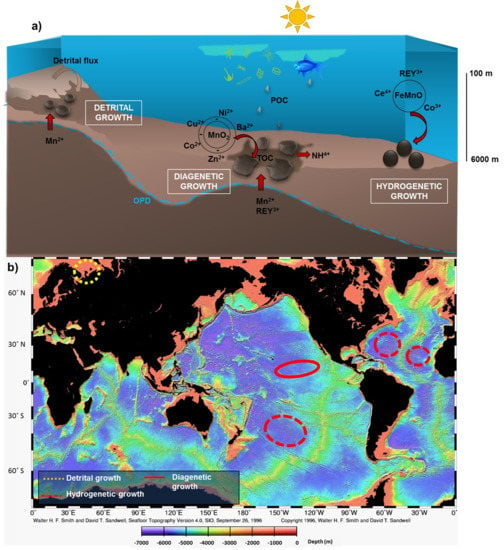
Linkages between the Genesis and Resource Potential of Ferromanganese Deposits in the Atlantic, Pacific, and Arctic Oceans
Abstract
:1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Samples
3.2. Analytical Methods
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Laser Ablation Inductively-Coupled Plasma Mass Spectrometry (LA ICP-MS) Analysis
4. Results
4.1. Internal Structure of Nodules and Crust
4.2. Chemical Composition of Internal Layers
4.3. Factor Analysis
5. Discussion
5.1. Genesis of Deep-Sea Ferromanganese Deposits
5.2. Controls on the Resource Potential of Deep-Sea Ferromanganese Deposits
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| JC120−104 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.48 | 1.00 | |||||||||||||||||||||||||
| Cr | 0.05 | −0.09 | 1.00 | ||||||||||||||||||||||||
| Mn | −0.84 | −0.13 | −0.17 | 1.00 | |||||||||||||||||||||||
| Fe | 0.91 | 0.52 | −0.01 | −0.82 | 1.00 | ||||||||||||||||||||||
| Co | 0.12 | 0.36 | 0.19 | −0.14 | 0.09 | 1.00 | |||||||||||||||||||||
| Ni | −0.56 | −0.54 | 0.04 | 0.39 | −0.62 | −0.13 | 1.00 | ||||||||||||||||||||
| Cu | −0.62 | −0.56 | 0.01 | 0.54 | −0.70 | −0.12 | 0.85 | 1.00 | |||||||||||||||||||
| Zn | −0.79 | −0.17 | −0.24 | 0.92 | −0.71 | −0.32 | 0.26 | 0.36 | 1.00 | ||||||||||||||||||
| Ba | 0.22 | 0.69 | 0.09 | 0.02 | 0.18 | 0.56 | −0.23 | −0.14 | −0.19 | 1.00 | |||||||||||||||||
| La | 0.40 | 0.46 | −0.17 | −0.41 | 0.50 | 0.42 | −0.45 | −0.45 | −0.37 | 0.26 | 1.00 | ||||||||||||||||
| Ce | 0.95 | 0.60 | −0.06 | −0.80 | 0.95 | 0.22 | −0.61 | −0.68 | −0.74 | 0.27 | 0.55 | 1.00 | |||||||||||||||
| Pr | 0.23 | 0.32 | −0.13 | −0.28 | 0.30 | 0.44 | −0.31 | −0.30 | −0.26 | 0.20 | 0.97 | 0.36 | 1.00 | ||||||||||||||
| Nd | 0.20 | 0.31 | −0.13 | −0.26 | 0.27 | 0.44 | −0.30 | −0.29 | −0.25 | 0.20 | 0.96 | 0.34 | 1.00 | 1.00 | |||||||||||||
| Sm | 0.17 | 0.29 | −0.12 | −0.23 | 0.23 | 0.44 | −0.28 | −0.27 | −0.22 | 0.18 | 0.95 | 0.30 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Eu | 0.17 | 0.28 | −0.12 | −0.23 | 0.22 | 0.44 | −0.27 | −0.26 | −0.22 | 0.18 | 0.95 | 0.29 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| Gd | 0.19 | 0.28 | −0.12 | −0.26 | 0.25 | 0.44 | −0.29 | −0.28 | −0.24 | 0.17 | 0.95 | 0.32 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Tb | 0.21 | 0.29 | −0.12 | −0.27 | 0.26 | 0.43 | −0.29 | −0.29 | −0.25 | 0.18 | 0.96 | 0.34 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Dy | 0.22 | 0.30 | −0.13 | −0.28 | 0.28 | 0.43 | −0.30 | −0.30 | −0.27 | 0.18 | 0.96 | 0.35 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Y | 0.10 | 0.15 | −0.10 | −0.20 | 0.13 | 0.43 | −0.19 | −0.17 | −0.21 | 0.11 | 0.90 | 0.21 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 1.00 | |||||||
| Ho | 0.23 | 0.29 | −0.13 | −0.29 | 0.28 | 0.43 | −0.30 | −0.29 | −0.28 | 0.18 | 0.96 | 0.35 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | ||||||
| Er | 0.24 | 0.29 | −0.13 | −0.30 | 0.28 | 0.43 | −0.29 | −0.29 | −0.29 | 0.18 | 0.96 | 0.36 | 1.00 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 | |||||
| Tm | 0.26 | 0.30 | −0.13 | −0.31 | 0.30 | 0.43 | −0.30 | −0.30 | −0.30 | 0.19 | 0.96 | 0.38 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 | 1.00 | ||||
| Yb | 0.27 | 0.30 | −0.13 | −0.31 | 0.30 | 0.43 | −0.30 | −0.30 | −0.31 | 0.19 | 0.96 | 0.38 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| Lu | 0.28 | 0.31 | −0.13 | −0.33 | 0.32 | 0.43 | −0.31 | −0.31 | −0.32 | 0.19 | 0.96 | 0.40 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 0.98 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Th | 0.84 | 0.45 | 0.00 | −0.80 | 0.86 | 0.24 | −0.59 | −0.62 | −0.73 | 0.16 | 0.75 | 0.88 | 0.63 | 0.61 | 0.58 | 0.58 | 0.60 | 0.61 | 0.62 | 0.52 | 0.63 | 0.63 | 0.65 | 0.65 | 0.67 | 1.00 | |
| U | 0.88 | 0.47 | −0.10 | −0.75 | 0.88 | 0.14 | −0.68 | −0.66 | −0.72 | 0.22 | 0.48 | 0.91 | 0.31 | 0.28 | 0.24 | 0.24 | 0.27 | 0.28 | 0.30 | 0.18 | 0.30 | 0.31 | 0.33 | 0.33 | 0.35 | 0.84 | 1.00 |
| EW9602-09RD | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.55 | 1.00 | |||||||||||||||||||||||||
| Cr | 0.001 | −0.03 | 1.00 | ||||||||||||||||||||||||
| Mn | 0.38 | 0.45 | −0.59 | 1.00 | |||||||||||||||||||||||
| Fe | 0.19 | 0.63 | 0.06 | −0.002 | 1.00 | ||||||||||||||||||||||
| Co | −0.33 | −0.41 | −0.46 | 0.62 | −0.26 | 1.00 | |||||||||||||||||||||
| Ni | −0.20 | −0.34 | −0.30 | 0.46 | −0.65 | 0.12 | 1.00 | ||||||||||||||||||||
| Cu | 0.56 | 0.37 | 0.08 | −0.05 | −0.25 | −0.40 | 0.58 | 1.00 | |||||||||||||||||||
| Zn | 0.26 | 0.55 | 0.30 | −0.10 | 0.36 | −0.03 | −0.13 | 0.21 | 1.00 | ||||||||||||||||||
| Ba | 0.28 | 0.66 | 0.13 | 0.07 | 0.88 | −0.25 | −0.48 | −0.09 | 0.54 | 1.00 | |||||||||||||||||
| La | 0.39 | 0.81 | −0.30 | 0.43 | 0.62 | 0.39 | −0.40 | −0.62 | 0.22 | 0.54 | 1.00 | ||||||||||||||||
| Ce | 0.42 | 0.69 | 0.04 | 0.12 | 0.74 | 0.13 | −0.63 | −0.43 | 0.34 | 0.74 | 0.68 | 1.00 | |||||||||||||||
| Pr | 0.48 | 0.80 | −0.22 | 0.40 | 0.40 | 0.58 | −0.33 | −0.67 | 0.33 | 0.36 | 0.92 | 0.56 | 1.00 | ||||||||||||||
| Nd | 0.50 | 0.79 | −0.19 | 0.38 | 0.37 | 0.57 | −0.33 | −0.68 | 0.33 | 0.35 | 0.91 | 0.55 | 1.00 | 1.00 | |||||||||||||
| Sm | 0.50 | 0.75 | −0.14 | 0.32 | 0.29 | 0.59 | −0.30 | −0.69 | 0.37 | 0.29 | 0.83 | 0.49 | 0.98 | 0.99 | 1.00 | ||||||||||||
| Eu | 0.54 | 0.78 | −0.12 | 0.34 | 0.35 | 0.57 | −0.32 | −0.67 | 0.39 | 0.35 | 0.86 | 0.54 | 0.98 | 0.99 | 0.99 | 1.00 | |||||||||||
| Gd | 0.57 | 0.76 | −0.10 | 0.32 | 0.32 | 0.56 | −0.32 | −0.69 | 0.37 | 0.33 | 0.84 | 0.54 | 0.96 | 0.98 | 0.99 | 0.99 | 1.00 | ||||||||||
| Tb | 0.60 | 0.78 | −0.08 | 0.34 | 0.31 | 0.56 | −0.29 | −0.65 | 0.39 | 0.34 | 0.83 | 0.53 | 0.95 | 0.97 | 0.98 | 0.99 | 0.99 | 1.00 | |||||||||
| Dy | 0.65 | 0.80 | −0.09 | 0.37 | 0.35 | 0.56 | −0.31 | −0.66 | 0.38 | 0.38 | 0.84 | 0.57 | 0.95 | 0.96 | 0.96 | 0.98 | 0.99 | 0.99 | 1.00 | ||||||||
| Y | 0.69 | 0.80 | 0.08 | 0.27 | 0.53 | 0.31 | −0.42 | −0.58 | 0.40 | 0.58 | 0.79 | 0.65 | 0.82 | 0.84 | 0.82 | 0.86 | 0.88 | 0.89 | 0.93 | 1.00 | |||||||
| Ho | 0.70 | 0.82 | −0.06 | 0.38 | 0.40 | 0.51 | −0.33 | −0.63 | 0.37 | 0.45 | 0.84 | 0.60 | 0.91 | 0.93 | 0.92 | 0.94 | 0.96 | 0.97 | 0.99 | 0.97 | 1.00 | ||||||
| Er | 0.74 | 0.84 | −0.03 | 0.40 | 0.44 | 0.49 | −0.34 | −0.60 | 0.38 | 0.50 | 0.83 | 0.62 | 0.89 | 0.90 | 0.89 | 0.91 | 0.93 | 0.95 | 0.97 | 0.97 | 0.99 | 1.00 | |||||
| Tm | 0.75 | 0.85 | −0.01 | 0.40 | 0.45 | 0.49 | −0.35 | −0.59 | 0.39 | 0.52 | 0.82 | 0.64 | 0.87 | 0.88 | 0.86 | 0.89 | 0.91 | 0.93 | 0.96 | 0.97 | 0.98 | 0.99 | 1.00 | ||||
| Yb | 0.73 | 0.87 | −0.01 | 0.40 | 0.56 | 0.39 | −0.38 | −0.53 | 0.41 | 0.62 | 0.83 | 0.69 | 0.83 | 0.84 | 0.81 | 0.85 | 0.86 | 0.88 | 0.92 | 0.98 | 0.96 | 0.98 | 0.98 | 1.00 | |||
| Lu | 0.69 | 0.84 | 0.04 | 0.34 | 0.62 | 0.25 | −0.42 | −0.51 | 0.39 | 0.68 | 0.81 | 0.69 | 0.78 | 0.79 | 0.75 | 0.79 | 0.81 | 0.83 | 0.87 | 0.98 | 0.93 | 0.95 | 0.96 | 0.99 | 1.00 | ||
| Th | 0.01 | 0.34 | −0.08 | 0.02 | 0.32 | 0.24 | −0.35 | −0.37 | 0.28 | 0.19 | 0.41 | 0.43 | 0.50 | 0.48 | 0.50 | 0.49 | 0.45 | 0.41 | 0.37 | 0.21 | 0.29 | 0.25 | 0.22 | 0.20 | 0.17 | 1.00 | |
| U | 0.90 | 0.75 | −0.04 | 0.52 | 0.26 | 0.62 | −0.20 | −0.31 | 0.36 | 0.37 | 0.57 | 0.50 | 0.63 | 0.63 | 0.61 | 0.65 | 0.66 | 0.69 | 0.74 | 0.75 | 0.78 | 0.82 | 0.84 | 0.82 | 0.77 | 0.03 | 1.00 |
| RC12-192GS | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.89 | 1.00 | |||||||||||||||||||||||||
| Cr | −0.47 | −0.47 | 1.00 | ||||||||||||||||||||||||
| Mn | 0.59 | 0.82 | −0.38 | 1.00 | |||||||||||||||||||||||
| Fe | 0.87 | 0.85 | −0.36 | 0.47 | 1.00 | ||||||||||||||||||||||
| Co | 0.90 | 0.92 | −0.43 | 0.77 | 0.78 | 1.00 | |||||||||||||||||||||
| Ni | −0.38 | −0.12 | 0.03 | 0.43 | −0.48 | −0.17 | 1.00 | ||||||||||||||||||||
| Cu | −0.32 | −0.05 | −0.02 | 0.48 | −0.42 | −0.11 | 0.98 | 1.00 | |||||||||||||||||||
| Zn | 0.04 | 0.30 | −0.16 | 0.72 | −0.04 | 0.21 | 0.87 | 0.87 | 1.00 | ||||||||||||||||||
| Ba | 0.87 | 0.92 | −0.45 | 0.79 | 0.69 | 0.92 | −0.10 | −0.04 | 0.24 | 1.00 | |||||||||||||||||
| La | 0.98 | 0.91 | −0.46 | 0.60 | 0.90 | 0.89 | −0.38 | −0.32 | 0.04 | 0.87 | 1.00 | ||||||||||||||||
| Ce | 0.94 | 0.91 | −0.44 | 0.62 | 0.93 | 0.90 | −0.37 | −0.32 | 0.06 | 0.83 | 0.95 | 1.00 | |||||||||||||||
| Pr | 0.97 | 0.93 | -0.46 | 0.62 | 0.93 | 0.89 | −0.38 | −0.31 | 0.06 | 0.86 | 1.00 | 0.96 | 1.00 | ||||||||||||||
| Nd | 0.97 | 0.92 | -0.46 | 0.61 | 0.92 | 0.89 | −0.39 | −0.33 | 0.04 | 0.86 | 1.00 | 0.96 | 1.00 | 1.00 | |||||||||||||
| Sm | 0.97 | 0.92 | -0.45 | 0.61 | 0.94 | 0.89 | −0.39 | −0.33 | 0.05 | 0.85 | 0.99 | 0.97 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Eu | 0.97 | 0.93 | -0.46 | 0.62 | 0.93 | 0.89 | −0.38 | −0.32 | 0.06 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| Gd | 0.97 | 0.92 | -0.45 | 0.61 | 0.93 | 0.89 | −0.39 | −0.33 | 0.04 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Tb | 0.97 | 0.93 | -0.45 | 0.62 | 0.93 | 0.90 | −0.38 | −0.32 | 0.06 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Dy | 0.97 | 0.93 | -0.45 | 0.63 | 0.93 | 0.90 | −0.38 | −0.31 | 0.06 | 0.87 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Y | 0.97 | 0.94 | −0.45 | 0.63 | 0.93 | 0.90 | −0.38 | −0.32 | 0.05 | 0.87 | 0.99 | 0.96 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Ho | 0.97 | 0.94 | -0.45 | 0.64 | 0.92 | 0.91 | -0.37 | −0.30 | 0.07 | 0.88 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Er | 0.97 | 0.94 | −0.45 | 0.65 | 0.92 | 0.91 | −0.36 | −0.30 | 0.07 | 0.88 | 0.99 | 0.96 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Tm | 0.97 | 0.94 | −0.45 | 0.66 | 0.91 | 0.91 | −0.35 | −0.29 | 0.08 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yb | 0.96 | 0.95 | −0.45 | 0.66 | 0.91 | 0.91 | −0.35 | −0.29 | 0.08 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| Lu | 0.97 | 0.94 | −0.45 | 0.65 | 0.91 | 0.91 | −0.36 | −0.30 | 0.07 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Th | 0.02 | 0.03 | 0.04 | −0.15 | 0.36 | −0.01 | −0.29 | −0.29 | −0.19 | −0.22 | 0.03 | 0.25 | 0.09 | 0.08 | 0.11 | 0.11 | 0.10 | 0.09 | 0.08 | 0.10 | 0.07 | 0.06 | 0.05 | 0.04 | 0.04 | 1.00 | |
| U | 0.94 | 0.97 | −0.44 | 0.70 | 0.91 | 0.93 | −0.30 | −0.24 | 0.13 | 0.90 | 0.96 | 0.95 | 0.97 | 0.97 | 0.97 | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.05 | 1.00 |
| E ATL N4 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.67 | 1.00 | |||||||||||||||||||||||||
| Cr | 0.32 | 0.47 | 1.00 | ||||||||||||||||||||||||
| Mn | 0.22 | 0.38 | −0.13 | 1.00 | |||||||||||||||||||||||
| Fe | 0.78 | 0.95 | 0.46 | 0.34 | 1.00 | ||||||||||||||||||||||
| Co | 0.23 | 0.12 | −0.20 | 0.70 | 0.19 | 1.00 | |||||||||||||||||||||
| Ni | 0.05 | 0.16 | −0.18 | 0.90 | 0.12 | 0.72 | 1.00 | ||||||||||||||||||||
| Cu | −0.03 | 0.22 | 0.01 | 0.67 | 0.10 | 0.20 | 0.68 | 1.00 | |||||||||||||||||||
| Zn | 0.28 | 0.60 | 0.17 | 0.80 | 0.47 | 0.29 | 0.67 | 0.86 | 1.00 | ||||||||||||||||||
| Ba | 0.66 | 0.75 | 0.14 | 0.67 | 0.80 | 0.45 | 0.46 | 0.26 | 0.57 | 1.00 | |||||||||||||||||
| La | 0.66 | 0.69 | 0.06 | 0.63 | 0.76 | 0.57 | 0.42 | 0.08 | 0.41 | 0.92 | 1.00 | ||||||||||||||||
| Ce | 0.67 | 0.62 | 0.05 | 0.61 | 0.70 | 0.71 | 0.46 | 0.12 | 0.41 | 0.80 | 0.90 | 1.00 | |||||||||||||||
| Pr | 0.67 | 0.75 | 0.10 | 0.62 | 0.80 | 0.54 | 0.42 | 0.11 | 0.46 | 0.91 | 0.99 | 0.90 | 1.00 | ||||||||||||||
| Nd | 0.67 | 0.74 | 0.10 | 0.61 | 0.79 | 0.52 | 0.40 | 0.09 | 0.44 | 0.91 | 0.99 | 0.89 | 1.00 | 1.00 | |||||||||||||
| Sm | 0.68 | 0.75 | 0.12 | 0.59 | 0.80 | 0.51 | 0.39 | 0.09 | 0.45 | 0.90 | 0.98 | 0.89 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Eu | 0.68 | 0.75 | 0.11 | 0.60 | 0.79 | 0.54 | 0.41 | 0.11 | 0.46 | 0.88 | 0.97 | 0.90 | 0.99 | 0.99 | 1.00 | 1.00 | |||||||||||
| Gd | 0.67 | 0.72 | 0.10 | 0.58 | 0.78 | 0.52 | 0.38 | 0.07 | 0.42 | 0.88 | 0.98 | 0.89 | 0.99 | 0.99 | 1.00 | 0.99 | 1.00 | ||||||||||
| Tb | 0.67 | 0.73 | 0.11 | 0.56 | 0.78 | 0.53 | 0.38 | 0.07 | 0.42 | 0.86 | 0.96 | 0.89 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 | |||||||||
| Dy | 0.66 | 0.73 | 0.11 | 0.57 | 0.77 | 0.53 | 0.39 | 0.07 | 0.43 | 0.87 | 0.96 | 0.89 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | ||||||||
| Y | 0.63 | 0.70 | 0.08 | 0.60 | 0.76 | 0.55 | 0.41 | 0.07 | 0.41 | 0.88 | 0.98 | 0.88 | 0.98 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 | |||||||
| Ho | 0.64 | 0.71 | 0.10 | 0.58 | 0.76 | 0.55 | 0.41 | 0.07 | 0.41 | 0.87 | 0.97 | 0.89 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 0.99 | 1.00 | ||||||
| Er | 0.62 | 0.69 | 0.08 | 0.58 | 0.74 | 0.56 | 0.42 | 0.06 | 0.40 | 0.86 | 0.96 | 0.88 | 0.97 | 0.98 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | |||||
| Tm | 0.61 | 0.68 | 0.07 | 0.59 | 0.73 | 0.58 | 0.43 | 0.06 | 0.40 | 0.85 | 0.95 | 0.89 | 0.97 | 0.97 | 0.97 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | ||||
| Yb | 0.61 | 0.68 | 0.07 | 0.60 | 0.73 | 0.58 | 0.44 | 0.06 | 0.40 | 0.86 | 0.96 | 0.89 | 0.97 | 0.98 | 0.98 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| Lu | 0.60 | 0.62 | 0.03 | 0.60 | 0.69 | 0.59 | 0.44 | 0.03 | 0.36 | 0.86 | 0.96 | 0.88 | 0.96 | 0.97 | 0.96 | 0.96 | 0.98 | 0.97 | 0.98 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | ||
| Th | 0.65 | 0.53 | 0.03 | 0.47 | 0.64 | 0.55 | 0.28 | 0.03 | 0.28 | 0.69 | 0.80 | 0.86 | 0.80 | 0.80 | 0.79 | 0.78 | 0.79 | 0.77 | 0.75 | 0.75 | 0.74 | 0.73 | 0.72 | 0.73 | 0.73 | 1.00 | |
| U | 0.65 | 0.88 | 0.22 | 0.62 | 0.84 | 0.45 | 0.44 | 0.25 | 0.62 | 0.82 | 0.83 | 0.81 | 0.89 | 0.87 | 0.88 | 0.89 | 0.85 | 0.87 | 0.86 | 0.84 | 0.85 | 0.84 | 0.84 | 0.84 | 0.79 | 0.67 | 1.00 |
| NAP N4 | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.89 | 1.00 | |||||||||||||||||||||||||
| Cr | −0.47 | −0.47 | 1.00 | ||||||||||||||||||||||||
| Mn | 0.59 | 0.82 | −0.38 | 1.00 | |||||||||||||||||||||||
| Fe | 0.87 | 0.85 | −0.36 | 0.47 | 1.00 | ||||||||||||||||||||||
| Co | 0.90 | 0.92 | −0.43 | 0.77 | 0.78 | 1.00 | |||||||||||||||||||||
| Ni | −0.38 | −0.12 | 0.03 | 0.43 | −0.48 | −0.17 | 1.00 | ||||||||||||||||||||
| Cu | −0.32 | −0.05 | −0.02 | 0.48 | −0.42 | −0.11 | 0.98 | 1.00 | |||||||||||||||||||
| Zn | 0.04 | 0.30 | −0.16 | 0.72 | −0.04 | 0.21 | 0.87 | 0.87 | 1.00 | ||||||||||||||||||
| Ba | 0.87 | 0.92 | −0.45 | 0.79 | 0.69 | 0.92 | −0.10 | −0.04 | 0.24 | 1.00 | |||||||||||||||||
| La | 0.98 | 0.91 | −0.46 | 0.60 | 0.90 | 0.89 | −0.38 | −0.32 | 0.04 | 0.87 | 1.00 | ||||||||||||||||
| Ce | 0.94 | 0.91 | −0.44 | 0.62 | 0.93 | 0.90 | −0.37 | −0.32 | 0.06 | 0.83 | 0.95 | 1.00 | |||||||||||||||
| Pr | 0.97 | 0.93 | −0.46 | 0.62 | 0.93 | 0.89 | −0.38 | −0.31 | 0.06 | 0.86 | 1.00 | 0.96 | 1.00 | ||||||||||||||
| Nd | 0.97 | 0.92 | −0.46 | 0.61 | 0.92 | 0.89 | −0.39 | −0.33 | 0.04 | 0.86 | 1.00 | 0.96 | 1.00 | 1.00 | |||||||||||||
| Sm | 0.97 | 0.92 | −0.45 | 0.61 | 0.94 | 0.89 | −0.39 | −0.33 | 0.05 | 0.85 | 0.99 | 0.97 | 1.00 | 1.00 | 1.00 | ||||||||||||
| Eu | 0.97 | 0.93 | −0.46 | 0.62 | 0.93 | 0.89 | −0.38 | −0.32 | 0.06 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||
| Gd | 0.97 | 0.92 | −0.45 | 0.61 | 0.93 | 0.89 | −0.39 | −0.33 | 0.04 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||
| Tb | 0.97 | 0.93 | −0.45 | 0.62 | 0.93 | 0.90 | −0.38 | −0.32 | 0.06 | 0.86 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||
| Dy | 0.97 | 0.93 | −0.45 | 0.63 | 0.93 | 0.90 | −0.38 | −0.31 | 0.06 | 0.87 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Y | 0.97 | 0.94 | −0.45 | 0.63 | 0.93 | 0.90 | −0.38 | −0.32 | 0.05 | 0.87 | 0.99 | 0.96 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||
| Ho | 0.97 | 0.94 | −0.45 | 0.64 | 0.92 | 0.91 | −0.37 | −0.30 | 0.07 | 0.88 | 0.99 | 0.96 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Er | 0.97 | 0.94 | −0.45 | 0.65 | 0.92 | 0.91 | −0.36 | −0.30 | 0.07 | 0.88 | 0.99 | 0.96 | 1.00 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| Tm | 0.97 | 0.94 | −0.45 | 0.66 | 0.91 | 0.91 | −0.35 | −0.29 | 0.08 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Yb | 0.96 | 0.95 | −0.45 | 0.66 | 0.91 | 0.91 | −0.35 | −0.29 | 0.08 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||
| Lu | 0.97 | 0.94 | −0.45 | 0.65 | 0.91 | 0.91 | −0.36 | −0.30 | 0.07 | 0.89 | 0.99 | 0.95 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||
| Th | 0.02 | 0.03 | 0.04 | −0.15 | 0.36 | −0.01 | −0.29 | −0.29 | −0.19 | −0.22 | 0.03 | 0.25 | 0.09 | 0.08 | 0.11 | 0.11 | 0.10 | 0.09 | 0.08 | 0.10 | 0.07 | 0.06 | 0.05 | 0.04 | 0.04 | 1.00 | |
| U | 0.94 | 0.97 | −0.44 | 0.70 | 0.91 | 0.93 | −0.30 | −0.24 | 0.13 | 0.90 | 0.96 | 0.95 | 0.97 | 0.97 | 0.97 | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.05 | 1.00 |
| PSh-125.29(1) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ba | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | Th | U | |
| Ti | 1.00 | ||||||||||||||||||||||||||
| V | 0.30 | 1.00 | |||||||||||||||||||||||||
| Cr | 0.57 | 0.35 | 1.00 | ||||||||||||||||||||||||
| Mn | 0.07 | 0.11 | −0.05 | 1.00 | |||||||||||||||||||||||
| Fe | 0.12 | 0.63 | 0.22 | −0.57 | 1.00 | ||||||||||||||||||||||
| Co | 0.04 | 0.20 | 0.01 | 0.66 | −0.30 | 1.00 | |||||||||||||||||||||
| Ni | 0.22 | 0.15 | 0.05 | 0.73 | −0.45 | 0.45 | 1.00 | ||||||||||||||||||||
| Cu | 0.10 | 0.10 | −0.04 | 0.75 | −0.48 | 0.52 | 0.86 | 1.00 | |||||||||||||||||||
| Zn | 0.44 | 0.62 | 0.36 | 0.37 | 0.15 | 0.42 | 0.50 | 0.47 | 1.00 | ||||||||||||||||||
| Ba | 0.19 | 0.40 | 0.03 | 0.66 | −0.23 | 0.69 | 0.70 | 0.68 | 0.66 | 1.00 | |||||||||||||||||
| La | 0.26 | 0.86 | 0.38 | −0.07 | 0.74 | 0.20 | 0.03 | −0.03 | 0.54 | 0.30 | 1.00 | ||||||||||||||||
| Ce | 0.24 | 0.57 | 0.47 | 0.08 | 0.35 | 0.29 | 0.08 | 0.06 | 0.42 | 0.23 | 0.63 | 1.00 | |||||||||||||||
| Pr | 0.30 | 0.85 | 0.40 | −0.06 | 0.70 | 0.17 | 0.08 | 0.01 | 0.53 | 0.27 | 0.97 | 0.64 | 1.00 | ||||||||||||||
| Nd | 0.31 | 0.85 | 0.40 | −0.07 | 0.69 | 0.16 | 0.08 | 0.01 | 0.53 | 0.25 | 0.95 | 0.62 | 0.99 | 1.00 | |||||||||||||
| Sm | 0.27 | 0.82 | 0.36 | −0.12 | 0.68 | 0.12 | 0.06 | −0.01 | 0.48 | 0.21 | 0.91 | 0.58 | 0.97 | 0.99 | 1.00 | ||||||||||||
| Eu | 0.25 | 0.83 | 0.35 | −0.09 | 0.69 | 0.15 | 0.07 | 0.00 | 0.49 | 0.25 | 0.93 | 0.60 | 0.98 | 0.99 | 0.99 | 1.00 | |||||||||||
| Gd | 0.26 | 0.85 | 0.35 | −0.13 | 0.72 | 0.09 | 0.04 | −0.02 | 0.49 | 0.22 | 0.93 | 0.52 | 0.97 | 0.98 | 0.98 | 0.98 | 1.00 | ||||||||||
| Tb | 0.25 | 0.83 | 0.33 | −0.17 | 0.74 | 0.07 | 0.01 | −0.06 | 0.45 | 0.18 | 0.92 | 0.52 | 0.96 | 0.98 | 0.98 | 0.98 | 0.99 | 1.00 | |||||||||
| Dy | 0.25 | 0.83 | 0.34 | −0.18 | 0.75 | 0.05 | −0.02 | −0.08 | 0.44 | 0.16 | 0.92 | 0.50 | 0.96 | 0.97 | 0.98 | 0.97 | 0.99 | 0.99 | 1.00 | ||||||||
| Y | 0.19 | 0.83 | 0.31 | −0.33 | 0.88 | −0.09 | −0.20 | −0.24 | 0.39 | 0.05 | 0.90 | 0.42 | 0.89 | 0.90 | 0.89 | 0.89 | 0.93 | 0.94 | 0.95 | 1.00 | |||||||
| Ho | 0.24 | 0.84 | 0.34 | −0.22 | 0.78 | 0.01 | −0.05 | −0.11 | 0.44 | 0.13 | 0.92 | 0.48 | 0.95 | 0.96 | 0.96 | 0.96 | 0.98 | 0.99 | 0.99 | 0.97 | 1.00 | ||||||
| Er | 0.24 | 0.84 | 0.33 | −0.24 | 0.81 | −0.01 | −0.08 | −0.14 | 0.43 | 0.12 | 0.92 | 0.47 | 0.94 | 0.95 | 0.95 | 0.95 | 0.97 | 0.98 | 0.99 | 0.98 | 0.99 | 1.00 | |||||
| Tm | 0.22 | 0.84 | 0.32 | −0.21 | 0.77 | 0.02 | −0.07 | −0.13 | 0.42 | 0.13 | 0.90 | 0.46 | 0.92 | 0.93 | 0.93 | 0.93 | 0.96 | 0.96 | 0.97 | 0.96 | 0.97 | 0.98 | 1.00 | ||||
| Yb | 0.24 | 0.83 | 0.32 | −0.26 | 0.81 | −0.04 | −0.10 | −0.16 | 0.41 | 0.10 | 0.90 | 0.43 | 0.92 | 0.93 | 0.93 | 0.93 | 0.96 | 0.97 | 0.98 | 0.98 | 0.99 | 0.99 | 0.98 | 1.00 | |||
| Lu | 0.25 | 0.85 | 0.32 | −0.23 | 0.82 | 0.01 | −0.09 | −0.13 | 0.45 | 0.14 | 0.92 | 0.47 | 0.92 | 0.93 | 0.92 | 0.93 | 0.96 | 0.96 | 0.97 | 0.98 | 0.98 | 0.99 | 0.97 | 0.99 | 1.00 | ||
| Th | 0.47 | 0.43 | 0.54 | 0.00 | 0.25 | 0.05 | 0.09 | 0.01 | 0.41 | 0.09 | 0.41 | 0.40 | 0.44 | 0.41 | 0.38 | 0.38 | 0.37 | 0.36 | 0.36 | 0.34 | 0.37 | 0.36 | 0.34 | 0.35 | 0.35 | 1.00 | |
| U | 0.11 | 0.78 | 0.15 | 0.05 | 0.55 | 0.17 | −0.12 | −0.10 | 0.43 | 0.33 | 0.67 | 0.35 | 0.65 | 0.64 | 0.60 | 0.63 | 0.64 | 0.63 | 0.64 | 0.71 | 0.66 | 0.68 | 0.66 | 0.66 | 0.69 | 0.31 | 1.00 |
References
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Halbach, P.; Kriete, C.; Prause, B.; Puteanus, D. Mechanisms to explain the platinum concentration in ferromanganese seamount crusts. Chem. Geol. 1989, 76, 95–106. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.-K.; Roberts, L. Cobalt-Rich Ferromanganese Crusts in the Pacific. In Handbook of Marine Mineral Deposits; Cronan, D.S., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 239–279. ISBN 9780849384295. [Google Scholar]
- Baturin, G.N.; Dubinchuk, V.T. Composition of ferromanganese nodules from Riga Bay (Baltic Sea). Oceanology 2009, 49, 111–120. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Lunar, R.; Martínez-Frías, J.; Martín Rubí, J.A.; Torres, T.; Ortiz, J.E.; Díaz del Río, V.; Pinheiro, L.M.; Magalhães, V.H. Hydrocarbon-derived ferromanganese nodules in carbonate-mud mounds from the Gulf of Cadiz: mud-breccia sediments and clasts as nucleation sites. Mar. Geol. 2009, 261, 64–81. [Google Scholar] [CrossRef]
- Von Stackelberg, U.; Beiersdorf, H. The formation of manganese nodules between the Clarion and Clipperton fracture zones southeast of Hawaii. Mar. Geol. 1991, 98, 411–423. [Google Scholar] [CrossRef]
- Opdyke, N.; Foster, J. The paleomagnetism of cores from the North Pacific. Geol. Soc. Am. Mem. 1970, 126, 83–119. [Google Scholar]
- Halbach, P.; Friedrich, G.; von Stackelberg, U. The Manganese Nodule Belt of the Pacific Ocean. Geological Environment, Nodule Formation, and Mining Aspects; Ferdinand Enke, 1988; ISBN 3432963815. [Google Scholar]
- Reyss, J.L.; Lemaitre, N.; Ku, T.L.; Marchig, V.; Southon, J.R.; Nelson, D.E.; Vogel, J.S. Growth of a manganese nodule from Peru basin: A radiochemical anatomy. Geochim. Cosmochim. Acta 1985, 49, 2401–2408. [Google Scholar] [CrossRef]
- Kuhn, T.; Bau, M.; Blum, N.; Halbach, P. Origin of negative Ce anomalies in mixed hydrothermal-hydrogenetic Fe-Mn crusts from the Central Indian Ridge. Earth Planet. Sci. Lett. 1998, 163, 207–220. [Google Scholar] [CrossRef]
- Halbach, P.; Hebisch, U.; Scherhag, C. Geochemical variations of ferromanganese nodules and crusts from different provinces of the Pacific Ocean and their genetic control. Chem. Geol. 1981, 34, 3–17. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Mills, R.A.; Wells, D.M.; Roberts, S. Genesis of ferromanganese crusts from the TAG hydrothermal field. Chem. Geol. 2001, 176, 283–293. [Google Scholar] [CrossRef]
- Marino, E.; Gonzalez, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (Northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- Hein, J.R.; Konstantinova, N.; Mikesell, M.; Mizell, K.; Fitzsimmons, J.N.; Lam, P.J.; Jensen, L.T.; Xiang, Y.; Gartman, A.; Cherkashov, G.; et al. Arctic Deep Water Ferromanganese-Oxide Deposits Reflect the Unique Characteristics of the Arctic Ocean. Geochem. Geophys. Geosyst. 2017, 18, 3771–3800. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Marine ferromanganese encrustations: Archives of changing oceans. Elem. Int. Mag. Miner. Geochem. Petrol. 2017, 13, 177–182. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Biscaye, P.E.; Kolla, V.; Turekian, K.K. Distribution of calcium carbonate in surface sediments of the Atlantic Ocean. J. Geophys. Res. 1976, 81, 2595–2603. [Google Scholar] [CrossRef]
- De Lange, G.J.; van Os, B.; Poorter, R. Geochemical composition and inferred accretion rates of sediments and manganese nodules from a submarine hill in the Madeira Abyssal Plain, eastern North Atlantic. Mar. Geol. 1992, 109, 171–194. [Google Scholar] [CrossRef]
- Addy, S.K. Rare earth element patterns in manganese nodules and micronodules from northwest Atlantic. Geochim. Cosmochim. Acta 1979, 43, 1105–1115. [Google Scholar] [CrossRef]
- Rothwell, R.G.; Pearce, T.J.; Weaver, P.P. Late Quaternary evolution of the Madeira Abyssal Plain, Canary Basin, NE Atlantic. Basin Res. 1992, 4, 103–131. [Google Scholar] [CrossRef]
- D’Hondt, S.; Inagaki, F.; Zarikian, C.A.; Abrams, L.J.; Dubois, N.; Engelhardt, T.; Evans, H.; Ferdelman, T.; Gribsholt, B.; Harris, R.N.; et al. Presence of oxygen and aerobic communities from sea floor to basement in deep-sea sediments. Nat. Geosci. 2015, 8, 299–304. [Google Scholar] [CrossRef]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Glasby, G.P. Mechanisms of enrichment of the rarer elements in marine manganese nodules. Mar. Chem. 1973, 1, 105–125. [Google Scholar] [CrossRef]
- Piper, D.Z. Rare earth elements in ferromanganese nodules and other marine phases. Geochem. Cosmochim. Acta 1974, 38, 1007–1022. [Google Scholar] [CrossRef]
- Antoine, D.; André, J.M.; Morel, A. Oceanic primary production: 2. Estimation at global scale from satellite (coastal zone color scanner) chlorophyll. Glob. Biogeochem. Cycles 1996, 10, 57–69. [Google Scholar] [CrossRef]
- Morgan, C. A Geological Model of Polymetallic Nodule Deposits in the Clarion-Clipperton Fracture Zone (No. 6); International Seabed Authority: Kingston, Jamaica, 2010. [Google Scholar]
- Fuyuan, Z.; Weiyan, Z.; Kechao, Z.; Shuitu, G.; Haisheng, Z.; Xiaoyu, Z.; Benduo, Z. Distribution Characteristics of Cobalt-rich Ferromanganese Crust Resources on Submarine Seamounts in the Western Pacific. Acta Geol. Sin. (Eng. Ed.) 2008, 82, 796–803. [Google Scholar] [CrossRef]
- Rozanov, A.G. Redox system of the bottom sediments of the western Kara Sea. Geochem. Int. 2015, 53, 987–1001. [Google Scholar] [CrossRef]
- Levitan, M.A.; Lavrushin, Y.A.; Stein, R. Outlines of Sedimentation History of the Arctic Ocean and Subarctic Seas for the Last 130 ka; GEOS: Moscow, Russia, 2007. (In Russian) [Google Scholar]
- Baturin, G.N. Variations in the composition of the ferromanganese concretions of the Kara Sea. Oceanology 2011, 51, 148–156. [Google Scholar] [CrossRef]
- Smith, W.H.; Sandwell, D.T. Global sea floor topography from satellite altimetry and ship depth soundings. Science 1997, 277, 1956–1962. [Google Scholar] [CrossRef]
- Van Der Loeff, M.R. Oxygen in pore waters of deep-sea sediments. Phil. Trans. R. Soc. Lond. A 1990, 331, 69–84. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T.; Novigatsky, A.N. Phase distribution of elements in ferromanganese nodules of the Kara Sea. Dokl. Earth Sci. 2016, 471, 1199–1203. [Google Scholar] [CrossRef]
- Gueguen, B.; Rouxel, O.; Rouget, M.L.; Bollinger, C.; Ponzevera, E.; Germain, Y.; Fouquet, Y. Comparative geochemistry of four ferromanganese crusts from the Pacific Ocean and significance for the use of Ni isotopes as paleoceanographic tracers. Geochem. Cosmochim. Acta 2016, 189, 214–235. [Google Scholar] [CrossRef]
- Hein, J.R.; Morgenson, L.A.; Clague, D.A.; Koski, R.A. Cobalt-Rich Ferromanganese Crusts from the Exclusive Economic Zone of the United States and Nodules from the Oceanic Pacific; Circum~Pacific Council for Energy and Mineral Resources: Houston, TX, USA, 1987. [Google Scholar]
- Winters, G.V.; Buckley, D.E. Factor Analyses as a Method of Evaluation Sediment Environmental Quality in Halifax Harbour, Nova Scotia; Current Research, Part D, Paper 92-1D; Geological Survey of Canada: Ottawa, ON, Canada, 1992; pp. 165–171. [Google Scholar]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochem. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and Genesis of Submarine Iron–Manganese Deposits. In Papers from a Conference on Ferromanganese Deposits on the Ocean Floor; Horn, D.R., Ed.; National Science Foundation: New York, NY, USA, 1972; pp. 149–166. [Google Scholar]
- Nath, B.N.; Balaram, V.; Sudhakar, M.; Plüger, W.L. Rare earth element geochemistry of ferromanganese deposits from the Indian Ocean. Mar. Chem. 1992, 38, 185–208. [Google Scholar] [CrossRef]
- Kasten, S.; Glasby, G.P.; Schulz, H.D.; Friedrich, G.; Andreev, S.I. Rare earth elements in manganese nodules from the South Atlantic Ocean as indicators of oceanic bottom water flow. Mar. Geol. 1998, 146, 33–52. [Google Scholar] [CrossRef]
- Bau, M.; Möller, P.; Dulski, P. Yttrium and lanthanides in eastern Mediterranean seawater and their fractionation during redox-cycling. Mar. Chem. 1997, 56, 123–131. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Landing, W.M.; Lewis, B.L. Ocean particle chemistry: The fractionation of rare earth elements between suspended particles and seawater. Geochim. Cosmochim. Acta 1994, 58, 1567–1579. [Google Scholar] [CrossRef]
- Schijf, J.; Marshall, K.S. YREE sorption on hydrous ferric oxide in 0.5 M NaCl solutions: A model extension. Mar. Chem. 2011, 123, 32–43. [Google Scholar] [CrossRef]
- Ohta, A.; Ishii, S.; Sakakibara, M.; Mizuno, A.; Kawabe, I. Systematic correlation of the Ce anomaly with the Co/(Ni+Cu) ratio and Y fractionation from Ho in distinct types of Pacific deep-sea nodules. Geochem. J. 1999, 3, 399–417. [Google Scholar] [CrossRef]
- Nance, W.B.; Taylor, S.R. Rare earth element patterns and crustal evolution—I. Australian post-Archean sedimentary rocks. Geochim. Cosmochim. Acta 1976, 40, 1539–1551. [Google Scholar] [CrossRef]
- Manheim, F.T.; Lane-Bostwick, C.M. Cobalt in ferromanganese crusts as a monitor of hydrothermal discharge on the Pacific sea floor. Nature 1988, 35, 59–62. [Google Scholar] [CrossRef]
- Hatch, G.P. Dynamics in the global market for rare earths. Elements 2012, 8, 341–346. [Google Scholar] [CrossRef]
- Cobalt Institute—Formerly the Cobalt Development Institute (CDI). Available online: https://www.cobaltinstitute.org/ (accessed on 4 May 2018).
- International Nickel Study Group. Available online: http://www.insg.org/ (accessed on 4 May 2018).
- Futures & Options Trading for Risk Management—CME Group. Available online: http://www.cmegroup.com/ (accessed on 4 May 2018).
- Jordahl, K.A.; McNutt, M.K.; Caress, D.W. Multiple episodes of volcanism in the Southern Austral Islands: Flexural constraints from bathymetry, seismic reflection, and gravity data. J. Geophys. Res. Sol. Earth 2004, 109. [Google Scholar] [CrossRef]
- Weaver, P.P.E.; Thomson, J. Geology and Geochemistry of Abyssal Plains; Blackwell Scientific Publications: Oakland, CA, USA, 1987; p. 246, ISBN 0632017449, 9780632017447. [Google Scholar]












| Sample | Lat (°N) | Long (°E) | Location | Water Depth (m) | OPD (m) | Description |
|---|---|---|---|---|---|---|
| Pacific | ||||||
| EW9602-09RD | −27.0 | −143.2 | Taukina Seamount (Cook-Austral volcanic chain) | 1520–1540 | ~75 a | ~3 cm botryoidal crust around volcanic clast |
| JC120-104 | 13.3 | −116.3 | UK claim area, CCFZ | 4130 | ~1 b | ~10 cm nodule, low sphericity, irregular bottom |
| RC12-192GS | −39.5 | −157.7 | Southwest Pacific Basin | 4830 | ~75 a | ~1 cm nodule, low sphericity |
| Atlantic | ||||||
| EATL N4 | 31.2 | −25.2 | Great Meteor East | 5100–5400 | >3 c | ~3 cm, round, smooth nodule |
| NAP N4 | 23.5 | −63.0 | NW Atlantic | 5900–6200 | ~18 d | ~3 cm, round, smooth nodule |
| Arctic | ||||||
| PSh-125.29(1) | 75.5 | 89.4 | NE Kara Sea | 51 | ~0.5 e | ~5 cm flat nodule |
| Mn | Fe | Co | Ni | Cu | Ti | V | Zn | Ba | Th | U | Mn/Fe | GR (mm/Ma) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Layer # | wt % | ppm | |||||||||||
| EW9602-09RD (crust) | 1 | 38.6 | 29.6 | 1.27 | 0.61 | 0.19 | 1.66 | 841 | 1060 | 2640 | 17.3 | 19.0 | 1.30 | 0.77 |
| 2 | 39.8 | 27.9 | 0.67 | 1.02 | 0.29 | 2.12 | 953 | 1510 | 2900 | 6.77 | 24.1 | 1.43 | 2.21 | |
| 3 | 51.8 | 18.2 | 3.67 | 1.06 | 0.13 | 1.87 | 1166 | 1370 | 1710 | 33.4 | 23.7 | 2.84 | 0.14 | |
| Avg. | 42.2 | 24.5 | 1.58 | 0.93 | 0.23 | 1.84 | 888 | 1250 | 2570 | 14.1 | 21.0 | 1.88 | 0.88 | |
| SD (n = 44) | 6.80 | 6.10 | 0.73 | 0.28 | 0.05 | 0.55 | 175 | 236 | 614 | 7.40 | 5.76 | 0.71 | 0.88 | |
| [37] | Bulk Avg. | 16 | 20 | 0.33 | 0.22 | 0.05 | 1.0 | - | 574 | 1.26 | - | |||
| JC120-104 | 1 | 18.4 | 11.5 | 0.21 | 0.41 | 0.18 | 0.53 | 499 | 617 | 1040 | 37.3 | 7.34 | 1.60 | 4.01 |
| 2 | 34.4 | 0.51 | 0.10 | 0.93 | 0.91 | 0.04 | 172 | 3770 | 330 | 1.27 | 5.84 | 67.8 | 17.3 | |
| 3 | 45.1 | 0.56 | 0.17 | 1.94 | 1.63 | 0.05 | 341 | 4970 | 1500 | 1.41 | 0.96 | 80.4 | 10.9 | |
| Avg | 35.9 | 4.08 | 0.20 | 1.10 | 0.89 | 0.24 | 476 | 3740 | 1580 | 18.8 | 7.54 | 8.79 | 6.84 | |
| SD (n = 37) | 10.9 | 4.79 | 0.16 | 0.62 | 0.49 | 0.22 | 177 | 2890 | 830 | 16.5 | 2.71 | 2.27 | 2.17 | |
| [18] | Bulk Avg. | 30.9 | 5.86 | 0.15 | 1.32 | 1.21 | 0.25 | 580 | 1500 | 5020 | - | - | 5.31 | - |
| L.T. 1 *,a | 23.1 | 14.2 | 0.3 | 0.46 | 0.34 | 0.80 | - | 900 | 2600 | - | - | 1.8 | - | |
| L.T. 2.1 *,b | 44.0 | 0.81 | 0.08 | 1.97 | 1.92 | 0.07 | - | 2600 | 3300 | - | - | 95.9 | - | |
| RC12-192GS | 1 | 15.6 | 10.2 | 0.38 | 0.26 | 0.20 | 0.72 | 324 | 418 | 1290 | 255 | 3.75 | 1.52 | 1.15 |
| 2 | 21.4 | 12.6 | 0.37 | 0.24 | 0.21 | 1.20 | 569 | 512 | 3510 | 38.6 | 8.20 | 1.70 | 1.88 | |
| 3 | 31.6 | 5.27 | 0.24 | 2.01 | 1.13 | 0.35 | 359 | 1310 | 1250 | 48.3 | 3.59 | 6.00 | 4.49 | |
| Avg. | 21.8 | 11.0 | 0.34 | 0.57 | 0.41 | 0.92 | 470 | 651 | 2700 | 79.8 | 6.49 | 1.99 | 2.08 | |
| SD (n = 92) | 10.1 | 5.36 | 0.23 | 0.49 | 0.30 | 0.58 | 243 | 310 | 2290 | 62.9 | 3.95 | 1.88 | 1.12 | |
| [24] | Bulk Avg. | 21.7 | 18.1 | 0.62 | 0.47 | 0.11 | 1.12 | 660 | 698 | 1700 | 15 | 12 | 1.20 | - |
| EATL N4 | 1 | 21.4 | 10.3 | 0.78 | 0.41 | 0.15 | 0.73 | 555 | 675 | 1090 | 125 | 10.8 | 2.07 | 0.48 |
| 2 | 26.1 | 12.2 | 0.73 | 0.46 | 0.16 | 0.83 | 752 | 683 | 843 | 201 | 13.5 | 2.14 | 0.73 | |
| 3 | 45.5 | 5.09 | 0.37 | 0.63 | 0.76 | 0.37 | 474 | 2170 | 937 | 53.2 | 7.19 | 8.95 | 3.69 | |
| Avg. | 17.9 | 12.6 | 0.47 | 0.28 | 0.16 | 0.96 | 680 | 741 | 1190 | 128 | 10.4 | 1.42 | 1.04 | |
| SD (n = 61) | 8.84 | 4.81 | 0.29 | 0.18 | 0.16 | 0.34 | 292 | 373 | 497 | 51.1 | 4.53 | 1.84 | 0.60 | |
| [1] | Bulk Avg. | 14.5 | 20.9 | 0.36 | 0.26 | 0.09 | 0.92 | 849 | 614 | 1560 | 52 | 11 | 0.69 | - |
| NAP N4 | 1 | 14.5 | 17.0 | 0.40 | 0.19 | 0.09 | 1.21 | 482 | 462 | 1090 | 136 | 6.22 | 0.85 | 1.45 |
| 2 | 22.8 | 17.8 | 0.80 | 0.26 | 0.11 | 1.43 | 565 | 600 | 1730 | 158 | 8.51 | 1.28 | 0.70 | |
| 3 | 32.2 | 19.8 | 0.61 | 0.68 | 0.24 | 1.04 | 762 | 1050 | 1700 | 106 | 13.7 | 1.63 | 1.64 | |
| Avg. | 22.9 | 19.5 | 0.62 | 0.41 | 0.21 | 1.12 | 721 | 847 | 1360 | 139 | 11.4 | 1.18 | 1.15 | |
| SD (n = 35) | 6.95 | 4.40 | 0.22 | 0.19 | 0.14 | 0.21 | 198 | 219 | 373 | 28.7 | 3.75 | 1.58 | 0.74 | |
| [1] | Bulk Avg. | 14.5 | 20.9 | 0.36 | 0.26 | 0.09 | 0.92 | 849 | 614 | 1560 | 52 | 11 | 0.69 | - |
| PSh-125.29(1) | 1 | 14.8 | 19.9 | 0.003 | 0.01 | 0.004 | 0.08 | 437 | 56.9 | 588 | 12.4 | 9.18 | 0.74 | 8080 |
| 2 | 29.2 | 10.8 | 0.02 | 0.01 | 0.004 | 0.03 | 224 | 51.1 | 1810 | 4.64 | 5.79 | 2.72 | 226 | |
| 3 | 43.3 | 4.52 | 0.03 | 0.02 | 0.01 | 0.09 | 306 | 54.8 | 1110 | 6.21 | 5.58 | 9.57 | 256 | |
| Avg. | 23.0 | 7.51 | 0.01 | 0.01 | 0.01 | 0.10 | 228 | 51.2 | 773 | 6.34 | 4.73 | 3.06 | 495 | |
| SD (n = 89) | 17.7 | 9.63 | 0.01 | 0.01 | 0.00 | 0.06 | 121 | 30.9 | 591 | 1.28 | 2.22 | 1.83 | 433 | |
| [32] | Bulk Avg. | 16.5 | 11.5 | 0.016 | 0.016 | 0.004 | 0.0001 | 325 | 80 | 616 | 4.2 | 5.9 | 1.43 | - |
| La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Y | Ho | Er | Tm | Yb | Lu | ΣREY | Ce * | LREE/HREE | Y/Ho | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Layer # | ppm | ||||||||||||||||||
| EW9602-09RD (crust) | 1 | 359 | 1609 | 41.4 | 184 | 34.1 | 8.17 | 44.0 | 6.11 | 45.9 | 262 | 10.7 | 35.5 | 5.28 | 35.6 | 6.36 | 2690 | 3.39 | 0.53 | 24.5 |
| 2 | 233 | 1407 | 29.2 | 134 | 27.2 | 7.02 | 38.8 | 5.85 | 44.7 | 313 | 11.4 | 38.6 | 5.80 | 42.3 | 7.51 | 2350 | 4.45 | 0.32 | 27.5 | |
| 3 | 398 | 1365 | 64.9 | 293 | 62.0 | 14.0 | 74.3 | 10.3 | 72.3 | 325 | 15.7 | 47.4 | 7.01 | 43.5 | 6.81 | 2800 | 2.31 | 0.60 | 20.7 | |
| Avg. | 302 | 1561 | 38.2 | 175 | 34.1 | 8.26 | 46.1 | 6.64 | 48.8 | 287 | 11.6 | 37.8 | 5.64 | 38.7 | 6.73 | 2610 | 3.85 | 0.45 | 24.9 | |
| SD (n = 44) | 72.9 | 410 | 10.6 | 48.6 | 10.4 | 2.37 | 13.0 | 1.84 | 12.7 | 64.6 | 2.84 | 8.74 | 1.30 | 8.55 | 1.43 | 600 | 0.82 | 0.06 | 1.52 | |
| Bulk Avg. | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| JC120-104 | 1 | 195 | 660 | 52.4 | 248 | 65.4 | 14.2 | 68.3 | 9.95 | 59.1 | 175 | 10.6 | 29.1 | 3.96 | 24.8 | 3.81 | 1620 | 1.46 | 0.74 | 16.6 |
| 2 | 23.3 | 40.9 | 6.18 | 27.5 | 6.83 | 1.49 | 5.91 | 0.83 | 4.77 | 15.6 | 0.89 | 2.50 | 0.35 | 2.23 | 0.35 | 140 | 0.79 | 0.97 | 17.4 | |
| 3 | 50.7 | 83.3 | 14.9 | 64.2 | 16.7 | 3.59 | 13.9 | 2.23 | 12.4 | 35.5 | 2.17 | 6.27 | 0.90 | 6.12 | 0.90 | 314 | 0.71 | 0.87 | 16.3 | |
| Avg. | 137 | 292 | 38.5 | 181 | 46.8 | 10.3 | 46.2 | 6.60 | 39.1 | 127 | 7.01 | 19.5 | 7.98 | 24.9 | 5.06 | 988 | 0.90 | 0.46 | 18.2 | |
| SD (n = 37) | 151 | 285 | 50.1 | 242 | 64.5 | 14.3 | 66.9 | 9.13 | 53.5 | 255 | 9.66 | 26.7 | 3.37 | 21.6 | 3.25 | 1250 | 0.70 | 0.78 | 26.4 | |
| [38] | Bulk Avg. | 114 | 284 | 33.4 | 140 | 34.0 | 8.03 | 31.8 | 4.98 | 28.5 | 96 | 5.35 | 14.6 | 2.11 | 13.7 | 2.05 | 813 | 1.10 | 0.84 | 17.9 |
| RC12-192GS | 1 | 247 | 1970 | 61.6 | 286 | 75.3 | 16.0 | 72.4 | 10.6 | 59.6 | 162 | 10.4 | 27.8 | 3.72 | 24.7 | 3.88 | 3030 | 3.64 | 0.90 | 15.6 |
| 2 | 445 | 1560 | 103 | 475 | 121 | 27.4 | 119 | 17.0 | 99.3 | 264 | 17.8 | 50.0 | 6.82 | 46.0 | 7.16 | 3360 | 1.67 | 0.84 | 14.8 | |
| 3 | 94.8 | 597 | 28.0 | 126 | 34.0 | 7.57 | 30.9 | 4.80 | 28.6 | 78.0 | 4.93 | 14.4 | 1.91 | 13.6 | 1.97 | 1070 | 2.64 | 0.75 | 15.8 | |
| Avg. | 323 | 1290 | 78.8 | 367 | 93.7 | 20.7 | 92.0 | 13.6 | 79.2 | 213 | 14.1 | 38.6 | 5.30 | 34.9 | 5.41 | 2670 | 1.84 | 0.83 | 15.1 | |
| SD (n = 92) | 227 | 772 | 51.5 | 240 | 59.5 | 13.1 | 59.7 | 8.71 | 50.9 | 133 | 9.11 | 25.0 | 3.42 | 22.7 | 3.56 | 1680 | 1.63 | 0.85 | 14.6 | |
| [24] | Bulk Avg. | 213 | 1481 | 51.4 | 189 | 43.4 | 10.4 | 43.8 | 7.69 | 42.0 | 163 | 8.62 | 22.8 | 3.76 | 23.3 | 3.61 | 2310 | 3.67 | 0.77 | 18.9 |
| EATL N4 | 1 | 313 | 2380 | 78.0 | 324 | 75.9 | 18.1 | 77.1 | 12.3 | 73.4 | 238 | 13.4 | 37.9 | 5.42 | 35.7 | 5.16 | 3690 | 3.69 | 0.79 | 17.8 |
| 2 | 346 | 2400 | 85.2 | 367 | 83.9 | 19.0 | 84.1 | 12.2 | 72.8 | 245 | 13.5 | 37.0 | 5.07 | 32.4 | 4.91 | 3810 | 3.32 | 0.93 | 18.2 | |
| 3 | 130 | 670 | 33.7 | 142 | 37.0 | 7.93 | 36.2 | 5.25 | 31.5 | 113 | 6.04 | 16.1 | 2.20 | 14.0 | 2.10 | 1250 | 2.42 | 0.84 | 18.7 | |
| Avg. | 308 | 2020 | 77.3 | 336 | 81.1 | 18.1 | 82.8 | 12.7 | 75.7 | 249 | 14.1 | 39.3 | 5.47 | 34.9 | 5.31 | 3360 | 3.10 | 0.78 | 17.6 | |
| SD (n = 61) | 117 | 824 | 30.2 | 128 | 30.3 | 6.87 | 29.9 | 4.58 | 27.3 | 88.3 | 5.10 | 14.3 | 1.97 | 13.0 | 1.94 | 1320 | 3.32 | 0.83 | 17.3 | |
| [1] | Bulk Avg. | 272 | 1392 | 63.8 | 243 | 55.5 | 11.5 | 57.9 | 9.17 | 47.1 | 181 | 9.61 | 28.0 | 3.91 | 23.9 | 3.74 | 2400 | 2.69 | 0.90 | 18.8 |
| NAP N4 | 1 | 328 | 2000 | 76.6 | 343 | 81.9 | 18.0 | 84.3 | 11.9 | 72.2 | 247 | 13.1 | 37.1 | 4.91 | 32.7 | 5.04 | 3360 | 2.94 | 0.86 | 18.8 |
| 3 | 386 | 2130 | 88.0 | 386 | 86.1 | 19.4 | 86.8 | 12.3 | 74.4 | 254 | 13.8 | 39.2 | 5.37 | 36.0 | 5.53 | 3620 | 2.73 | 0.90 | 18.4 | |
| 2 | 411 | 2290 | 104 | 446 | 105 | 24.3 | 107 | 15.9 | 96.9 | 336 | 18.2 | 52.7 | 7.38 | 49.4 | 7.41 | 4070 | 2.64 | 0.76 | 18.4 | |
| Bulk Avg. | 357 | 2350 | 89.2 | 387 | 92.9 | 21.0 | 94.8 | 13.9 | 84.7 | 283 | 15.6 | 44.2 | 6.06 | 40.0 | 5.94 | 3880 | 3.11 | 0.80 | 18.1 | |
| SD (n = 35) | 75.7 | 618 | 20.5 | 84.6 | 20.4 | 4.96 | 20.8 | 3.21 | 19.8 | 63.6 | 3.75 | 10.9 | 1.53 | 10.2 | 1.47 | 960 | 3.80 | 0.71 | 17.0 | |
| [1] | Bulk Avg. | 272 | 1392 | 63.8 | 243 | 55.5 | 11.5 | 57.9 | 9.17 | 47.1 | 181 | 9.61 | 28.0 | 3.91 | 23.9 | 3.74 | 2400 | 2.69 | 0.90 | 18.8 |
| N29 | 1 | 27.0 | 32.3 | 5.34 | 25.5 | 5.98 | 1.37 | 3.58 | 1.06 | 6.88 | 42.2 | 1.44 | 4.22 | 0.57 | 3.65 | 0.53 | 162 | 0.61 | 0.57 | 29.3 |
| 3 | 27.6 | 27.9 | 6.08 | 29.5 | 6.41 | 1.57 | 8.43 | 1.15 | 6.78 | 29.3 | 1.43 | 3.95 | 0.54 | 3.24 | 0.47 | 154 | 0.48 | 0.69 | 20.4 | |
| 2 | 19.4 | 27.9 | 3.88 | 18.0 | 4.01 | 0.95 | 2.32 | 0.66 | 4.22 | 20.7 | 0.84 | 2.40 | 0.34 | 2.02 | 0.31 | 108 | 0.74 | 0.71 | 24.6 | |
| Avg. | 20.8 | 27.1 | 4.11 | 19.4 | 4.44 | 1.03 | 2.60 | 0.76 | 4.92 | 27.7 | 1.01 | 2.92 | 0.40 | 2.50 | 0.37 | 123 | 0.67 | 0.63 | 27.4 | |
| SD (n = 89) | 11.5 | 16.8 | 2.38 | 11.5 | 2.78 | 0.64 | 3.49 | 0.50 | 3.28 | 20.1 | 0.69 | 2.02 | 0.27 | 1.74 | 0.24 | 70.7 | 0.72 | 0.53 | 29.1 | |
| [32] | Bulk Avg. | 44.4 | 65.9 | 9.2 | 40.6 | 10.0 | 2.3 | 10.4 | 1.4 | 8.8 | 42 | 1.6 | 4.8 | 0.63 | 3.6 | 0.48 | 247 | 0.77 | 0.90 | 26.3 |
| EW9602-09RD | JC120-104 | RC12-192GS | EATL N4 | NAP N4 | PSh-125.29(1) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 1 | Factor 2 | Factor 3 | |
| Ti | 0.43 | 0.86 | 0.11 | 0.95 | 0.16 | 0.15 | 0.97 | 0.56 | 0.48 | 0.97 | 0.17 | 0.13 | 0.92 | ||||||||||||
| V | 0.31 | 0.82 | 0.27 | 0.16 | 0.18 | 0.29 | 0.85 | 0.32 | 0.95 | 0.19 | 0.2 | 0.59 | 0.24 | 0.71 | 0.95 | 0.19 | 0.2 | 0.8 | 0.2 | ||||||
| Cr | −0.39 | −0.34 | −0.17 | −0.12 | −0.47 | −0.12 | 0.66 | −0.47 | −0.12 | 0.25 | 0.29 | ||||||||||||||
| Mn | 0.4 | 0.11 | 0.34 | 0.36 | −0.17 | −0.92 | 0.19 | 0.68 | 0.67 | 0.28 | 0.46 | 0.79 | −0.12 | 0.32 | 0.68 | 0.67 | 0.28 | −0.19 | 0.81 | ||||||
| Fe | 0.17 | 0.75 | 0.12 | −0.20 | 0.13 | 0.91 | 0.14 | 0.26 | 0.91 | −0.18 | 0.3 | 0.65 | 0.11 | 0.71 | 0.91 | −0.18 | 0.3 | 0.75 | −0.45 | ||||||
| Co | 0.86 | 0.2 | 0.11 | 0.37 | 0.37 | 0.82 | 0.91 | 0.11 | 0.27 | 0.45 | 0.27 | −0.24 | 0.74 | 0.91 | 0.11 | 0.27 | 0.52 | ||||||||
| Ni | 0.24 | 0.91 | −0.18 | −0.42 | −0.18 | −0.78 | −0.30 | 0.94 | −0.11 | 0.28 | 0.74 | −0.21 | 0.48 | −0.30 | 0.94 | −0.11 | 0.96 | ||||||||
| Cu | −0.13 | 0.96 | −0.17 | −0.50 | −0.11 | −0.81 | −0.24 | 0.95 | −0.11 | 0.94 | −0.24 | 0.95 | −0.11 | 0.94 | |||||||||||
| Zn | 0.13 | 0.67 | −0.15 | −0.87 | 0.13 | −0.18 | 0.13 | 0.96 | 0.26 | 0.92 | 0.28 | 0.13 | 0.96 | 0.39 | 0.5 | 0.2 | |||||||||
| Ba | −0.49 | 0.62 | 0.29 | 0.11 | 0.12 | 0.11 | 0.82 | 0.23 | 0.89 | 0.15 | −0.23 | 0.26 | 0.8 | 0.34 | 0.25 | 0.89 | 0.15 | −0.23 | 0.26 | 0.14 | 0.77 | ||||
| La | −0.30 | 0.69 | 0.64 | 0.91 | 0.32 | 0.16 | 0.15 | 0.99 | 0.93 | 0.16 | 0.11 | 0.13 | 0.99 | 0.9 | |||||||||||
| Ce | 0.94 | 0.23 | 0.19 | 0.91 | 0.23 | 0.2 | 0.95 | 0.21 | 0.79 | 0.17 | 0.13 | 0.38 | 0.95 | 0.21 | 0.43 | ||||||||||
| Pr | 0.98 | 0.98 | 0.15 | 1 | 0.93 | 0.19 | 0.17 | 0.11 | 1 | 0.94 | |||||||||||||||
| Nd | 0.17 | 0.27 | 0.94 | 0.13 | 0.98 | 0.12 | 0.99 | 0.94 | 0.16 | 0.16 | 0.99 | 0.95 | |||||||||||||
| Sm | 1 | 0.99 | 0.99 | 0.95 | 0.16 | 0.18 | 0.99 | 0.96 | |||||||||||||||||
| Eu | 1 | 0.99 | 0.99 | 0.94 | 0.18 | 0.18 | 0.11 | 0.99 | 0.96 | ||||||||||||||||
| Gd | 0.15 | 0.24 | 0.95 | 0.99 | 0.11 | 0.99 | 0.96 | 0.14 | 0.15 | 0.99 | 0.98 | ||||||||||||||
| Tb | 1 | 0.99 | 0.13 | 1 | 0.96 | 0.14 | 0.17 | 0.11 | 1 | 0.99 | |||||||||||||||
| Dy | 0.37 | 0.92 | 0.98 | 0.14 | 1 | 0.96 | 0.14 | 0.16 | 0.11 | 1 | 0.99 | ||||||||||||||
| Y | 0.93 | 0.13 | 0.99 | 0.99 | 0.97 | 0.14 | 0.13 | 0.13 | 0.99 | 0.94 | −0.18 | ||||||||||||||
| Ho | 1 | 0.98 | 0.15 | 1 | 0.97 | 0.14 | 0.14 | 0.14 | 1 | 0.99 | |||||||||||||||
| Er | 1 | 0.98 | 0.16 | 1 | 0.97 | 0.13 | 0.12 | 0.15 | 1 | 0.98 | |||||||||||||||
| Tm | 0.96 | 0.11 | −0.18 | 0.14 | 0.98 | 0.18 | 1 | 0.97 | 0.14 | 0.12 | 0.17 | 1 | 0.97 | ||||||||||||
| Yb | 0.79 | 0.46 | 0.41 | 0.98 | 0.18 | 1 | 0.97 | 0.14 | 0.11 | 0.18 | 1 | 0.98 | |||||||||||||
| Lu | 0.55 | 0.42 | 0.42 | −0.41 | 0.42 | 0.97 | 0.2 | 1 | 0.97 | 0.12 | 0.19 | 1 | 0.96 | ||||||||||||
| Th | 0.92 | 0.15 | 0.2 | 0.51 | 0.8 | 0.18 | −0.18 | 0.98 | 0.67 | 0.11 | 0.22 | −0.18 | 0.98 | 0.27 | 0.21 | ||||||||||
| U | 0.94 | 0.18 | -0.1 | 0.14 | 0.14 | 0.87 | 0.15 | 0.28 | 0.98 | 0.16 | 0.74 | 0.33 | 0.4 | 0.15 | 0.98 | 0.16 | 0.6 | ||||||||
| Resource | Cook-Austral Volcanic Chain | UK Claim (CCFZ) | SW Pacific Basin | East Atlantic | North West Atlantic | Kara Sea | |
|---|---|---|---|---|---|---|---|
| Avg. nodule resource (kg/m2) | 50–75 e1 | 1.7–57 f1 | 0.6–58 g1 | 2.6–8.8 h1 | 2.6–8.9 i1 | 0.5–2.5 j1 | |
| Surface area (km2) | 1197 e2 | 60,000 f2 | 20,000 g2 | 54,000 h2 | 110,000 i2 | 30,800 j2 | |
| REY | Avg. content (ppm) | 2610 | 988 | 2670 | 3360 | 3880 | 123 |
| Mass of metals (T × 105) | 1.56–2.34 | 1.01–33.8 | 3.20–31.0 | 4.71–16.1 | 11.1–38.0 | ≤0.09 | |
| Mass/Global annual demand a | 1.49–2.23 | 0.96–32.1 | 3.05–29.5 | 4.49–15.4 | 10.6–36.1 | ≤0.09 | |
| Co | Avg. content (ppm) | 15,800 | 2000 | 3400 | 4700 | 8000 | 100 |
| Mass of metals (T × 105) | 9.45–14.2 | 2.04–68.4 | 4.08–39.4 | 6.6–22.6 | 22.9–72.3 | ≤0.07 | |
| Mass/Global annual demand b | 10.9–16.3 | 2.34–78.6 | 4.69–45.3 | 7.58–26.0 | 26.3–90.0 | ≤0.09 | |
| Ni | Avg. content (ppm) | 9300 | 11,000 | 5700 | 2800 | 2600 | 100 |
| Mass of metals (T × 105) | 5.57–8.35 | 11.2–376 | 6.84–66.1 | 3.93–13.5 | 7.43–25.4 | ≤0.08 | |
| Mass/Global annual demand c | ≤0.001 | ≤0.03 | ≤0.005 | ≤0.001 | ≤0.002 | ≤5 × 10−6 | |
| Cu | Avg. content (ppm) | 2300 | 8900 | 4100 | 1600 | 1100 | 100 |
| Mass of metals (T × 105) | 1.38–2.06 | 9.08–304 | 4.92–47.6 | 2.25–7.69 | 3.15–10.8 | ≤0.08 | |
| Mass/Global annual demand d | ≤0.009 | ≤1.35 | ≤0.21 | ≤0.03 | ≤0.05 | ≤3 × 10−4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menendez, A.; James, R.; Shulga, N.; Connelly, D.; Roberts, S. Linkages between the Genesis and Resource Potential of Ferromanganese Deposits in the Atlantic, Pacific, and Arctic Oceans. Minerals 2018, 8, 197. https://doi.org/10.3390/min8050197
Menendez A, James R, Shulga N, Connelly D, Roberts S. Linkages between the Genesis and Resource Potential of Ferromanganese Deposits in the Atlantic, Pacific, and Arctic Oceans. Minerals. 2018; 8(5):197. https://doi.org/10.3390/min8050197
Chicago/Turabian StyleMenendez, Amaya, Rachael James, Natalia Shulga, Doug Connelly, and Steve Roberts. 2018. "Linkages between the Genesis and Resource Potential of Ferromanganese Deposits in the Atlantic, Pacific, and Arctic Oceans" Minerals 8, no. 5: 197. https://doi.org/10.3390/min8050197