Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. X-ray Diffraction Characterization
3.1.1. Chrysotile
3.1.2. Amosite
3.1.3. Crocidolite
3.2. Thermal Analysis Characterization
3.2.1. Chrysotile
3.2.2. Amosite
3.2.3. Crocidolite
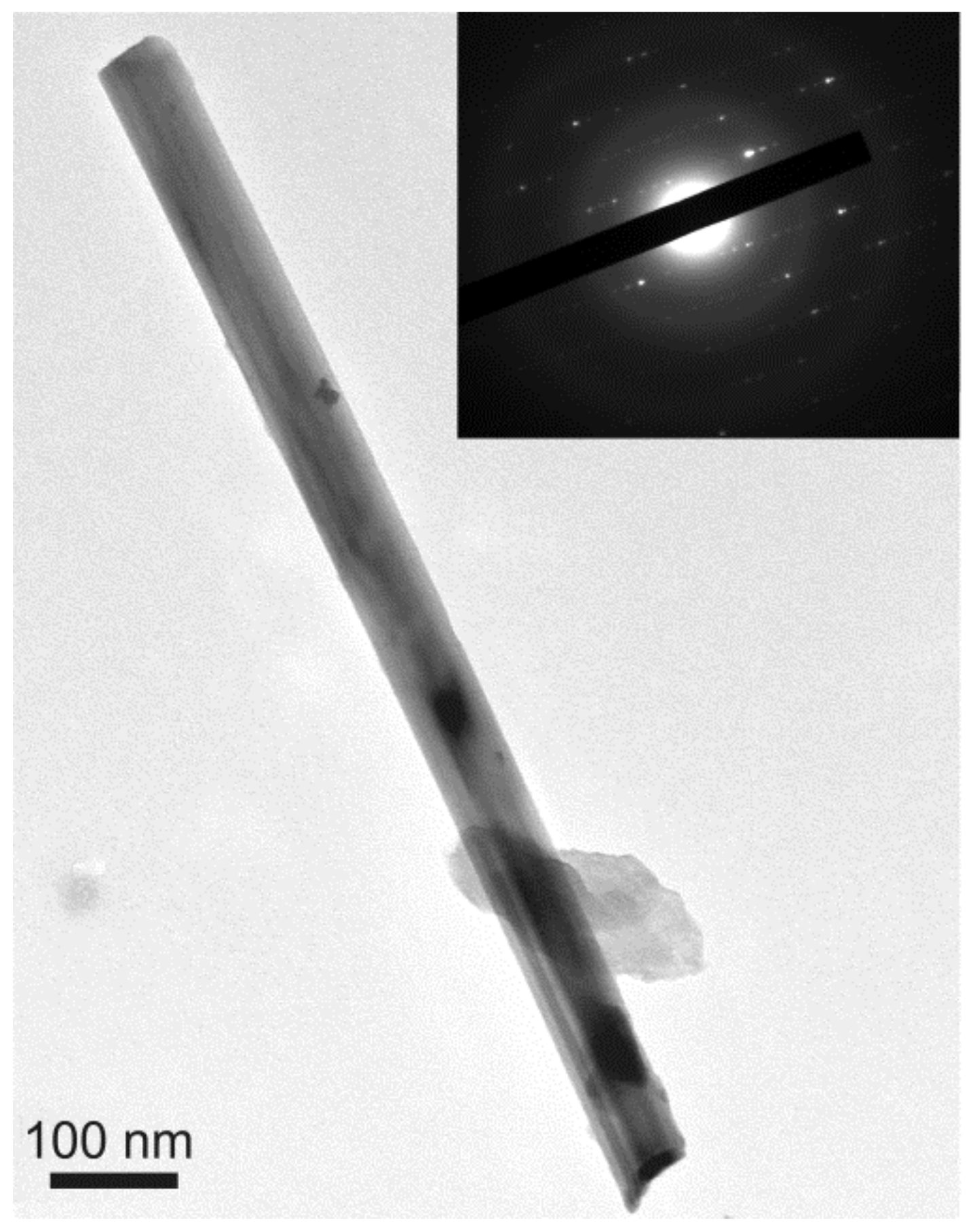
3.3. TEM Characterization
3.3.1. Chrysotile
3.3.2. Amosite
3.3.3. Crocidolite
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). Asbestos and Other Natural Mineral Fibres. Environmental Health Criteria, 53; World Health Organization: Geneva, Switzerland, 1986; 194p. [Google Scholar]
- The European Parliament and the Council of the European Union. Directive 2003/18/EC of the European Parliament and of the Council of 27 March 2003 amending Council Directive 83/477/EEC on the protection of workers from the risks related to exposure to asbestos at work. Off. J. Eur. Union 2003, L97, 48–52. [Google Scholar]
- Gualtieri, A.F. Mineral fibre-based building materials and their health hazards. In Toxicity of Building Materials; Pacheco-Torgal, F., Jalali, S., Fucic, A., Eds.; Woodhead Publishing: Cambridge, UK, 2012; pp. 166–195. [Google Scholar]
- Røe, O.D.; Stella, G.M. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. [Google Scholar] [CrossRef] [PubMed]
- IARC. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42; IARC: Lyon, France, 1987; 440p. [Google Scholar]
- Gualtieri, A.F. (Ed.) Introduction. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 1–15. [Google Scholar]
- Anastasiadou, K.; Axiotis, D.; Gidarakos, E. Hydrothermal conversion of chrysotile asbestos using near supercritical conditions. J. Hazard. Mater. 2010, 179, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Giacobbe, C.; Sardisco, L.; Saraceno, M.; Gualtieri, M.L.; Lusvardi, G.; Cavenati, C.; Zanatto, I. Recycling of the product of thermal inertization of cement–asbestos for various industrial applications. Waste Manag. 2011, 31, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Boccaletti, M. Recycling of the product of thermal inertization of cement–asbestos for the production of concrete. Constr. Build. Mater. 2011, 25, 3561–3569. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F. Recycling the product of thermal transformation of cement-asbestos for the preparation of calcium sulfoaluminate clinker. J. Hazard. Mater. 2013, 260, 813–818. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F. Preparation of magnesium phosphate cement by recycling the product of thermal transformation of asbestos containing wastes. Cem. Concr. Res. 2014, 58, 56–66. [Google Scholar] [CrossRef]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Podwórny, J. Utilisation of cement-asbestos wastes by thermal treatment and the potential possibility use of obtained product for the clinker bricks manufacture. J. Mater. Sci. 2015, 50, 6757–6767. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives (Text with EEA relevance). Off. J. Eur. Union 2008, L312, 3–30. [Google Scholar]
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 2000, 20, 1409–1418. [Google Scholar] [CrossRef]
- Leonelli, C.; Veronesi, P.; Boccaccini, D.N.; Rivasi, M.R.; Barbieri, L.; Andreola, F.; Lancellotti, I.; Rabitti, D.; Pellacani, G.C. Microwave thermal inertisation of asbestos containing waste and its recycling in traditional ceramics. J. Hazard. Mater. 2006, 135, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, A.F.; Cavenati, C.; Zanatto, I.; Meloni, M.; Elmi, G.; Gualtieri, M.L. The transformation sequence of cement–asbestos slates up to 1200 °C and safe recycling of the reaction product in stoneware tile mixtures. J. Hazard. Mater. 2008, 152, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dellisanti, F.; Rossi, P.L.; Valdrè, G. Remediation of asbestos containing materials by Joule heating vitrification performed in a pre-pilot apparatus. Int. J. Miner. Process. 2009, 91, 61–67. [Google Scholar] [CrossRef]
- Giacobbe, C.; Gualtieri, A.F.; Quartieri, S.; Rinaudo, C.; Allegrina, M.; Andreozzi, G.B. Spectroscopic study of the product of thermal transformation of chrysotile-asbestos containing materials (ACM). Eur. J. Mineral. 2010, 22, 535–546. [Google Scholar] [CrossRef]
- Viani, A.; Gualtieri, A.F.; Pollastri, S.; Rinaudo, C.; Croce, A.; Urso, G. Crystal chemistry of the high temperature product of transformation of cement-asbestos. J. Hazard. Mater. 2013, 248, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Croce, A.; Allegrina, M.; Trivero, P.; Rinaudo, C.; Viani, A.; Pollastri, S.; Gualtieri, A.F. The concept of ‘end of waste’ and recycling of hazardous materials: In depth characterization of the product of thermal transformation of cement-asbestos. Mineral. Mag. 2014, 78, 1177–1191. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kida, A.; Noma, Y.; Terazono, A.; Sakai, S. Evaluation of thermally treated asbestos based on fiber number concentration determined by transmission electron microscopy. J. Mater. Cycles Waste Manag. 2016, 20, 214–222. [Google Scholar] [CrossRef]
- Pawełczyk, A.; Božek, F.; Grabas, K.; Chęcmanowski, J. Chemical elimination of the harmful properties of asbestos from military facilities. Waste Manag. 2017, 61, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Witek, J.; Kusiorowski, R. Neutralization of cement-asbestos waste by melting in an arc-resistance furnace. Waste Manag. 2017, 69, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Sugama, T.; Sabatini, R.; Petrakis, L. Decomposition of chrysotile asbestos by fluorosulfonic acid. Ind. Eng. Chem. Res. 1998, 37, 79–88. [Google Scholar] [CrossRef]
- Candela, P.A.; Crummett, C.D.; Earnest, D.J.; Frank, M.R.; Wylie, A.G. Low-pressure decomposition of chrysotile as a function of time and temperature. Am. Mineral. 2007, 92, 1704–1713. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Kozawa, T.; Onda, A.; Kanazawa, M.; Shinohara, J.; Takanami, T.; Shiraishi, M. A novel decomposition technique of friable asbestos by CHClF 2-decomposed acidic gas. J. Hazard. Mater. 2009, 163, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Yvon, Y.; Sharrock, P. Characterization of thermochemical inactivation of asbestos containing wastes and recycling the mineral residues in cement products. Waste Biomass Valoriz. 2011, 2, 169–181. [Google Scholar] [CrossRef]
- Belardi, G.; Piga, L. Influence of calcium carbonate on the decomposition of asbestos contained in end-of-life products. Thermochim. Acta 2013, 573, 220–228. [Google Scholar] [CrossRef]
- Yao, M.; Lian, B.; Teng, H.H.; Tian, Y.; Yang, X. Serpentine dissolution in the presence of bacteria Bacillus mucilaginosus. Geomicrobiol. J. 2013, 30, 72–80. [Google Scholar] [CrossRef]
- Spasiano, D.; Pirozzi, F. Treatments of asbestos containing wastes. J. Environ. Manag. 2017, 204, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Spasiano, D.; Luongo, V.; Petrella, A.; Alfè, M.; Pirozzi, F.; Fratino, U.; Piccinni, A.F. Preliminary study on the adoption of dark fermentation as pretreatment for a sustainable hydrothermal denaturation of cement-asbestos composites. J. Clean. Prod. 2017, 166, 172–180. [Google Scholar] [CrossRef]
- Spasiano, D. Dark fermentation process as pretreatment for a sustainable denaturation of asbestos containing wastes. J. Hazard. Mater. 2018, 349, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Berbenni, V.; Marini, A.; Bruni, G. Effect of mechanical milling on solid state formation of BaTiO3 from BaCO3–TiO2 (rutile) mixtures. Thermochim. Acta 2001, 374, 151–158. [Google Scholar] [CrossRef]
- Miriello, D.; Bloise, A.; Crisci, G.M.; Barrese, E.; Apollaro, C. Effects of milling: A possible factor influencing the durability of historical mortars. Archaeometry 2010, 52, 668–679. [Google Scholar] [CrossRef]
- Sánchez-Soto, P.J.; Carmen Jiménez de Haro, M.; Pérez-Maqueda, L.A.; Varona, I.; Pérez-Rodríguez, J.L. Effects of dry grinding on the structural changes of kaolinite powders. J. Am. Ceram. Soc. 2000, 83, 1649–1657. [Google Scholar] [CrossRef]
- Haurie, L.; Fernandez, A.I.; Velasco, J.I.; Chimenos, J.M.; Lopez-Cuesta, J.M.; Espiell, F. Effects of milling on the thermal stability of synthetic hydromagnesite. Mater. Res. Bull. 2007, 42, 1010–1018. [Google Scholar] [CrossRef]
- Suquet, H. Effects of dry grinding and leaching on the crystal structure of chrysotile. Clays Clay Miner. 1989, 37, 439–445. [Google Scholar] [CrossRef]
- Plescia, P.; Gizzi, D.; Benedetti, S.; Camilucci, L.; Fanizza, C.; De Simone, P.; Paglietti, F. Mechanochemical treatment to recycling asbestos-containing waste. Waste Manag. 2003, 23, 209–218. [Google Scholar] [CrossRef]
- Inoue, R.; Kano, J.; Shimme, K.; Saito, F. Safe decomposition of asbestos by mechano-chemical reaction. Mater. Sci. Forum 2007, 561, 2257–2260. [Google Scholar] [CrossRef]
- Hashimoto, S.; Takeda, H.; Okuda, A.; Kambayashi, A.; Honda, S.; Iwamoto, Y.; Fukuda, K. Detoxification of industrial asbestos waste by low-temperature heating in a vacuum. J. Ceram. Soc. Jpn. 2008, 116, 242–246. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Lavorgna, M.; Verdolotti, L.; De Stefano, L. Treatment and recycling of asbestos-cement containing waste. J. Hazard. Mater. 2011, 195, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Viani, A.; Gualtieri, A.F.; Secco, M.; Peruzzo, L.; Artioli, G.; Cruciani, G. Minerals in the Human. Body Crystal chemistry of cement-asbestos. Am. Mineral. 2013, 98, 1095–1105. [Google Scholar] [CrossRef]
- Martinelli, G.; Plescia, P. Mechanochemical dissociation of calcium carbonate: Laboratory data and relation to natural emissions of CO2. Phys. Earth Planet. Inter. 2004, 142, 205–214. [Google Scholar] [CrossRef]
- Weeber, A.W.; Haag, W.J.; Wester, A.; Bakker, H. Differences in the amorphization reaction by mechanical alloying of Ni-Zr resulting from different ball-milling techniques. J. Less Common Met. 1988, 140, 119–127. [Google Scholar] [CrossRef]
- Eckert, J.; Schultz, L.; Hellstern, E.; Urban, K. Glass-forming range in mechanically alloyed Ni-Zr and the influence of the milling intensity. J. Appl. Phys. 1988, 64, 3224–3228. [Google Scholar] [CrossRef]
- Bloise, A.; Catalano, M.; Barrese, E.; Gualtieri, A.F.; Gandolfi, N.B.; Capella, S.; Belluso, E. TG/DSC study of the thermal behaviour of hazardous mineral fibres. J. Therm. Anal. Calorim. 2016, 123, 2225–2239. [Google Scholar] [CrossRef]
- Bloise, A.; Barca, D.; Gualtieri, A.F.; Pollastri, S.; Belluso, E. Trace elements in hazardous mineral fibres. Environ. Pollut. 2016, 216, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Pollastri, S.; Perchiazzi, N.; Lezzerini, M.; Plaisier, J.; Cavallo, A.; Dalconi, M.; Bursi Gandolfi, N.; Gualtieri, A. The crystal structure of mineral fibres. 1. Chrysotile. Period. Mineral. 2016, 85, 249–259. [Google Scholar]
- Pollastri, S.; Perchiazzi, N.; Gigli, L.; Cavallo, A.; Bursi Gandolfi, N.; Pollok, K.; Gualtieri, A. The crystal structure of mineral fibres. 2. Amosite and fibrous anthophyllite. Period. Mineral. 2017, 86, 55–65. [Google Scholar]
- González, G.; Sagarzazu, A.; Villalba, R. Study of the mechano-chemical transformation of goethite to hematite by TEM and XRD. Mater. Res. Bull. 2000, 35, 2295–2308. [Google Scholar] [CrossRef]
- Liang, S.H.; Cameron, L.E. Differential Scanning Calorimetry (DSC) for the Analysis of Activated Carbon; Report No. 1098; Defense Research Establishment: Ottawa, ON, Canada, 1991. [Google Scholar]
- Bloise, A.; Kusiorowski, R.; Lassinantti Gualtieri, M.; Gualtieri, A.F. Thermal behaviour of mineral fibres. In Mineral Fibres: Crystal Chemistry, Chemical-Physical Properties, Biological Interaction and Toxicity; Gualtieri, A.F., Ed.; European Mineralogical Union: London, UK, 2017; Volume 18, pp. 215–252. [Google Scholar]
- Weber, J.N.; Greer, R.T. Dehydration of serpentine-heat of reaction and reaction kinetics at p H2O = 1 atm. Am. Mineral. 1965, 50, 450–464. [Google Scholar]
- Kusiorowski, R.; Zaremba, T.; Piotrowski, J.; Adamek, J. Thermal decomposition of different types of asbestos. J. Therm. Anal. Calorim. 2012, 109, 693–704. [Google Scholar] [CrossRef]
- Hodgson, A.A.; Freeman, A.G.; Taylor, H.F.W. The thermal decomposition of crocidolite from Koegas. South Africa. Mineral. Mag. 1965, 35, 5–30. [Google Scholar] [CrossRef]
- Whittaker, E.J.W. The structure of chrysotile. V. Diffuse reflections and fibre texture. Acta Crystallogr. 1957, 10, 149–156. [Google Scholar] [CrossRef]
- Bloise, A.; Fornero, E.; Belluso, E.; Barrese, E.; Rinaudo, C. Synthesis and characterization of tremolite asbestos fibres. Eur. J. Mineral. 2008, 20, 1027–1033. [Google Scholar] [CrossRef]
- Čičel, B.; Kranz, G. Mechanism of montmorillonite structure degradation by percussive grinding. Clay Miner. 1981, 16, 151–162. [Google Scholar] [CrossRef]
- Horváth, E.; Frost, R.L.; Makó, É.; Kristóf, J.; Cseh, T. Thermal treatment of mechanochemically activated kaolinite. Thermochim. Acta 2003, 404, 227–234. [Google Scholar] [CrossRef]
- Iguchi, Y.; Senna, M. Mechanochemical polymorphic transformation and its stationary state between aragonite and calcite I. Effects of preliminary annealing. Powder Technol. 1985, 43, 155–162. [Google Scholar] [CrossRef]
- Cornejo, J.; Hermosin, M.C. Structural alteration of sepiolite by dry grinding. Clay Miner. 1988, 23, 391–398. [Google Scholar] [CrossRef]
- Miller, J.G.; Oulton, T.D. Prototropy in kaolinite during percussive grinding. Clays Clay Miner. 1970, 18, 313–323. [Google Scholar] [CrossRef]
- Cattaneo, A.; Gualtieri, A.F.; Artioli, G. Kinetic study of the dehydroxylation of chrysotile asbestos with temperature by in situ XRPD. Phys. Chem. Miner. 2003, 30, 177–183. [Google Scholar] [CrossRef]
- Aglietti, E.F.; Lopez, J.P.; Pereira, E. Mechanochemical effects in kaolinite grinding. I. Textural and physicochemical aspects. Int. J. Miner. Process. 1986, 16, 125–133. [Google Scholar] [CrossRef]
- Al-Wakeel, M.I. Effect of mechanical treatment on the mineralogical constituents of Abu-Tartour phosphate ore, Egypt. Int. J. Miner. Process. 2005, 75, 101–112. [Google Scholar] [CrossRef]
- Henmi, T.; Yoshinaga, N. Alteration of imogolite by dry grinding. Clay Miner. 1981, 16, 139–149. [Google Scholar] [CrossRef]
- Dellisanti, F.; Valdrè, G.; Mondonico, M. Changes of the main physical and technological properties of talc due to mechanical strain. Appl. Clay Sci. 2009, 42, 398–404. [Google Scholar] [CrossRef]
- Juhasz, A.Z.; Opoczky, L. Mechanical Activation of Minerals by Grinding: Pulverizing and Morphology of Particles; Akadémiai Kiadó: Budapest, Hungary, 1990; 234p. [Google Scholar]











| Sample (Grinding Time) | Chr 30 s | Chr 5 min | Chr 10 min |
|---|---|---|---|
| DSC T (°C) | 660 endo s | 625 endo | 622 endo w |
| 822 exo s | 818 exo s | 810 exo s | |
| ΔH (J/g) | 345 | 35 | 12 |
| Onset-end (°C) | 500–725 | 556–656 | 556–656 |
| Grinding Time | 30 s | 5 min | 10 min |
|---|---|---|---|
| TG Loss (%) | TG Loss (%) | TG Loss (%) | |
| T Range 40–110 (°C) | T Range 40–110 (°C) | T Range 40–110 (°C) | |
| Chr | 0.52 | 2.67 | 1.08 |
| AM | 0.14 | 0.50 | 0.42 |
| CR | 0.26 | 2.57 | 0.95 |
| Sample (Grinding Time) | AM 30 s | AM 5 min | AM 10 min |
|---|---|---|---|
| DSC T (°C) | 740 endo | 591 endo | 536 endo |
| 880 exo | 683 exo | 682 exo | |
| ΔH (J/g) | 222 | 81 | 59 |
| Onset-end (°C) | 586–800 | 450–656 | 462–655 |
| Sample (Grinding Time) | CR 30 s | CR 5 min | CR 10 min |
|---|---|---|---|
| DSC T (°C) | 650 endo | 555 endo w | 538 endo w |
| 850 exo w | 838 exo s | 833 exo s | |
| ΔH (J/g) | 213 | 85 | 73 |
| Onset-end (°C) | 311–420 and 430–817 | 426–661 | 415–648 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloise, A.; Catalano, M.; Gualtieri, A.F. Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment. Minerals 2018, 8, 135. https://doi.org/10.3390/min8040135
Bloise A, Catalano M, Gualtieri AF. Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment. Minerals. 2018; 8(4):135. https://doi.org/10.3390/min8040135
Chicago/Turabian StyleBloise, Andrea, Manuela Catalano, and Alessandro Francesco Gualtieri. 2018. "Effect of Grinding on Chrysotile, Amosite and Crocidolite and Implications for Thermal Treatment" Minerals 8, no. 4: 135. https://doi.org/10.3390/min8040135





