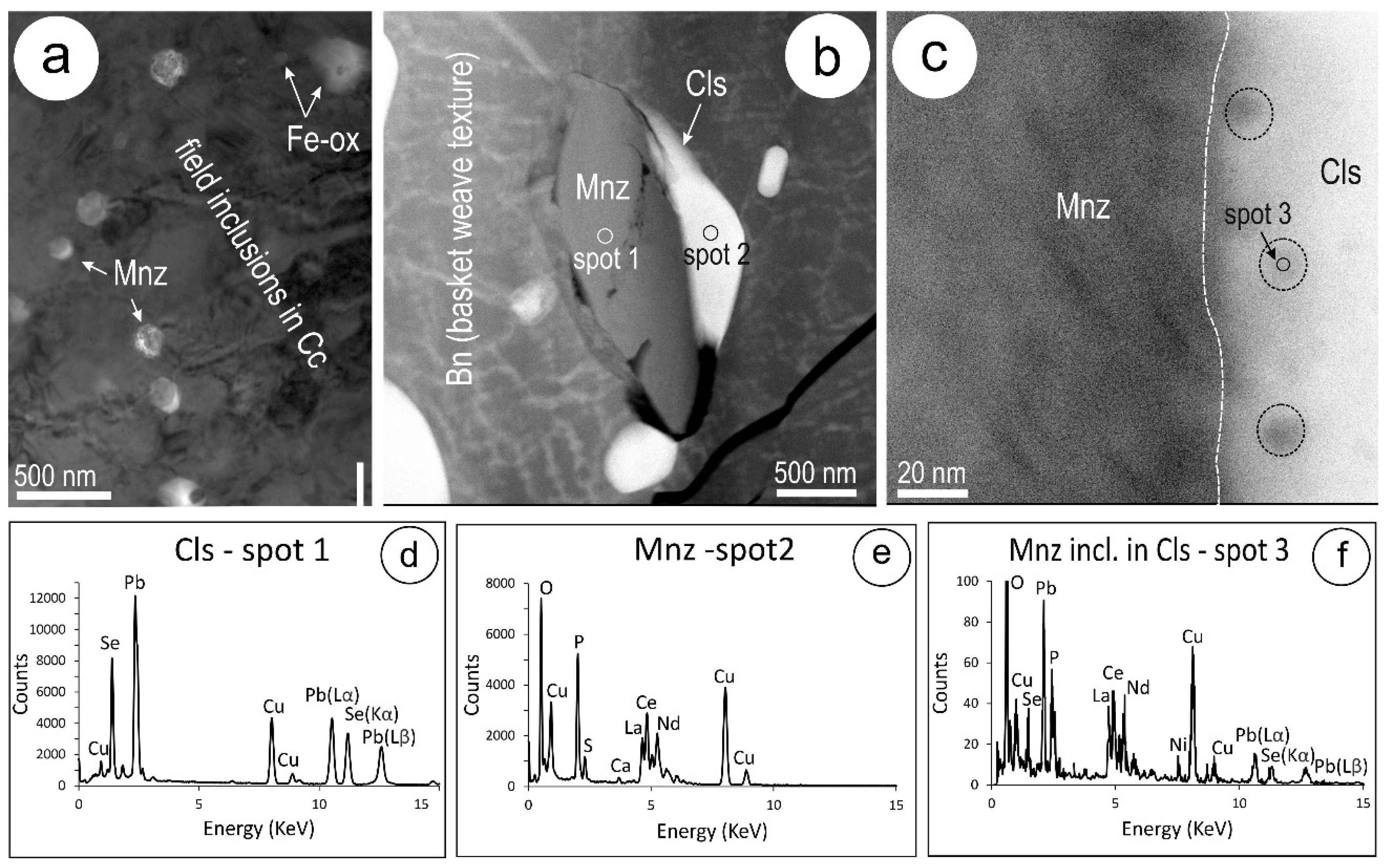
4.1. Characterisation of Symplectite Textures
4.2. Compositional Data for Pb-Chalcogenides and Host Cu-(Fe)-Sulphides
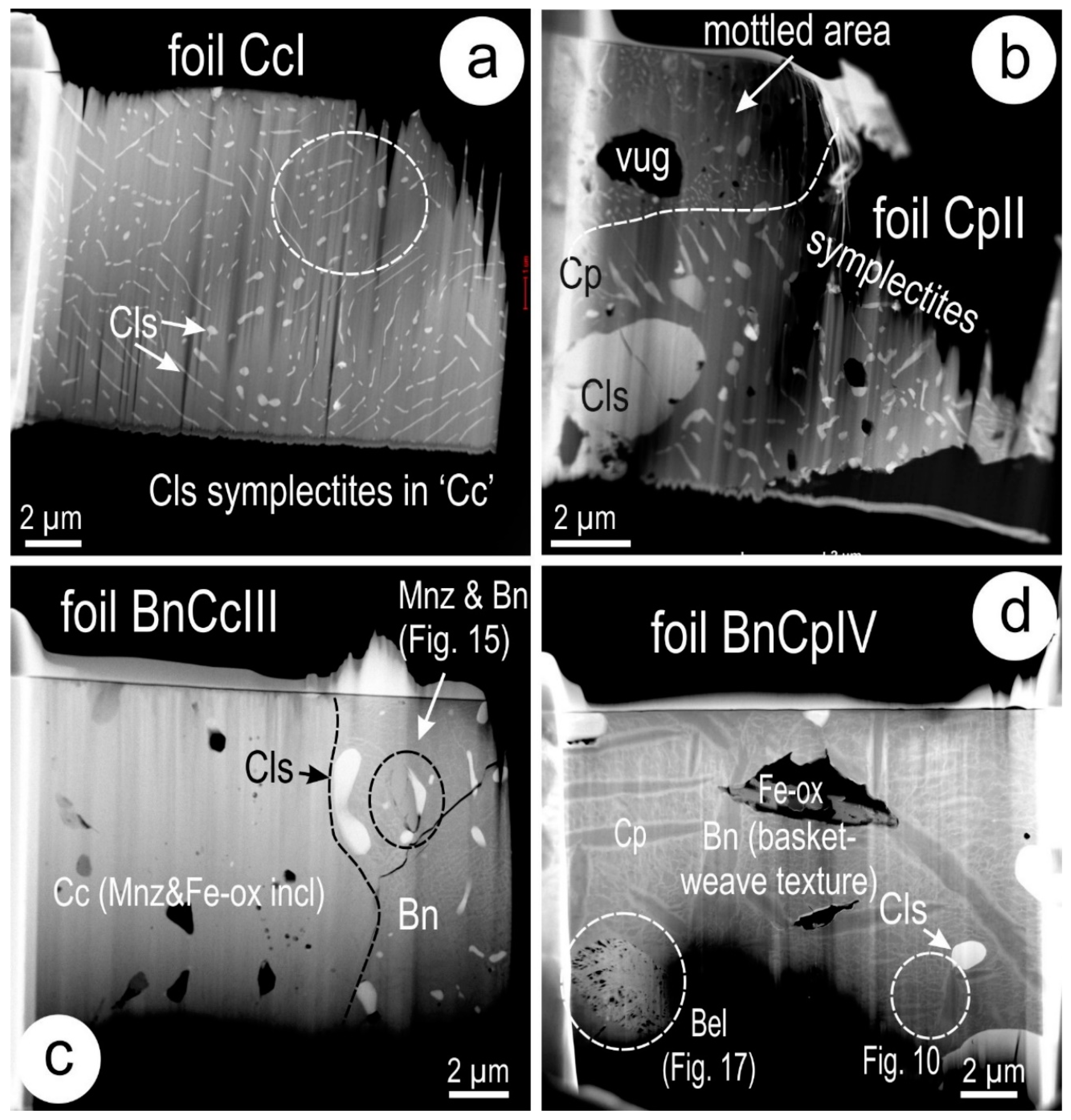
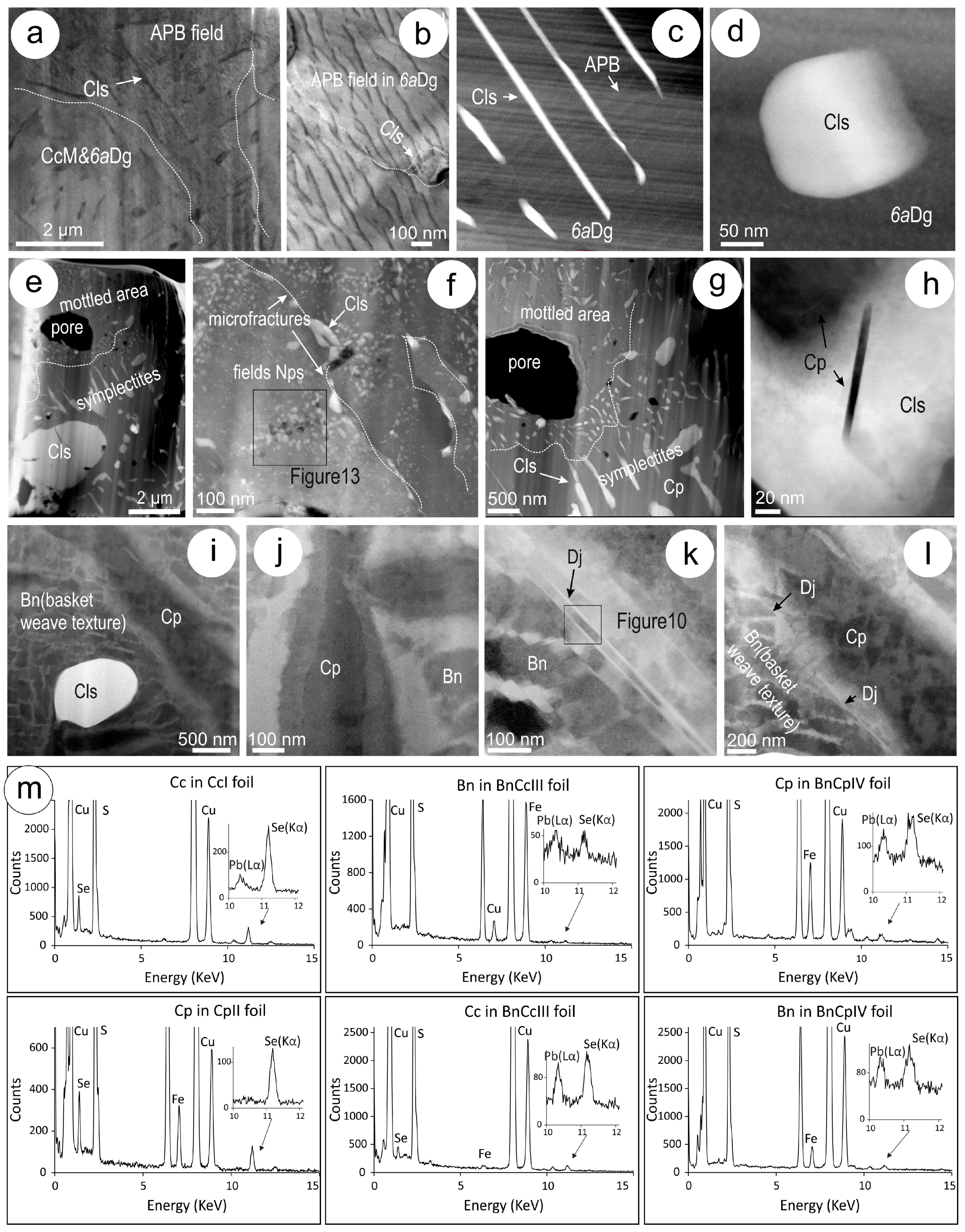
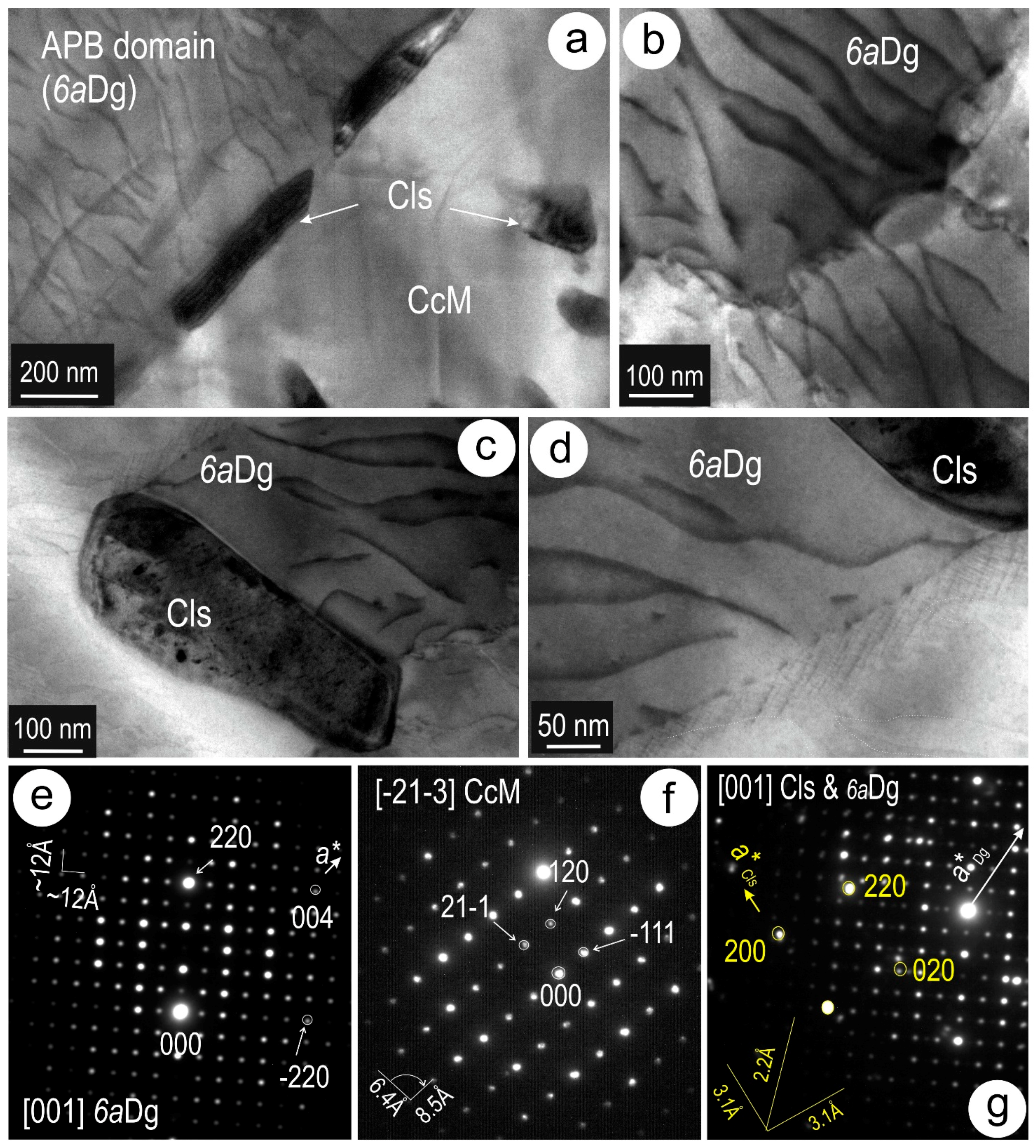
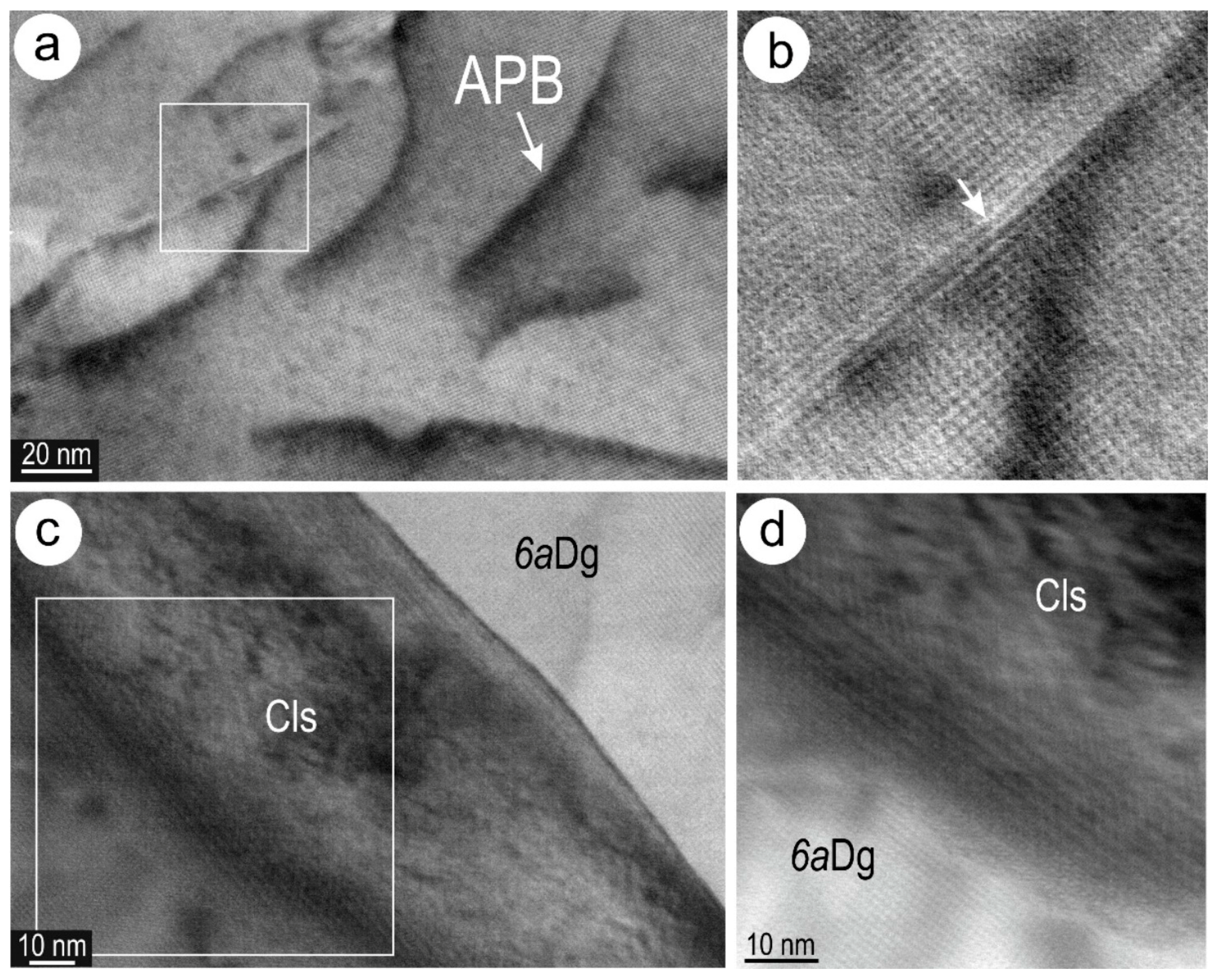
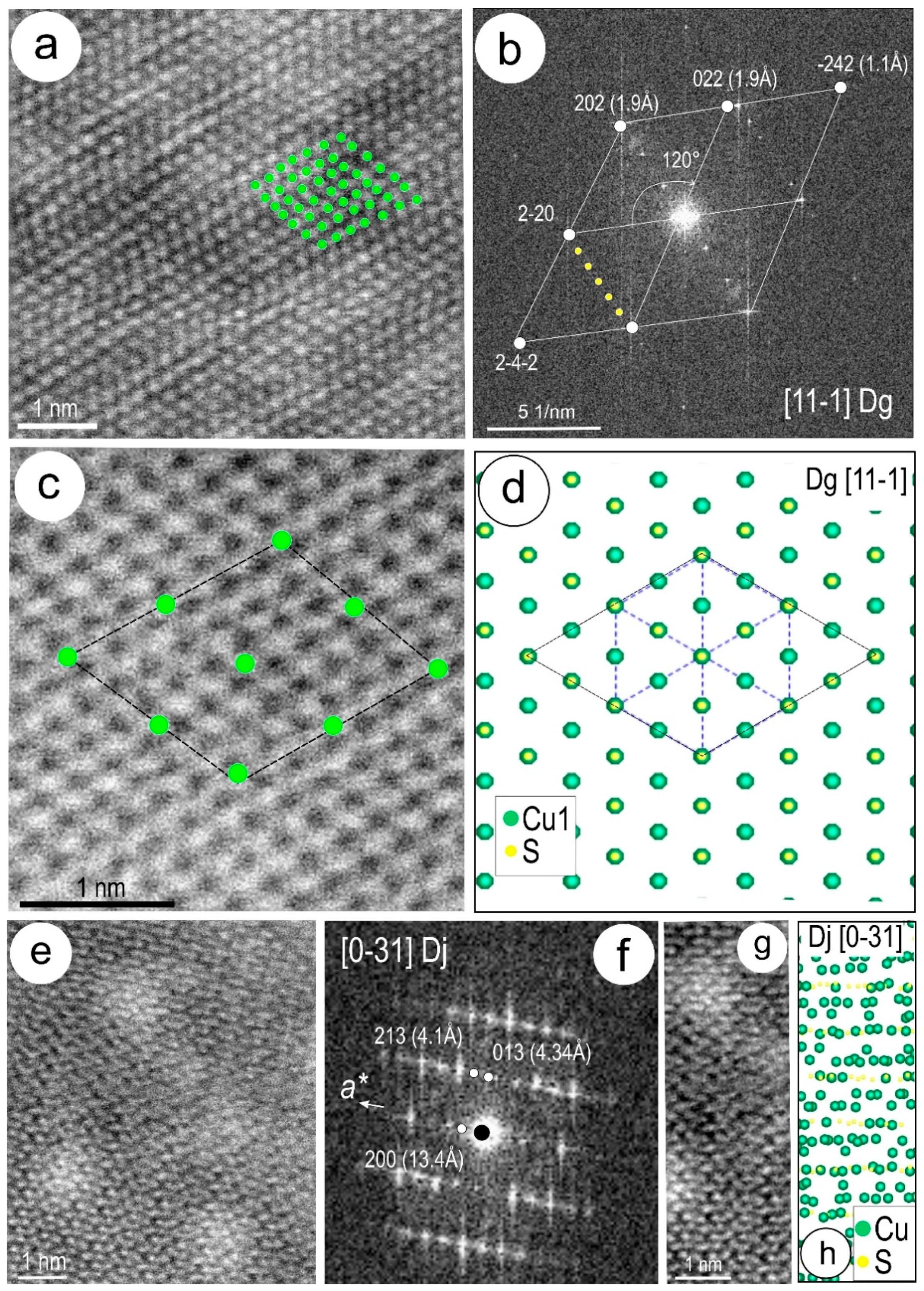
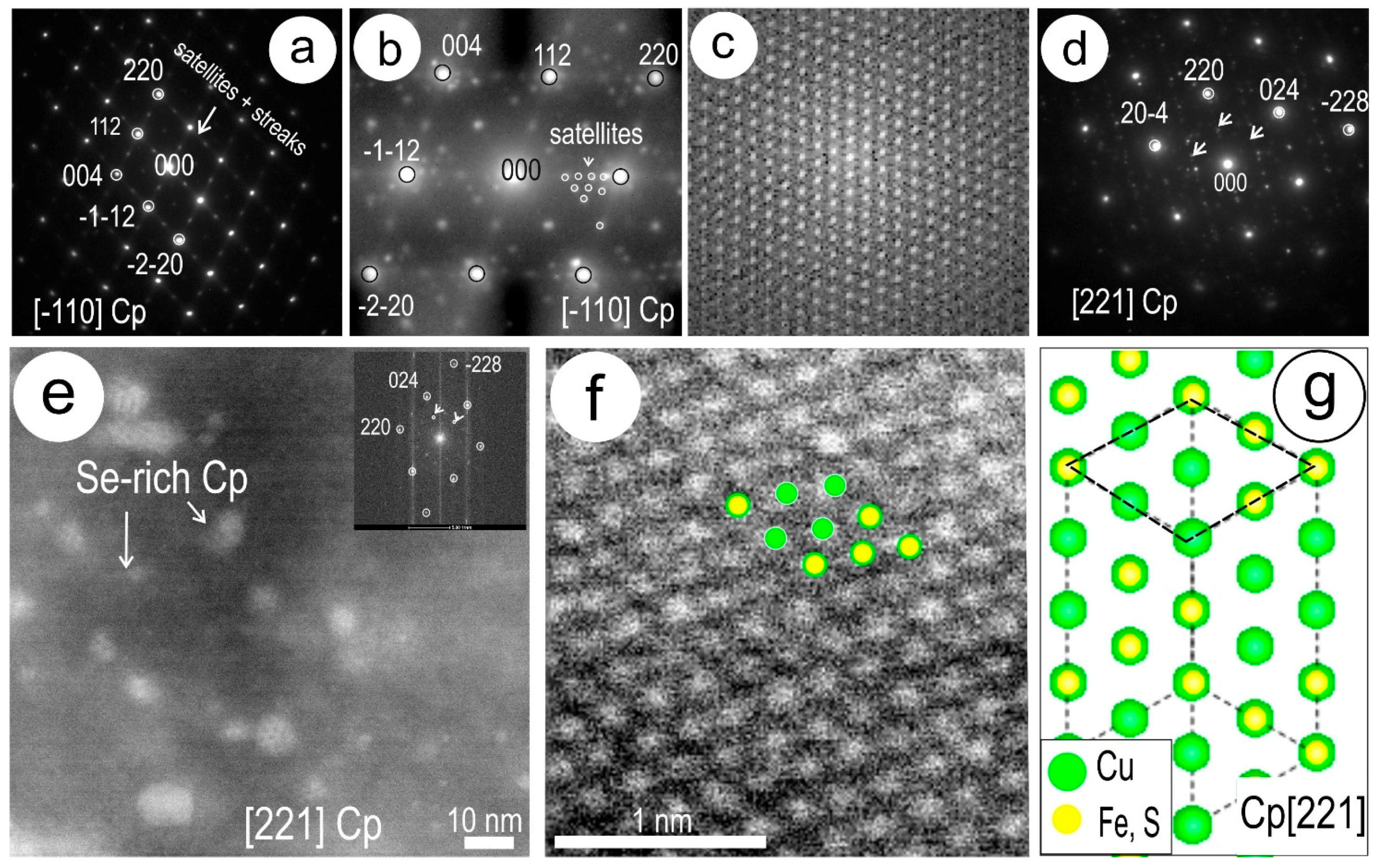
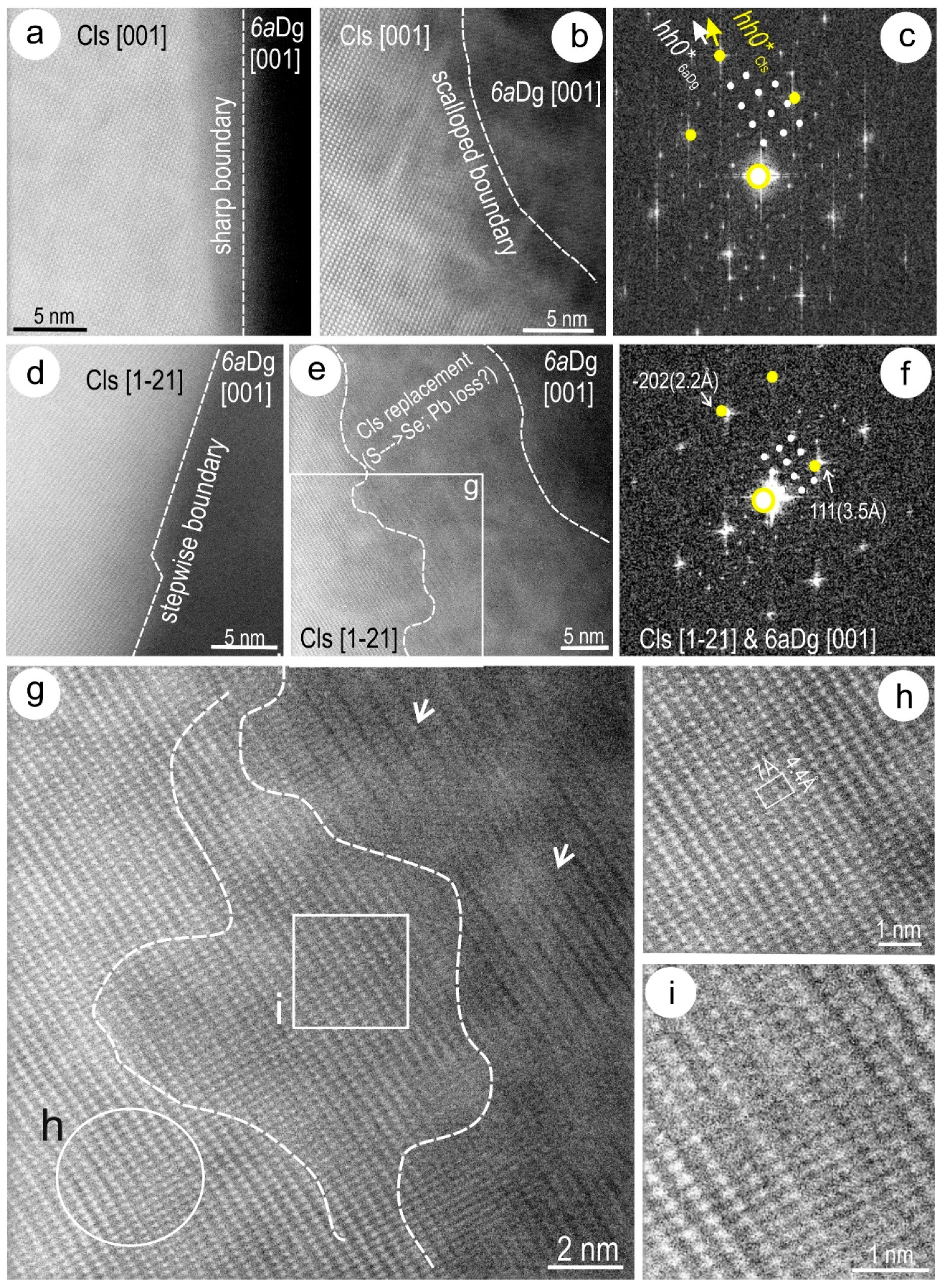
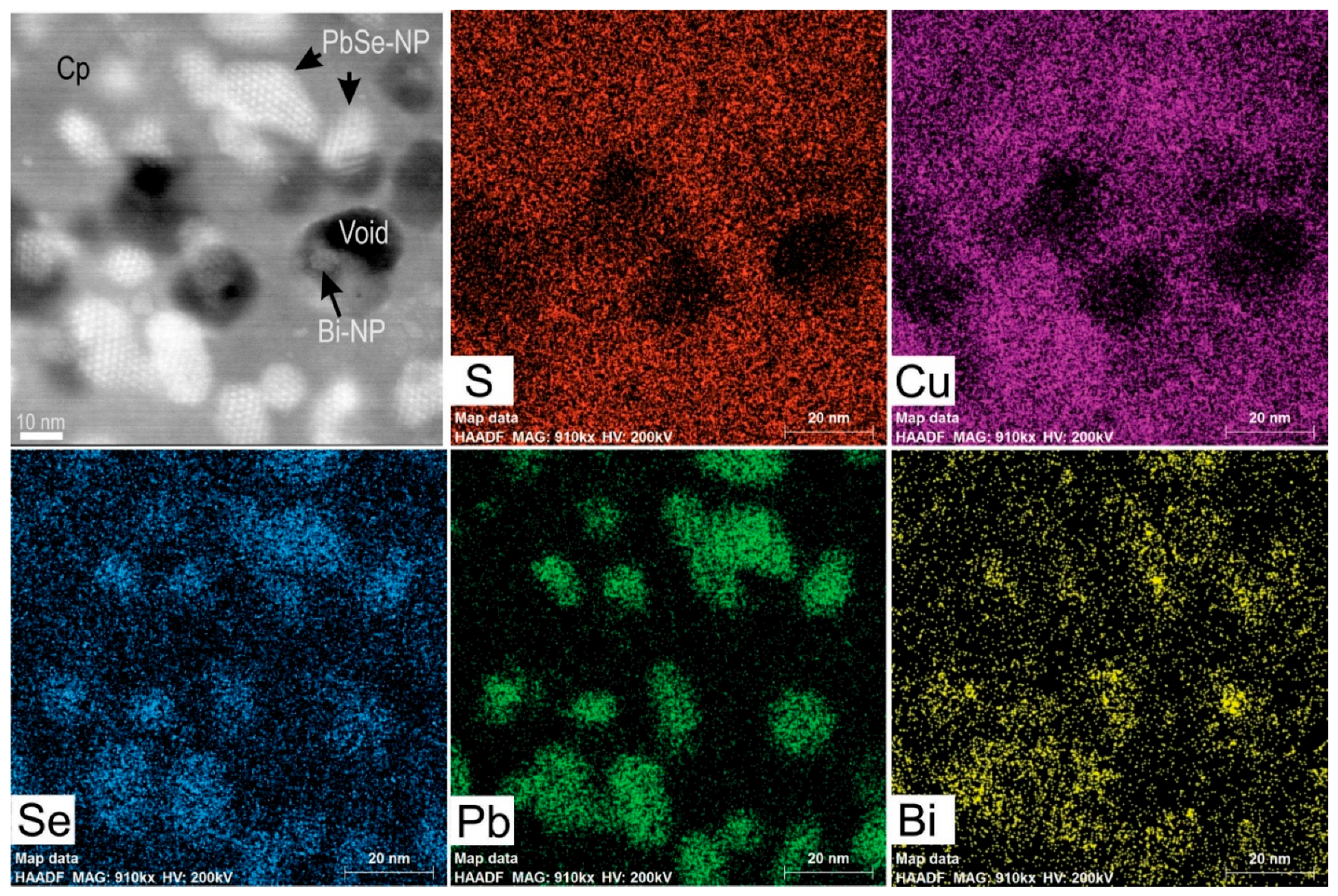
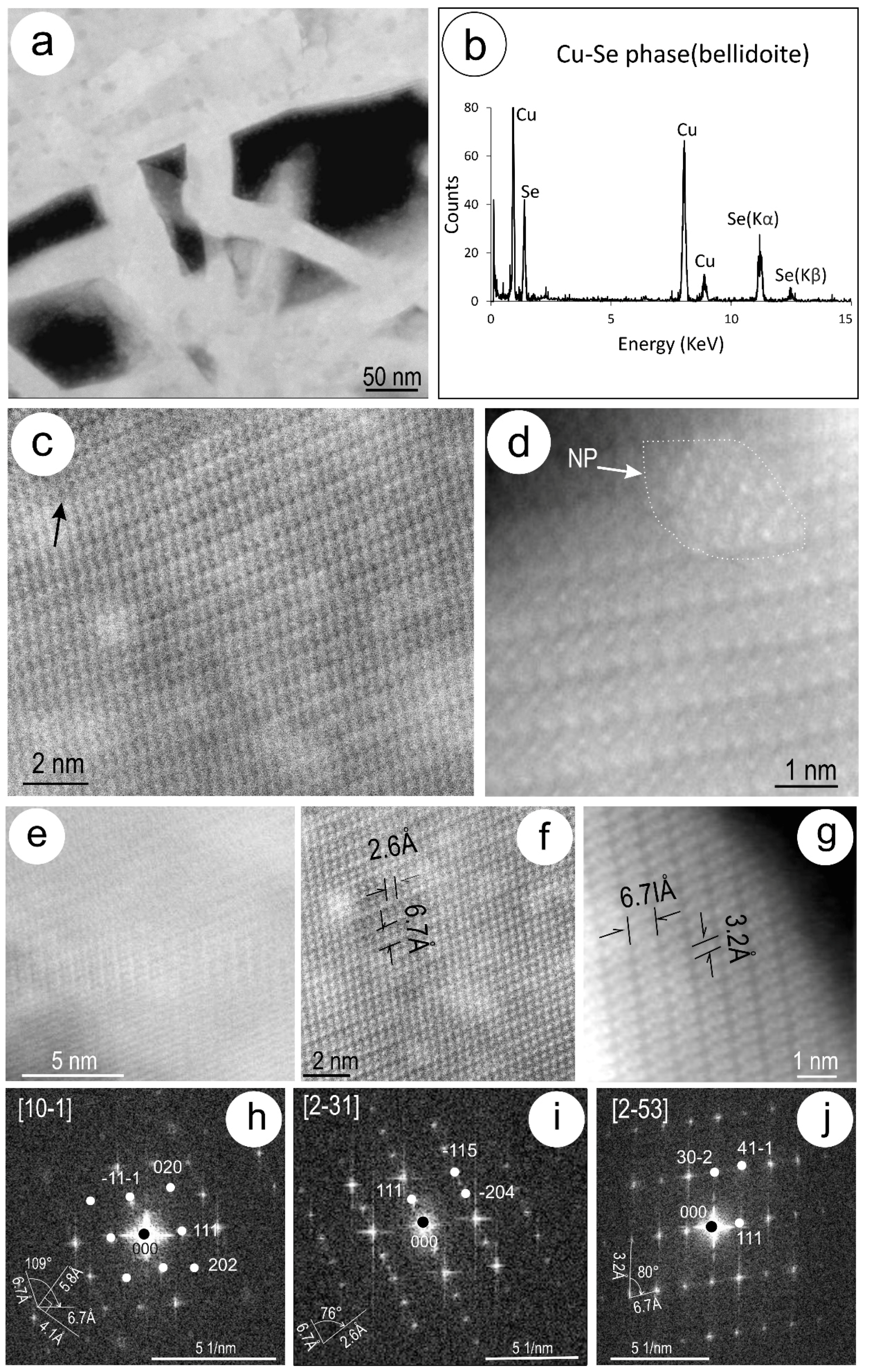
4.3. Nanoscale Characterisation (TEM Data)
4.3.1. Host Sulphides
4.3.2. Clausthalite and other Inclusions
5. Discussion
5.1. Evolution of Sulphide Assemblages
5.2. Formation of Clausthalite in Cu-(Fe)-Sulphides
6. Conclusions and Implications
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Element/Line | Diffracting Crystal/Sp | Peak Count Time (Sec) | Background Type/Fit | Bkgd Points Acquired (Lo/Hi) | Background Count Time (Lo/Hi) (Sec) | Standard |
|---|---|---|---|---|---|---|
| S Kα | LPET/1 | 10 | Multipoint | 2/2 | 10/10 | Astimex Marcasite |
| Pb Mα | LPET/1 | 200 | Multipoint | 4/3 | 20/20 | P & H block Galena |
| Cd Lα | LPET/1 | 10 | Multipoint | 2/2 | 10/10 | P & H block Greenockite |
| Bi Mα | LPET/1 | 10 | Multipoint | 2/2 | 10/10 | P & H block Bi2Se3 |
| As Lα | TAP/2 | 10 | Multipoint | 2/2 | 10/10 | Astimex GaAs |
| Se Lα | TAP/2 | 20 | Multipoint | 2/3 | 20/20 | P & H block Bi2Se3 |
| Fe Kα | LLIF/3 | 10 | Multipoint | 2/2 | 10/10 | P & H block Chalcopyrite |
| Cu Kα | LLIF/3 | 10 | Linear | - | 5/5 | P & H block Chalcopyrite |
| Mn Kα | LLIF/3 | 10 | Multipoint | 2/2 | 10/10 | P & H block Rhodonite |
| Ag Lα | LPET/4 | 10 | Multipoint | 1/2 | 10/10 | P & H block AgTe |
| Sb Lα | LPET/4 | 10 | Multipoint | 2/2 | 10/10 | Astimex Stibnite |
| Te Lα | LPET/4 | 10 | Multipoint | 2/2 | 10/10 | P & H block AgTe |
| Hg Lα | LLIF/5 | 10 | Multipoint | 3/3 | 15/15 | P & H Cinnabar |
| Zn Kα | LLIF/5 | 10 | Multipoint | 2/2 | 10/10 | P & H Spahlerite |
| Ni Kα | LLIF/5 | 10 | Linear | - | 5/5 | Astimex Pentlandite |
| Co. Kα | LLIF/5 | 10 | Multipoint | 2/2 | 10/10 | Astimex Co. metal |
References
- Simon, G.; Essene, E.J. Phase relations among selenides, sulfides, tellurides, and oxides: I. Thermodynamic properties and calculated equilibria. Econ. Geol. 1996, 91, 1183–1208. [Google Scholar] [CrossRef]
- Simon, G.; Kesler, S.E.; Essene, E.J. Phase relations among selenides, tellurides, and oxides; II, Applications to selenide-bearing ore deposits. Econ. Geol. 1997, 92, 468–484. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Spry, P.G.; Voudouris, P. Understanding gold-(silver)-telluride-(selenide) mineral deposits. Episodes 2009, 32, 249–263. [Google Scholar]
- Ramdohr, P. The Ore Minerals and Their Intergrowths; English Translation of the 3rd Edition; Pergamon Press: Oxford, UK, 1969; p. 1174. [Google Scholar]
- Bogdanov, K.; Filipov, A.; Kehayov, R. Au-Ag-Te-Se minerals in the Elatsite porphyry-copper deposit, Bulgaria. Geochem. Miner. Petrol. 2005, 43, 13–19. [Google Scholar]
- Belogub, E.V.; Novoselov, K.A.; Yakovleva, V.A.; Spiro, B. Supergene sulphides and related minerals in the supergene profiles of VHMS deposits from the South Urals. Ore Geol. Rev. 2008, 33, 239–254. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Eliopoulos, D.G.; Chryssoulis, S. A comparison of high-Au massive sulfide ores hosted in ophiolite complexes of the Balkan Peninsula with modern analogues: Genetic significance. Ore Geol. Rev. 2008, 33, 81–100. [Google Scholar] [CrossRef]
- Förster, H.J. Mineralogy of the U-Se-polymetallic deposit Niederschlema–Alberoda, Erzgebirge, Germany. IV. The continuous clausthalite-galena solid-solution series. N. Jahrb. Mineral. Abh. 2005, 181, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Cepedal, A.; Fuertes-Fuente, M.; Martin-Izard, A.; Gonzalez-Nistal, S.; Rodriguez-Pevida, L. Tellurides, selenides and Bi-mineral assemblages from the Río Narcea Gold Belt, Asturias, Spain: genetic implications in Cu-Au and Au skarns. Mineral. Petrol. 2006, 87, 277–304. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.L.Y. Phase relations in the system PbS-PbSe-PbTe. Mineral. Mag. 1994, 58, 567–578. [Google Scholar] [CrossRef]
- Williams, P.J.; Pollard, P.J. Australian Proterozoic iron oxide-Cu-Au deposits; an overview with new metallogenic and exploration data from the Cloncurry District, Northwest Queensland. Explor. Min. Geol. 2001, 10, 191–213. [Google Scholar] [CrossRef]
- Piestrzynski, A.; Pieczonka, J. Low temperature ore minerals associations in the Kupferschiefer type deposit, Lubin-Sieroszowice Mining District SW Poland. Mineral. Rev. 2012, 62, 59–66. [Google Scholar]
- Skirrow, R.G.; Bastrakov, E.N.; Barovcich, K.; Fraser, G.L.; Creaser, R.A.; Fanning, C.M.; Raymond, O.L.; Davidson, G.J. Timing of iron oxide Cu-Au-(U) hydrothermal activity and Nd isotope constraints on metal sources in the Gawler craton, South Australia. Econ. Geol. 2007, 102, 1441–1470. [Google Scholar] [CrossRef]
- Ehrig, K.; McPhie, J.; Kamenetsky, V.S. Geology and mineralogical zonation of the Olympic Dam iron oxide Cu-U-Au-Ag deposit, South Australia. In Geology and Genesis of Major Copper Deposits and Districts of the World, a Tribute to Richard Sillitoe; Hedenquist, J.W., Harris, M., Camus, F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2012; Volume 16, pp. 237–268. [Google Scholar]
- Skirrow, R.G.; Raymond, O.L.; Bastrakov, E.; Davidson, G.J.; Heithersay, P. The geological framework, distribution and controls of Fe oxide Cu-Au mineralisation in the Gawler Craton, South Australia. Part II—alteration and mineralisation. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2002; Volume 2, pp. 33–48. [Google Scholar]
- Kirchenbaur, M.; Maas, R.; Ehrig, K.; Kamenetsky, V.S.; Strub, E.; Ballhaus, C.; Münker, C. Uranium and Sm isotope studies of the supergiant Olympic Dam Cu-Au-U-Ag deposit, South Australia. Geochim. Cosmochim. Acta 2016, 180, 15–32. [Google Scholar] [CrossRef]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Chemical zoning and lattice distortion in uraninite from Olympic Dam, South Australia. Am. Mineral. 2016, 101, 2351–2354. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Pring, A. Chemical and textural interpretation of late-stage coffinite and brannerite from the Olympic Dam IOCG-Ag-U deposit. Mineral. Mag. 2017, 81, 1323–1366. [Google Scholar] [CrossRef]
- Betts, P.G.; Valenta, R.K.; Finlay, J. Evolution of the Mount Woods Inlier, northern Gawler Craton, Southern Australia: An integrated structural and aeromagnetic analysis. Tectonophysics 2003, 366, 83–111. [Google Scholar] [CrossRef]
- Huang, Q.; Kamenetsky, V.S.; McPhie, J.; Ehrig, K.; Meffre, S.; Maas, R.; Thompson, J.; Kamenetsky, M.; Chambefort, I.; Apukhtina, O.; et al. Neoproterozoic (ca. 820–830 Ma) mafic dykes at Olympic Dam, South Australia: Links with the Gairdner Large Igneous Province. Precambrain Res. 2015, 271, 160–172. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Danyushevsky, L.V.; Gilbert, S. Minor elements in bornite and associated Cu-(Fe)-sulfides: A LA-ICPMS study. Geochim. Cosmochim. Acta 2011, 73, 4761–4791. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite-galena-chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Crowe, B.B.P.; Ciobanu, C.L. Trace elements in hydrothermal chalcopyrite. Mineral. Mag. 2018, in press. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Ore minerals down to the nanoscale: Cu-(Fe)-sulphides from the iron oxide copper gold deposit at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 81, 1218–1235. [Google Scholar] [CrossRef]
- Noda, Y.; Masumoto, K.; Ohba, S.; Saito, Y.; Toriumi, K.; Iwata, Y.; Shibuya, I. Temperature dependence of atomic thermal parameters of lead chalcogenides, PbS, PbSe and PbTe. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1987, 43, 1443–1445. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. Crystal chemistry of the monazite and xenotime structures. Am. Mineral. 1995, 80, 21–26. [Google Scholar] [CrossRef]
- Hall, S.R.; Stewart, J.M. The crystal structure refinement of chalcopyrite, CuFeS2. Acta Cryst. 1973, 29, 579–585. [Google Scholar] [CrossRef]
- Tunell, G.; Adams, C.E. On the symmetry and crystal structure of bornite. Am. Mineral. 1949, 34, 824–829. [Google Scholar]
- Kanazawa, Y.; Koto, K.; Morimoto, N. Bornite (Cu5FeS4): Stability and crystal structure of the intermediate form. Can. Mineral. 1978, 16, 397–404. [Google Scholar]
- Ding, Y.; Veblen, D.R.; Prewitt, C.T. High-resolution transmission electron microscopy (HRTEM) study of 4a and 6a superstructure of bornite Cu5FeS4. Am. Mineral. 2005, 90, 1256–1264. [Google Scholar] [CrossRef]
- Ding, Y.; Veblen, D.R.; Prewitt, C.T. Possible Fe/Cu ordering schemes in the 2a superstructure of bornite (Cu5FeS4). Am. Mineral. 2005, 90, 1265–1269. [Google Scholar] [CrossRef]
- Pierce, L.; Buseck, P.R. Superstructuring in the bornite-digenite series: A high-resolution electron microscopy study. Am. Mineral. 1978, 63, 1–6. [Google Scholar]
- Koto, K.; Morimoto, N. Superstructure investigation of bornite, Cu5FeS4, by the modified partial Patterson function. Acta Crystallogr. 1975, 31, 2268–2273. [Google Scholar] [CrossRef]
- Evans, H.T. The crystal structures of low chalcocite and djurleite. Z. Krist. Cryst. Mater. 1979, 150, 299–320. [Google Scholar] [CrossRef]
- Buerger, M.J.; Buerger, N.W. Low-chalcocite and high-chalcocite. Am. Mineral. 1944, 29, 55–65. [Google Scholar]
- Morimoto, N.; Kullerud, G. Polymorphism in digenite. Am. Mineral. 1963, 48, 110–123. [Google Scholar]
- Will, G.; Hinze, E.; Abdelrahman, A.R.M. Crystal structure analysis and refinement of digenite, Cu1.8S, in the temperature range 20 to 500 °C under controlled sulfur partial pressure. Eur. J. Mineral. 2002, 14, 591–598. [Google Scholar] [CrossRef]
- De Montreuil, L.A. Bellidoite, a new copper selenide. Econ. Geol. 1975, 70, 384–387. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Pring, A.; Green, L. Focussed ion beam-transmission electron microscopy applications in ore mineralogy: Bridging micron-and nanoscale observations. Ore Geol. Rev. 2011, 42, 6–31. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Maunders, C.; Wade, B.P.; Ehrig, K. Focused ion beam and advanced electron microscopy for minerals: insights and outlook from bismuth sulphosalts. Minerals 2016, 6, 112. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in Mixed-Layer compounds: A HAADF-STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.; Verdugo-Ihl, M.R.; Courtney-Davies, L.; Gao, W. Advances and opportunities in ore mineralogy. Minerals 2017, 7, 233. [Google Scholar] [CrossRef]
- Downs, R.T.; Hall-Wallace, M. The American mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Yund, R.A.; Kullerud, G. Thermal stability of assemblages in the Cu-Fe-S system. J. Petrol. 1966, 7, 454–488. [Google Scholar] [CrossRef]
- Posfai, M.; Buseck, P.R. Djurleite, digenite, and chalcocite: intergrowths and transformations. Am. Mineral. 1994, 79, 308–315. [Google Scholar]
- Van Dyck, D.; Conde-Amiano, C.; Amelinckx, S. The diffraction pattern of crystals presenting a digenite type of disorder. II. The structure of the digenite-related phases derived by means of the Cluster Theory. Phys. Status Solidi 1980, 58, 451–468. [Google Scholar] [CrossRef]
- Morimoto, N.; Kullerud, G. Polymorphism on the Cu9S5-Cu5FeS4 join. Z. Krist. 1966, 123, 235–254. [Google Scholar] [CrossRef]
- Echigoya, J.; Edington, J.W. A transmission electron microscope study of the chalcocite-djurleite transformation in topotactically grown thin films of CuxS. Phys. Status Solidi 1982, 72, 305–311. [Google Scholar] [CrossRef]
- Zhao, J.; Brugger, J.; Ngothai, Y.; Pring, A. The replacement of chalcopyrite by bornite under hydrothermal conditions. Am. Mineral. 2014, 99, 2389–2397. [Google Scholar] [CrossRef]
- Altree-Williams, A.L.; Pring, A.; Ngothai, Y.; Brugger, J. Textural and compositional complexities resulting from coupled dissolution-reprecipitation reactions in geomaterials. Earth Sci. Rev. 2015, 150, 628–651. [Google Scholar] [CrossRef]
- Quan, Z.; Wu, D.; Zhu, J.; Evers, W.H.; Boncella, J.M.; Siebbeles, L.D.A.; Wang, Z.; Navrotsky, A.; Xu, H. Energy landscape of self-assembled superlattices of PbSe nanocrystals. Proc. Nat. Acad. Sci. USA 2014, 111, 9054–9057. [Google Scholar] [CrossRef] [PubMed]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Greem, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Meffre, S.; Ehrig, K.; Kamenetsky, V.; Chambefort, I.; Maas, R.; McPhie, J. Pb isotopes at Olympic Dam: Constraining sulphide growth. In Proceedings of the 13th Quadrennial IAGOD Symposium 2010, Adelaide, Australia, 6–9 April 2010; pp. 78–79. [Google Scholar]
- Maas, R.; Kamenetsky, V.; Ehrig, K.; Meffre, S.; McPhie, J.; Diemar, G. Olympic Dam U-Cu-Au deposit, Australia: New age constraints. Mineral. Mag. 2011, 75, 1375. [Google Scholar]
- Paar, W.H.; Topa, D.; Roberts, A.C.; Criddle, A.J.; Amann, G.; Sureda, R.J. The new mineral species brodtkorbite, Cu2HgSe2, and the associated selenide assemblage from Tuminico, Sierra de Cacho, La Rioja, Argentina. Can. Mineral. 2002, 40, 225–237. [Google Scholar] [CrossRef]
- Škácha, P.; Sejkora, J.; Plášil, J. Selenide Mineralization in the Příbram Uranium and Base-Metal District (Czech Republic). Minerals 2017, 7, 91. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]





| Mineral Information | Formula | Cu/S Ratio | Symmetry | System | Superstructures | a (Å) | b (Å) | c (Å) | Angle (°) | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|
| Cu-Fe-sulphides | ||||||||||
| Chalcopyrite | CuFeS2 | - | Tetragonal | I42d | 5.289 | 5.289 | 10.423 | [27] | ||
| Bornite | ||||||||||
| >265 °C | Cu5FeS4 | - | Cubic | F23 | (a) | 5.47 | 5.47 | 5.47 | [28] | |
| 200–265 °C | - | Cubic | Fm3m | 2a | 10.981 | 10.981 | 10.981 | [29] | ||
| - | Cubic | F43m | 2a | 10.71 | 10.71 | 10.71 | [30,31] | |||
| - | Cubic | 3a | (3 × 5.5) | (3 × 5.5) | (3 × 5.5) | [32] | ||||
| - | Cubic | Fm3m | 4a | 21.88 | 21.88 | 21.88 | [30,31] | |||
| - | Cubic | 5a | (5 × 5.5) | (5 × 5.5) | (5 × 5.5) | [32] | ||||
| - | Cubic | 6a | (6 × 5.5) | (6 × 5.5) | (6 × 5.5) | [32] | ||||
| <200 °C | - | Orthorhombic | Pbca | 2a4a2a | 10.95 | 21.862 | 10.95 | [33] | ||
| Chalcocite | Cu2-xS | |||||||||
| High-T (104–435 °C) | Cu2S | 2 | Hexagonal | P63/mmc | 3.95 | 3.95 | 6.72 | γ = 120 | [34] | |
| Low-T (<104 °C) | Monoclinic | P21/c | 15.246 | 11.884 | 13.494 | β = 116.35 | [34] | |||
| Pseudo-orthorhombic | ABm2 | 11.884 | 27.324 | 13.494 | β = 90.08 | [35] | ||||
| Djurleite | Cu31S16 | 1.96 | Monoclinic | P21/n | 26.7 | 15.72 | 13.57 | β = 90.13 | [34] | |
| Digenite | ||||||||||
| High-T (>83 °C) | Cu1.8S | 1.8 | Cubic | Fm3m | (a) | 5.57 | 5.57 | 5.57 | [36,37] | |
| Low-T | Cubic | Fd3m | na | (n × 5.57) | (n × 5.57) | (n × 5.57) | [32,36] | |||
| Selenides and monazite | ||||||||||
| Clausthalite | PbSe | - | Cubic | Fm3m | 6.1054 | 6.1054 | 6.1054 | [25] | ||
| Bellidoite | Cu2Se | - | Tetragonal | P 41/m | 11.52 | 11.74 | [38] | |||
| Monazite | RE(PO4) | - | Monoclinic | P21/n | 6.7902 | 7.0203 | 6.4674 | β = 103.38 | [26] | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owen, N.D.; Ciobanu, C.L.; Cook, N.J.; Slattery, A.; Basak, A. Nanoscale Study of Clausthalite-Bearing Symplectites in Cu-Au-(U) Ores: Implications for Ore Genesis. Minerals 2018, 8, 67. https://doi.org/10.3390/min8020067
Owen ND, Ciobanu CL, Cook NJ, Slattery A, Basak A. Nanoscale Study of Clausthalite-Bearing Symplectites in Cu-Au-(U) Ores: Implications for Ore Genesis. Minerals. 2018; 8(2):67. https://doi.org/10.3390/min8020067
Chicago/Turabian StyleOwen, Nicholas D., Cristiana L. Ciobanu, Nigel J. Cook, Ashley Slattery, and Animesh Basak. 2018. "Nanoscale Study of Clausthalite-Bearing Symplectites in Cu-Au-(U) Ores: Implications for Ore Genesis" Minerals 8, no. 2: 67. https://doi.org/10.3390/min8020067
APA StyleOwen, N. D., Ciobanu, C. L., Cook, N. J., Slattery, A., & Basak, A. (2018). Nanoscale Study of Clausthalite-Bearing Symplectites in Cu-Au-(U) Ores: Implications for Ore Genesis. Minerals, 8(2), 67. https://doi.org/10.3390/min8020067







