Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment
Abstract
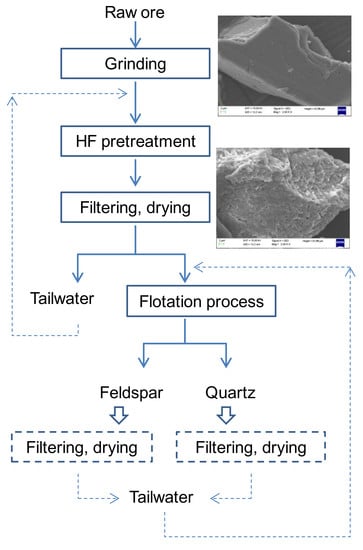
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. HF Pretreatment
2.3. Micro-Flotation Tests
2.4. Zeta Potential Measurements
2.5. Pyrene Fluorescence Probe
2.6. ATR-FTIR Measurements
2.7. SEM and XPS Tests
2.8. Bench Scale Flotation Tests
3. Results and Discussion
3.1. Micro-Flotation
3.2. Zeta Potential
3.3. Pyrene Fluorescence Spectroscopy
3.4. ATR-FTIR Analysis of DDA Adsorption
3.5. SEM Patterns
3.6. XPS Analysis
3.7. Effective Separation of Quartz and Feldspar
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abdel-Khalek, N.A.; Yehia, A.; Ibrahim, S.S. Technical note beneficiation of egyptian feldspar for application in the glass and ceramics industries. Miner. Eng. 1994, 7, 1193–1201. [Google Scholar] [CrossRef]
- Wu, J.-F.; Li, Z.; Huang, Y.-Q.; Li, F.; Yang, Q.-R. Fabrication and characterization of low temperature co-fired cordierite glass–ceramics from potassium feldspar. J. Alloys Compd. 2014, 583, 248–253. [Google Scholar] [CrossRef]
- Basnayaka, L.; Subasinghe, N.; Albijanic, B. Influence of clays on the slurry rheology and flotation of a pyritic gold ore. Appl. Clay Sci. 2017, 136, 230–238. [Google Scholar] [CrossRef]
- Han, J.; Jiao, F.; Liu, W.; Qin, W.; Xu, T.; Xue, K.; Zhang, T. Innovative methodology for comprehensive utilization of spent MgO-Cr2O3 bricks: Copper flotation. ACS Sustain. Chem. Eng. 2016, 4, 5503–5510. [Google Scholar] [CrossRef]
- Xia, W.; Zhou, C.; Peng, Y. Enhancing flotation cleaning of intruded coal dry-ground with heavy oil. J. Clean. Prod. 2017, 161, 591–597. [Google Scholar] [CrossRef]
- Elshall, H.; Vidanage, S.; Somasundaran, P. Grinding of quartz in amine solutions. Int. J. Miner. Process. 1979, 6, 105–121. [Google Scholar] [CrossRef]
- Scott, J.L.; Smith, R.W. Diamine flotation of quartz. Miner. Eng. 1991, 4, 141–150. [Google Scholar] [CrossRef]
- Vieira, A.M.; Peres, A.E.C. The effect of amine type, pH, and size range in the flotation of quartz. Miner. Eng. 2007, 20, 1008–1013. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Raghavan, S. The crystal chemistry, surface properties and flotation behaviour of silicate minerals. Proc. XII Int. Miner. Process. Congr. 1977, 2, 368–415. [Google Scholar]
- Hanumantha Rao, K.; Forssberg, K.S.E. Mixed collector systems in flotation. Int. J. Miner. Process. 1997, 51, 67–79. [Google Scholar] [CrossRef]
- Shehu, N.; Spaziani, E. Separation of feldspar from quartz using edta as modifier. Miner. Eng. 1999, 12, 1393–1397. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Hanumantha Rao, K.; Chernyshova, I.V.; Pradip; Forssberg, K.S.E. Mechanisms of amine–quartz interaction in the absence and presence of alcohols studied by spectroscopic methods. J. Colloid Interface Sci. 2002, 256, 59–72. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Hanumantha Rao, K.; Chernyshova, I.V. Mechanisms of amine–feldspar interaction in the absence and presence of alcohols studied by spectroscopic methods. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 127–142. [Google Scholar] [CrossRef]
- Sekulic, Z.; Canic, N.; Bartulovic, Z.; Dakovic, A. Application of different collectors in the flotation concentration of feldspar, mica and quartz sand. Miner. Eng. 2004, 17, 77–80. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Lee, B.-K. Combining ZnO/microwave treatment for changing wettability of WEEE styrene plastics (ABS and HIPS) and their selective separation by froth flotation. Appl. Surf. Sci. 2017, 420, 746–752. [Google Scholar] [CrossRef]
- Cao, S.; Cao, Y.; Liao, Y.; Ma, Z. Depression mechanism of strontium ions in bastnaesite flotation with salicylhydroxamic acid as collector. Minerals 2018, 8, 66. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, W.; Wei, D.; Wang, W.; Cui, B.; Liu, W. Effect of copper ions on the flotation separation of chalcopyrite and molybdenite using sodium sulfide as a depressant. Miner. Eng. 2018, 115, 44–52. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Y.; Xie, Y.; Shang, Y.; Zheng, G. A novel macromolecular depressant for reverse flotation: Synthesis and depressing mechanism in the separation of hematite and quartz. Sep. Purif. Technol. 2017, 186, 175–181. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Li, L.; Jin, S. Effect of energy input on flocculation process and flotation performance of fine scheelite using sodium oleate. Miner. Eng. 2017, 112, 27–35. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Pradip. Zeta potentials in the flotation of oxide and silicate minerals. Adv. Colloid Interface Sci. 2005, 114–115, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Baldi, L.D.C.; Iamazaki, E.T.; Atvars, T.D.Z. Evaluation of the polarity of polyamide surfaces using the fluorescence emission of pyrene. Dyes Pigment. 2008, 76, 669–676. [Google Scholar] [CrossRef]
- Arunima, C.; Sourav, H.; Amitabha, C. Organization and dynamics in micellar structural transition monitored by pyrene fluorescence. Biochem. Biophys. Res. Commun. 2009, 390, 728–732. [Google Scholar]
- Zhang, J.; Wang, W.-Q.; Liu, J.; Huang, Y.; Feng, Q.-M.; Zhao, H. Fe(III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- Wijnja, H.; Schulthess, C.P. ATR-FTIR and DRIFT spectroscopy of carbonate species at the aged γ-Al2O3/water interface. Spectrochim. Acta Part A 1999, 55, 861–872. [Google Scholar] [CrossRef]
- Xu, H.; van Deventer, J.S.J. The effect of alkali metals on the formation of geopolymeric gels from alkali-feldspars. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 27–44. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Ren, J.-W. The flotation of quartz from iron minerals with a combined quaternary ammonium salt. Int. J. Miner. Process. 2005, 77, 116–122. [Google Scholar]
- Wang, L.; Sun, W.; Hu, Y.-H.; Xu, L.-H. Adsorption mechanism of mixed anionic/cationic collectors in muscovite- quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Long, X.; Chen, J.; Chen, Y. Adsorption of ethyl xanthate on ZnS(110) surface in the presence of water molecules: A DFT study. Appl. Surf. Sci. 2016, 370, 11–18. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, Y.; Huang, J.; Liu, T.; Yuan, Y.; Xue, N. Eco-friendly leaching and separation of vanadium over iron impurity from vanadium-bearing shale using oxalic acid as a leachant. ACS Sustain. Chem. Eng. 2018, 6, 1900–1908. [Google Scholar] [CrossRef]
- Yin, W.Z.; Sun, C.Y. X-ray photoelectron spectrometric analysis on surface property of silicate minerals. J. Northeast. Univ. (Nat. Sci.) 2002, 23, 156–159. [Google Scholar]
- Shchukarev, A.; Sjöberg, S. XPS with fast-frozen samples: A renewed approach to study the real mineral/solution interface. Surf. Sci. 2005, 584, 106–112. [Google Scholar] [CrossRef]
- Baer, D.R.; Gaspar, D.J.; Nachimuthu, P.; Techane, S.D.; Castner, D.G. Application of surface chemical analysis tools for characterization of nanoparticles. Anal. Bioanal. Chem. 2010, 396, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Dorfner, S.; Trindle, H.; Jakobs, U. Electrostatic feldspar quartz separation without hydrofluoric acid reduces pollution. Dev. Miner. Process. 2000, 13, 30–33. [Google Scholar]











| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K(2p) | Na(1s) | Al(2p) | Si(2p) | O(1s) | C(1s) | K(2p) | Na(1s) | Al(2p) | Si(2p) | O(1s) | C(1s) | |
| Untreated feldspar | 299.3 | 1072.5 | 74.5 | 102.9 | 532.1 | 284.8 | - | - | - | - | - | - |
| Pretreated feldspar | 298.0 | 1072.4 | 74.4 | 102.8 | 532.0 | 284.8 | −1.3 | −0.1 | −0.1 | −0.1 | −0.1 | 0.0 |
| Pretreated feldspar + DDA | 293.1 | 1068.8 | 74.2 | 102.7 | 528.3 | 284.8 | −4.9 | −3.6 | −0.2 | −0.1 | −3.7 | 0.0 |
| Untreated quartz | - | - | - | 103.1 | 532.4 | 284.8 | - | - | - | - | - | - |
| Pretreated quartz | - | - | - | 103.1 | 532.3 | 284.8 | - | - | - | −0.0 | −0.1 | 0.0 |
| Pretreated quartz + DDA | - | - | - | 103.0 | 532.3 | 284.8 | - | - | - | −0.1 | −0.0 | −0.0 |
| Sample | Surface Atomic Composition (%) | ||||||
|---|---|---|---|---|---|---|---|
| C | O | Si | Al | Na | K | N | |
| Untreated feldspar | 21.8 | 50.1 | 17.9 | 6.5 | 3.7 | 0.1 | - |
| Pretreated feldspar | 22.2 | 49.8 | 17.6 | 6.4 | 3.9 | 0.1 | - |
| Pretreated feldspar + DDA | 22.9 | 49.5 | 17.6 | 5.8 | 3.4 | 0.1 | 0.8 |
| Untreated quartz | 21.1 | 53.2 | 25.8 | - | - | - | - |
| Pretreated quartz | 21.1 | 53.0 | 26.0 | - | - | - | - |
| Pretreated quartz + DDA | 22.6 | 51.5 | 25.2 | - | - | - | 0.7 |
| Flotation Technology | Products | Yield (%) | Grade (%) | Recovery (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Na2O | K2O | SiO2 | Na2O | K2O | SiO2 | |||
| Untreated | Concentrate | 10.94 | 7.83 | 0.32 | 78.64 | 15.21 | 21.92 | 10.15 |
| Tailing | 89.06 | 5.36 | 0.14 | 85.48 | 84.79 | 78.08 | 89.85 | |
| Feed | 100.00 | 5.63 | 0.16 | 84.73 | 100.00 | 100.00 | 100.00 | |
| Pretreated by HF (2000 g/t) | Concentrate | 50.79 | 10.24 | 0.29 | 72.26 | 92.39 | 90.89 | 43.32 |
| Tailing | 49.21 | 0.87 | 0.03 | 97.6 | 7.61 | 9.11 | 56.68 | |
| Feed | 100.00 | 5.63 | 0.16 | 84.51 | 100.00 | 100.00 | 100.00 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Cong, J.; Deng, J.; Weng, X.; Lin, Y.; Huang, Y.; Peng, T. Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment. Minerals 2018, 8, 149. https://doi.org/10.3390/min8040149
Wang W, Cong J, Deng J, Weng X, Lin Y, Huang Y, Peng T. Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment. Minerals. 2018; 8(4):149. https://doi.org/10.3390/min8040149
Chicago/Turabian StyleWang, Weiqing, Jinyao Cong, Jie Deng, Xiaoqing Weng, Yiming Lin, Yang Huang, and Tiefeng Peng. 2018. "Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment" Minerals 8, no. 4: 149. https://doi.org/10.3390/min8040149
APA StyleWang, W., Cong, J., Deng, J., Weng, X., Lin, Y., Huang, Y., & Peng, T. (2018). Developing Effective Separation of Feldspar and Quartz While Recycling Tailwater by HF Pretreatment. Minerals, 8(4), 149. https://doi.org/10.3390/min8040149