Physicochemical Model of Formation of Gold-Bearing Magnetite-Chlorite-Carbonate Rocks at the Karabash Ultramafic Massif (Southern Urals, Russia)
Abstract
:1. Introduction
2. Geological Background of the Studied Area
2.1. Geological Setting of the Karabash Massif and Hydrothermal-Metasomatic Rocks
2.2. Mineral Composition of Altered Ultramafic Rocks
2.3. Geochemical Characteristics of Rocks
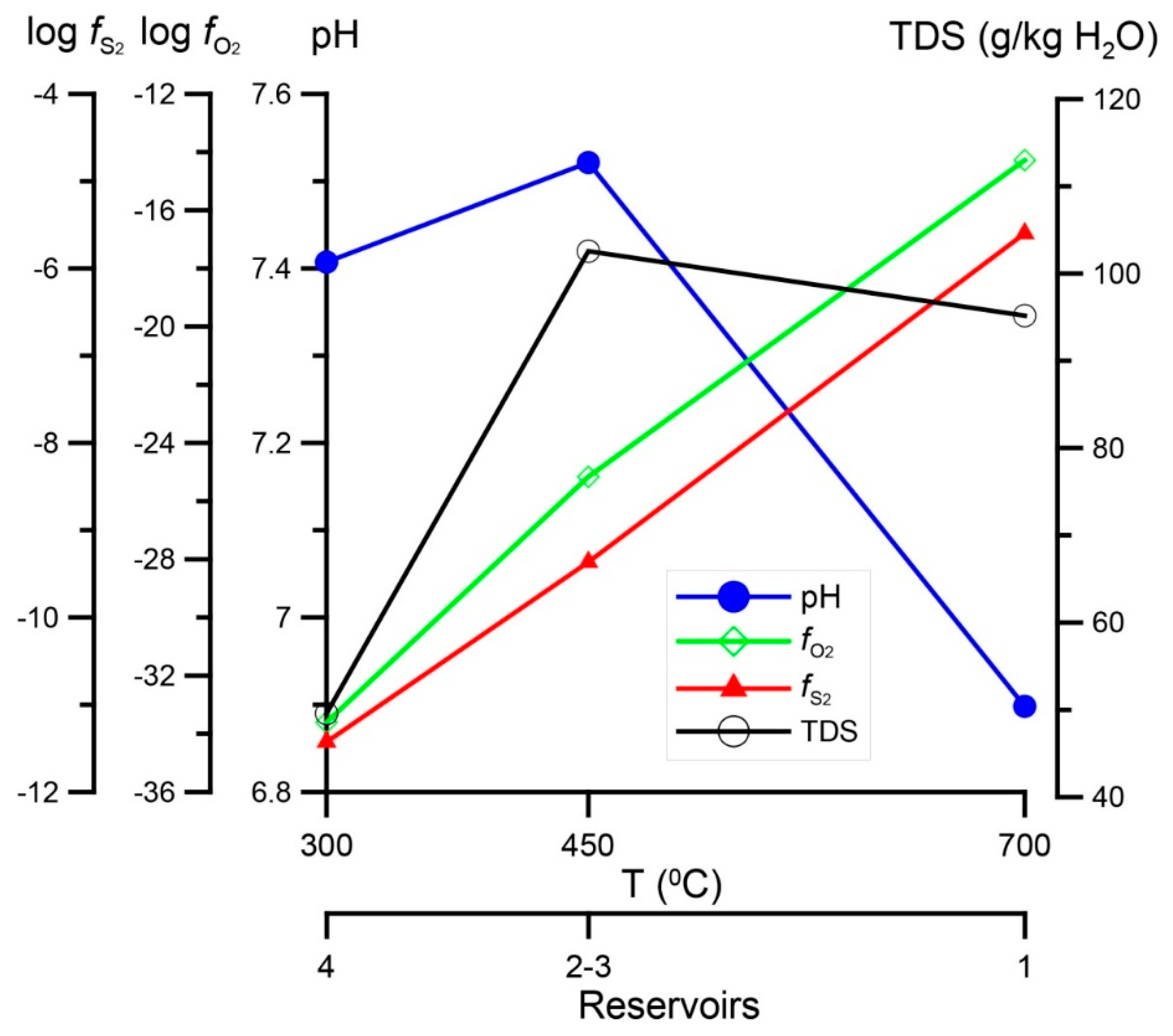
2.4. P,T-Conditions of Formation and Composition of Ore-Forming Fluid
2.5. Sources of Metals and Ore-Bearing Fluid
2.6. Geodynamic Model
3. Methods
3.1. Software and Thermodynamic Dataset for Modeling
3.2. Initial Data for Thermodynamic Modeling
4. Results of Thermodynamic Modeling
4.1. Fluid Composition and Forms of Transfer and Deposition of Au, Ag, Cu, and Hg
4.2. Specific Features of Mineral Composition of Rocks in Different Reservoirs of the Model
4.3. Uplift of Deep-Seated Rocks under Low T,P-Conditions
5. Discussion
5.1. Similarity of Natural and Model Composition of Rocks and Gold Mineralization
5.2. Scales of Gold Mineralization in the Karabash Massif and the Variations in the Compositions of Gold Minerals
6. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Puchkov, V.N. General features relating to the occurrence of mineral deposits in the Urals: What, where, when and why. Ore Geol. Rev. 2017, 85, 4–29. [Google Scholar] [CrossRef]
- Snachyov, A.V.; Kuznetsov, N.S.; Snachyov, V.I. The Chernoe ozero gold occurrence in carbonaceous deposits of the ophiolite association: The first object of such a type in the Soutern Urals. Dokl. Earth Sci. 2011, 439, 906–908. [Google Scholar] [CrossRef]
- Novgorodova, M.I.; Tsepin, A.I.; Kudrevich, I.M.; Vyal’sov, L.N. New data on crystal chemistry and properties of natural intermetallic compounds in the copper-gold system. Zap. Vses. Miner. Obsch. 1977, 106, 540–552. (In Russian) [Google Scholar]
- Spiridonov, E.M.; Pletniov, P.A. Zolotaya Gora Deposit of Cupriferous Gold (About “Golden-Rodingite” Formation); Scientific World: Moskow, Russia, 2002; p. 220. ISBN 5-89176-169-6. (In Russian) [Google Scholar]
- Murzin, V.V.; Varlamov, D.A.; Ronkin, Y.L.; Shanina, S.N. Origin of Au-bearing Rodingite in the Karabash Massif of Alpine-Type Ultramafic Rocks in the Southern Urals. Geol. Ore Depos. 2013, 55, 278–297. [Google Scholar] [CrossRef]
- Murzin, V.V.; Chudnenko, K.V.; Palyanova, G.A.; Varlamov, D.A.; Naumov, E.A.; Pirajno, F. Physicochemical model of formation of Cu-Ag-Au-Hg solid solutions and intermetallic alloys in the rodingites of the Zolotaya Gora gold deposit (Urals, Russia). Ore Geol. Rev. 2018, 93, 81–97. [Google Scholar] [CrossRef]
- Murzin, V.V.; Popov, V.A.; Erokhin, Y.V.; Rakhov, E.V. Mineralogic and Geochemical Features of Gold-Rare-Metal-Rare-Earth Mineralization of Chlorite-Carbonate Rocks from the KARABASH Massif of Ultrabasic Rocks (Southern Urals). In Ural’skii Mineralogicheskii Sbornik; IMin UrO, RAS: Miass, Russia, 2005; Volume 13, pp. 123–145. (In Russian) [Google Scholar]
- Murzin, V.V.; Varlamov, D.A.; Palyanova, G.A. Conditions of formation of gold-bearing magnetite–chlorite–carbonate rocks of the Karabash ultrabasic massif (South Urals). Russ. Geol. Geophys. 2017, 58, 803–814. [Google Scholar] [CrossRef]
- Murzin, V.V.; Shanina, S.N. Physic-chemical conditions of gold-bearing magnetite-chlorite-carbonate rocks’ formation of the Karabash ultramafic massif (the Southern Urals). Litosphera 2017, 17, 110–117. [Google Scholar] [CrossRef]
- Lozhechkin, M.P. The Karabash Deposit of Cupriferous Gold; Trudy UFAN SSSR: Sverdlovsk, Russia, 1935; pp. 35–44. (In Russian) [Google Scholar]
- Borisenko, A.S. Study of the salt composition of solutions in gasliquid inclusions in minerals by the cryometric method. Sov. Geol. Geophys. 1977, 18, 11–19. [Google Scholar]
- Bodnar, R.J.; Vityk, M.O. Interpretation of microthermometric data for H2O-NaCl fluid inclusions. In Fluid Inclusions in Minerals: Methods and Applications, 2nd ed.; De Vivo, B., Frezzotti, M.L., Eds.; Virginia Tech: Blacksburg, VA, USA, 1994; pp. 117–130. [Google Scholar]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 1–644. [Google Scholar]
- Kisin, A.Y.; Koroteev, V.A. Block Folding and Oreogenesis; IGG UrB RAS: Ekaterinburg, Russia, 2017; p. 349. (In Russian) [Google Scholar]
- Kissin, Y.; Murzin, V.V.; Pritchin, M.E. Tectonic position of the gold mineralization of the Karabash Mountain (Southern Urals): Examination of small structural forms. Litosphera 2016, 16, 79–91. (In Russian) [Google Scholar]
- Karpov, I.K.; Chudnenko, K.V.; Kulik, D.A. Modeling chemical mass-transfer in geochemical processes: Thermodynamic relations, conditions of equilibria and numerical algorithms. Am. J. Sci. 1997, 297, 767–806. [Google Scholar] [CrossRef]
- Chudnenko, K.V. Thermodynamic Modeling in Geochemistry: Theory, Algorithms, Software, Applications; Academic Publishing House Geo: Novosibirsk, Russia, 2010; 287p, ISBN 978-5-904682-18-7. (In Russian) [Google Scholar]
- Chudnenko, K.V.; Palyanova, G.A. Thermodynamic modeling of native formation Cu-Ag-Au-Hg solid solutions. Appl. Geochem. 2016, 66, 88–100. [Google Scholar] [CrossRef]
- Zhuravkova, T.V.; Palyanova, G.A.; Chudnenko, K.V.; Kravtsova, R.G.; Prokopyev, I.R.; Makshakov, A.S.; Borisenko, A.S. Physicochemical models of formation of gold–silver ore mineralization at the Rogovik deposit (Northeastern Russia). Ore Geol. Rev. 2017, 91, 1–20. [Google Scholar] [CrossRef]
- Tanger, J.C.; Helgeson, H.C. Calculation of the thermodynamic and transport properties of aqueous species at high pressures and temperatures: Revised equations of state for standard partial molal properties of ions and electrolytes. Am. J. Sci. 1988, 288, 19–98. [Google Scholar] [CrossRef]
- Lee, B.I.; Kesler, M.G. Generalized thermodynamic correlation based on three parameter corresponding states. AIChE J. 1975, 21, 510–527. [Google Scholar] [CrossRef]
- Walas, S.M. Phase Equilibria in Chemical Engineering; Butterworth Publishers: Boston, MA, USA, 1985; 671p, ISBN 978-0-409-95162-2. [Google Scholar]
- Breedveld, G.J.F.; Prausnitz, J.M. Thermodynamic properties of supercritical fluids and their mixtures at very high pressure. AIChE J. 1973, 19, 783–796. [Google Scholar] [CrossRef]
- Garuti, G.; Fershtater, G.B.; Bea, F.; Montero, P.; Pushkarev, E.V.; Zaccarini, F. Platinum Group Elements As a Pertrological Indicators in Mafic-Ultramafic Complexes of the Central and Southern Urals: Preliminary Results. Tectonophysics 1997, 276, 181–194. [Google Scholar] [CrossRef]
- Grigoriev, N.A. Chemical Element Distribution in the Upper Continental Crust; UB RAS: Ekaterinburg, Russia, 2009; p. 382. (In Russian) [Google Scholar]
- Karpov, I.K.; Chudnenko, K.V.; Kravtsova, R.G.; Bychinskiy, V.A. Simulation modeling of physical and chemical processes of dissolution, transport and deposition of gold in epithermal gold-silver deposits of the North-East Russia. Russ. Geol. Geophys. 2001, 3, 393–408. [Google Scholar]
- Gritsuk, N.A. Petrogeochemical Features and Pre Potential of the Talov Massif of Gabbro-Hyperbasic Rocks. Ph.D. Thesis, Moscow State University, Russia, 2003; p. 148. (In Russian). [Google Scholar]
- Berzon, R.O. Gold Resource Potential of Ultramafics; VIEMS: Moscow, Russia, 1983; p. 47. (In Russian) [Google Scholar]
- Zotov, A.; Kuzmin, N.; Reukov, V.; Tagirov, B. Stability of AuCl2− from 25 to 1000 °C at pressures to 5000 bar, and consequences for hydrothermal gold mobilization. Minerals 2018, 8, 286. [Google Scholar] [CrossRef]
- Seward, T.M.; Williams-Jones, A.E.; Migdisov, A.A. The chemistry of metal transport and deposition by ore-forming hydrothermal fluids. In Treatise on Geochemistry, 2nd ed.; Turekian, K., Holland, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 29–57. [Google Scholar]
- Belogub, E.V.; Melekestseva, I.Y.; Novoselov, K.A.; Zabotina, M.V.; Tret’yakov, G.A.; Zaykov, V.V.; Yuminov, A.M. Listvenite-related gold deposits of the South Urals (Russia): A review. Ore Geol. Rev. 2017, 85, 247–270. [Google Scholar] [CrossRef]
- Murzin, V.V.; Varlamov, D.A.; Shanina, S.N. New Data on the Gold–Antigorite Association of the Urals. Dokl. Earth Sci. 2007, 417A, 1436–1439. [Google Scholar] [CrossRef]










| Ultramafic Rocks | Mafic Rocks | |
|---|---|---|
| Au | 0.01135 1 | 0.003925 1 |
| Ag | 0.14 2 | 0.11 2 |
| Cu | 6.15 1 | 31.7 1 |
| Hg | 0.02 2 | 0.07 2 |
| Gabbro (n = 7) | Harzburgite (n = 9) | Limestone | Serpentinite | |
|---|---|---|---|---|
| SiO2 | 48.81 | 40.43 | 0.19 | 40.47 |
| TiO2 | 0.37 | 0.04 | - | 0.04 |
| Al2O3 | 14.65 | 1.52 | 0.18 | 1.44 |
| Fe2O3 | 7.97 | 4.26 | - | 5.31 |
| FeO | 7.20 | 3.79 | 0.23 | 1.61 |
| MnO | 0.18 | 0.09 | 0.17 | 0.09 |
| MgO | 8.57 | 38.41 | 0.39 | 38.21 |
| CaO | 12.03 | 1.62 | 55.13 | 0.48 |
| Na2O | 2.75 | 0.11 | - | 0.12 |
| K2O | 0.44 | 0.03 | - | 0.03 |
| P | 0.09 * | 0.035 * | - | 0.013 ** |
| S | - | - | - | 0.75 |
| CO2 | - | - | 43.75 | 0.46 |
| LOI | 1.96 | 6.77 | - | 10.59 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murzin, V.; Chudnenko, K.; Palyanova, G.; Kissin, A.; Varlamov, D. Physicochemical Model of Formation of Gold-Bearing Magnetite-Chlorite-Carbonate Rocks at the Karabash Ultramafic Massif (Southern Urals, Russia). Minerals 2018, 8, 306. https://doi.org/10.3390/min8070306
Murzin V, Chudnenko K, Palyanova G, Kissin A, Varlamov D. Physicochemical Model of Formation of Gold-Bearing Magnetite-Chlorite-Carbonate Rocks at the Karabash Ultramafic Massif (Southern Urals, Russia). Minerals. 2018; 8(7):306. https://doi.org/10.3390/min8070306
Chicago/Turabian StyleMurzin, Valery, Konstantin Chudnenko, Galina Palyanova, Aleksandr Kissin, and Dmitry Varlamov. 2018. "Physicochemical Model of Formation of Gold-Bearing Magnetite-Chlorite-Carbonate Rocks at the Karabash Ultramafic Massif (Southern Urals, Russia)" Minerals 8, no. 7: 306. https://doi.org/10.3390/min8070306





