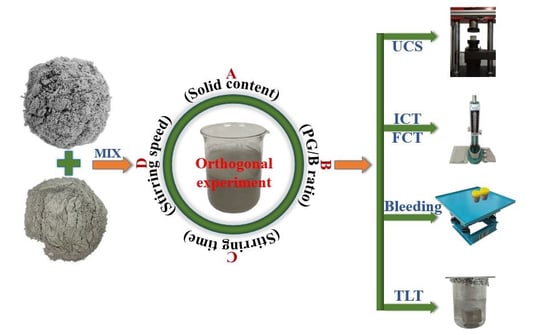
Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Orthogonal Experiment
2.3. Sample Preparation
2.4. Tank Leaching Test
2.5. Analytical Methods
2.5.1. Bleeding Rate and Setting Times
2.5.2. Unconfined Compressive Strength of Cemented PG Backfill Samples
2.5.3. Microstructural Analysis
2.5.4. Chemical Measurements
3. Results and Discussion
3.1. Differences of the Slurry Properties and Strength of Cemented PG Backfill
3.1.1. Effects of Slurry Preparation on Slurry Properties
3.1.2. Effects of Slurry Preparation on Unconfined Compressive Strength
3.2. Impurities in Bleeding Water
3.3. Impurities in the Leachates of Tank Leaching Test (TLT)
3.3.1. pH Variation
3.3.2. Cumulative Effects of Impurities on the Environment
3.3.3. Effects of Slurry Preparation on Leaching Behavior of Impurities
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, J.K.; Liu, W.C.; Zhang, L.L.; Xiao, B. Preparation of load-bearing building materials from autoclaved phosphogypsum. Constr. Build. Mater. 2009, 23, 687–693. [Google Scholar] [CrossRef]
- Contreras, M.; Teixeira, S.R.; Santos, G.T.A.; Gázquez, M.J.; Romero, M.; Bolívar, J.P. Influence of the addition of phosphogypsum on some properties of ceramic tiles. Constr. Build. Mater. 2018, 175, 588–600. [Google Scholar] [CrossRef]
- Lopez, F.A.; Gazquez, M.; Alguacil, F.J.; Bolivar, J.P.; Garcia-Diaz, I.; Lopez-Coto, I. Microencapsulation of phosphogypsum into a sulfur polymer matrix: Physico-chemical and radiological characterization. J. Hazard. Mater. 2011, 192, 234–245. [Google Scholar] [PubMed]
- Szlauer, B.; Szwanenfeld, M.; Werblanjakubiec, H.; Kolasa, K. Hydrobiological characteristics of ponds collecting effluents from a phosphogypsum tip of the Police Chemical Works near Szczecin. Acta Hydroch. Hydrob. 1990, 32, 27–34. [Google Scholar]
- Degirmenci, N. Utilization of phosphogypsum as raw and calcined material in manufacturing of building products. Constr. Build. Mater. 2008, 22, 1857–1862. [Google Scholar] [CrossRef]
- Perez-Lopez, R.; Alvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Tayibi, H.; Choura, M.; Lopez, F.A.; Alguacil, F.J.; Lopez-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef]
- Holanda, F.D.C.; Schmidt, H.; Quarcioni, V.A. Influence of phosphorus from phosphogypsum on the initial hydration of Portland cement in the presence of superplasticizers. Cem. Concr. Compos. 2017, 83, 384–393. [Google Scholar] [CrossRef]
- Degirmenci, N.; Okucu, A.; Turabi, A. Application of phosphogypsum in soil stabilization. Build. Environ. 2007, 42, 3393–3398. [Google Scholar] [CrossRef]
- Bensalah, H.; Bekheet, M.F.; Younssi, S.A.; Ouammou, M.; Gurlo, A. Hydrothermal synthesis of nanocrystalline hydroxyapatite from phosphogypsum waste. J. Environ. Chem. Eng. 2018, 6, 1347–1352. [Google Scholar] [CrossRef]
- Papastefanou, C.; Stoulos, S.; Ioannidou, A.; Manolopoulou, M. The application of phosphogypsum in agriculture and the radiological impact. J. Environ. Radioact. 2006, 89, 188–198. [Google Scholar] [CrossRef]
- Li, X.B.; Du, J.; Gao, L.; He, S.Y.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the effect of sulphate on strength development of paste backfill and binder mixture optimization. Cem. Concr. Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Ercikdi, B.; Kesimal, A.; Cihangir, F.; Deveci, H.; Alp, İ. Cemented paste backfill of sulphide-rich tailings: Importance of binder type and dosage. Cem. Concr. Compos. 2009, 31, 268–274. [Google Scholar] [CrossRef]
- Cao, S.; Yilmaz, E.; Song, W.D. Evaluation of viscosity, strength and microstructural properties of cemented Tailings backfill. Minerals 2018, 8, 352. [Google Scholar] [CrossRef]
- Kermani, M.; Hassani, F.P.; Aflaki, E.; Benzaazoua, M.; Nokken, M. Evaluation of the effect of sodium silicate addition to mine backfill, Gelfill—Part 2: Effects of mixing time and curing temperature. J. Rock. Mech. Geotech. Eng. 2015, 7, 668–673. [Google Scholar] [CrossRef]
- Zou, G.L.; Xu, J.; Wu, C. Evaluation of factors that affect rutting resistance of asphalt mixes by orthogonal experiment design. Int. J. Pavement. Res. Tech. 2017, 10, 282–288. [Google Scholar] [CrossRef]
- Zheng, C.S.; Kizil, M.S.; Chen, Z.W.; Aminossadati, S.M. Effects of coal properties on ventilation air leakage into methane gas drainage boreholes: Application of the orthogonal design. J. Nat. Gas. Sci. Eng. 2017, 45, 88–95. [Google Scholar] [CrossRef]
- Su, L.S.; Zhang, J.B.; Wang, C.J.; Zhang, Y.K.; Li, Z.; Song, Y.; Jin, T.; Ma, Z. Identifying main factors of capacity fading in lithium ion cells using orthogonal design of experiments. Appl. Eng. 2016, 163, 201–210. [Google Scholar] [CrossRef]
- Zhai, Y.N.; Sun, S.D.; Wang, J.Q.; Niu, G.G. Job shop bottleneck detection based on orthogonal experiment. Comput. Ind. Eng. 2011, 61, 872–880. [Google Scholar] [CrossRef]
- Yao, Z.Q. Technoligical Study and Reliability Analysis of Yellow Phosphorus Slag and Phosphgysum Backfill in Kaiyang Mine. Master’s Thesis, Central South University, Changsha, China, 2009. [Google Scholar]
- Cui, C.W.; Shi, F.; Li, Y.G.; Wang, S.Y. Orthogonal analysis for perovskite structure microwave dielectric ceramic thin films fabricated by the RF magnetron-sputtering method. J. Mater. Sci. Mater. Electronics 2010, 21, 349–354. [Google Scholar]
- Wu, X.; Leung, D.Y.C. Optimization of biodiesel production from camelina oil using orthogonal experiment. Appl. Eng. 2011, 88, 3615–3624. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Y.; Shao, B.; Qi, R.; Myoga, H. Precipitative Removal of Fluoride from Electronics Wastewater. J. Environ. Eng. 2001, 127, 902–907. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Edahbi, M.; Mansori, M.; Hakkou, R. Leaching and geochemical behavior of fired bricks containing coal wastes. J. Environ. Manag. 2018, 209, 227–235. [Google Scholar] [CrossRef]
- Chai, J.C.; Onitsuk, K.; Hayashi, S. Cr(VI) concentration from batch contact/tank leaching and column percolation test using fly ash with additives. J. Hazard Mater. 2009, 166, 67–73. [Google Scholar] [CrossRef]
- Ma, J.L.; Zhao, Y.C.; Wang, J.M.; Wang, L. Effect of magnesium oxychloride cement on stabilization/solidification of sewage sludge. Constr. Build. Mater. 2010, 24, 79–83. [Google Scholar]
- Wu, A.X.; Wang, Y.; Wang, H.J.; Yin, S.H.; Miao, X.X. Coupled effects of cement type and water quality on the properties of cemented paste backfill. Int. J. Miner. Process. 2015, 143, 65–71. [Google Scholar] [CrossRef]
- Standard for Test Method of Performance on Building Mortar; Chinses National Standard: JGJ/T70-2009; Ministry of Construction of PRC: Beijing, China, 2009.
- Chen, Q.S.; Zhang, Q.L.; Fourie, A.; Xin, C. Utilization of phosphogypsum and phosphate tailings for cemented paste backfill. J. Environ. Manag. 2017, 201, 19–27. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M.; Saa, E.G. Mix proportioning of underground cemented tailings backfill. Tunn. Underg. Sp. Tech. 2008, 23, 80–90. [Google Scholar] [CrossRef]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Yin, S.H.; Wu, A.X.; Hu, K.J.; Wang, Y.; Zhang, Y.K. The effect of solid components on the rheological and mechanical properties of cemented paste backfill. Miner Eng. 2012, 35, 61–66. [Google Scholar] [CrossRef]
- Kumer, S.; Kameswara Rao, C.V.S. Effect of sulfates on the setting time of cement and strength of concrete. Cem. Concr. Res. 1994, 24, 1237–1244. [Google Scholar] [CrossRef]
- Fall, M.; Adrien, D.; Célestin, J.C.; Pokharel, M.; Touré, M. Saturated hydraulic conductivity of cemented paste backfill. Miner Eng. 2009, 22, 1307–1317. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.L.; Liu, Y.C. Mechanical performance and ultrasonic properties of cemented gangue backfill with admixture of fly ash. Ultrasonics 2016, 64, 89–96. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cem. Concr. Compos. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Kitazume, M.; Grisolia, M.; Leder, E.; Marzano, I.P.; Correia, A.A.S.; Venda Oliveira, P.J.; Ahnberg, H.; Andersson, M. Applicability of molding procedures in laboratory mix tests for quality control and assurance of the deep mixing method. Soils. Found. 2015, 55, 761–777. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Fall, M.; Belem, T. A contribution to understanding the hardening process of cemented pastefill. Miner Eng. 2004, 17, 141–152. [Google Scholar] [CrossRef]
- Juez, J.M.; Cazacliu, B.; Cothenet, A.; Artoni, R.; Roquet, N. Recycled concrete aggregate attrition during mixing new concrete. Constr. Build. Mater. 2016, 116, 299–309. [Google Scholar] [CrossRef]
- Manjit, S. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar]
- Benzaazoua, M.; Fiset, J.F.; Bussière, B.; Villeneuve, M.; Plante, B. Sludge recycling within cemented paste backfill: Study of the mechanical and leachability properties. Miner Eng. 2006, 19, 420–432. [Google Scholar] [CrossRef]
- Hamberg, R.; Maurice, C.; Alakangas, L. The use of low binder proportions in cemented paste backfill—Effects on As-leaching. Miner Eng. 2015, 78, 74–82. [Google Scholar] [CrossRef]
- Sanchez, F.; Garrabrants, A.C.; Vandecasteele, C.; Moszkowicz, P.; Kosson, D.S. Environmental assessment of waste matrices contaminated with arsenic. J. Hazard. Mater. 2003, B96, 229–257. [Google Scholar] [CrossRef]
- Li, X.B.; Zhou, Z.L.; Zhao, G.Y.; Liu, Z.X. Utilization of phosphogypsum for backfilling, way to relieve its environmental impact. Gospod. Surowcami. Min. 2008, 24, 223–232. [Google Scholar]
- Shi, Y.; Gan, L.; Li, X.B.; He, S.Y.; Sun, C.; Gao, L. Dynamics of metals in backfill of a phosphate mine of guiyang, China using a three-step sequential extraction technique. Chemosphere 2018, 192, 354–361. [Google Scholar] [CrossRef]
- Coussy, S.; Benzaazoua, M.; Blanc, D.; Moszkowicz, P.; Bussiere, B. Assessment of arsenic immobilization in synthetically prepared cemented paste backfill specimens. J. Environ. Manag. 2012, 93, 10–21. [Google Scholar] [CrossRef] [PubMed]





| Chemical Composition | PG | Binder |
|---|---|---|
| % | % | |
| Na2O | 0.22 | 0.39 |
| MgO | 0.41 | 1.70 |
| Al2O3 | 0.47 | 4.97 |
| SiO2 | 1.76 | 23.31 |
| P2O5 | 2.61 | 1.58 |
| SO3 | 55.00 | 5.40 |
| K2O | 0.05 | 0.97 |
| CaO | 37.69 | 51.44 |
| TiO2 | 0.03 | 0.46 |
| Fe2O3 | 0.25 | 2.60 |
| Physical Characteristic | ||
| D10 (μm) | 19.40 | 6.08 |
| D30 (μm) | 54.78 | 14.18 |
| D60 (μm) | 102.41 | 33.66 |
| Cu = D60/D10 | 5.28 | 5.54 |
| Cc = D302/(D60 × D10) | 1.51 | 0.98 |
| Factors | Levels | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| A | Solid content (%) | 45 | 50 | 55 | 60 |
| B | PG/B ratio | 2:1 | 3:1 | 4:1 | 5:1 |
| C | Stirring time (min) | 5 | 30 | 60 | 120 |
| D | Stirring speed (rpm) | 300 | 400 | 500 | 600 |
| Batch No. | Factors | Evaluation Indexes | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A Solid Content | B PG/B Ratio | C Stirring Time | D Stirring Speed | Backfill | Slurry Properties | Bleeding Waters | Leachates of TLT | |||||||||
| UCS (MPa) | Wet Unit Weight (kN/m3) | IST (h) | FST (h) | Bleeding Rate (%) | F− (mg/L) | SO42− (mg/L) | PO43− (mg/L) | pH | F− (mg) | SO42− (mg) | PO43− (mg) | |||||
| 1 | 45% | 2:1 | 5 | 300 | 0.83 | 13.22 | 87 | 105 | 40.10 | 257 | 2149 | 2.55 | 10.62 | 28 | 876 | 0.25 |
| 2 | 45% | 3:1 | 30 | 400 | 0.81 | 13.42 | 98 | 112 | 35.17 | 267 | 2433 | 2.50 | 10.75 | 38 | 1482 | 0.26 |
| 3 | 45% | 4:1 | 60 | 500 | 0.74 | 12.89 | 103 | 122 | 46.40 | 282 | 2300 | 5.00 | 10.46 | 43 | 1597 | 0.28 |
| 4 | 45% | 5:1 | 120 | 600 | 0.77 | 12.65 | 99 | 124 | 42.54 | 454 | 2888 | 1.80 | 10.13 | 50 | 1751 | 0.32 |
| 5 | 50% | 2:1 | 30 | 500 | 1.57 | 13.26 | 80 | 95 | 38.70 | 277 | 2168 | 2.05 | 11.18 | 27 | 1080 | 0.33 |
| 6 | 50% | 3:1 | 5 | 600 | 0.92 | 13.44 | 99 | 115 | 35.73 | 287 | 2266 | 2.25 | 11.26 | 37 | 1601 | 0.37 |
| 7 | 50% | 4:1 | 120 | 300 | 0.87 | 13.02 | 98 | 106 | 43.78 | 356 | 2518 | 3.15 | 10.71 | 39 | 1341 | 0.36 |
| 8 | 50% | 5:1 | 60 | 400 | 0.80 | 12.58 | 92 | 108 | 42.29 | 533 | 2870 | 1.75 | 10.08 | 60 | 1848 | 0.41 |
| 9 | 55% | 2:1 | 60 | 600 | 2.06 | 14.22 | 76 | 88 | 37.49 | 305 | 2489 | 3.10 | 11.40 | 25 | 939 | 0.34 |
| 10 | 55% | 3:1 | 120 | 500 | 1.17 | 13.78 | 88 | 96 | 40.42 | 307 | 2168 | 2.85 | 11.02 | 44 | 1651 | 0.38 |
| 11 | 55% | 4:1 | 5 | 400 | 0.96 | 13.04 | 92 | 112 | 32.29 | 376 | 2540 | 2.35 | 10.51 | 47 | 1732 | 0.40 |
| 12 | 55% | 5:1 | 30 | 300 | 0.93 | 12.54 | 97 | 125 | 32.25 | 545 | 3167 | 2.75 | 10.35 | 48 | 1890 | 0.40 |
| 13 | 60% | 2:1 | 120 | 400 | 2.26 | 14.22 | 72 | 85 | 27.60 | 306 | 2395 | 2.75 | 11.52 | 27 | 1060 | 0.37 |
| 14 | 60% | 3:1 | 60 | 300 | 1.41 | 13.86 | 85 | 100 | 26.34 | 335 | 2462 | 2.40 | 11.09 | 41 | 1812 | 0.39 |
| 15 | 60% | 4:1 | 30 | 600 | 1.37 | 13.22 | 83 | 94 | 30.64 | 415 | 2529 | 2.65 | 10.71 | 49 | 2036 | 0.40 |
| 16 | 60% | 5:1 | 5 | 500 | 1.02 | 12.98 | 98 | 116 | 26.45 | 615 | 3483 | 4.10 | 10.28 | 51 | 2054 | 0.41 |
| Evaluation Index | Value Name | A Solid Content (%) | B PG/B Ratio | C Stirring Time (h) | D Stirring Speed (rpm) |
|---|---|---|---|---|---|
| UCS (MPa) | k1 | 0.79 | 1.68 | 0.93 | 1.01 |
| k2 | 1.04 | 1.08 | 1.17 | 1.21 | |
| k3 | 1.28 | 0.99 | 1.25 | 1.13 | |
| k4 | 1.52 | 0.88 | 1.27 | 1.28 | |
| Rj | 0.73 | 0.80 | 0.33 | 0.27 | |
| Ranking | B > A > C > D | ||||
| IST (h) | k1 | 97 | 79 | 94 | 92 |
| k2 | 92 | 93 | 90 | 89 | |
| k3 | 88 | 94 | 89 | 92 | |
| k4 | 85 | 97 | 89 | 89 | |
| Rj | 12 | 18 | 5 | 3 | |
| Ranking | B > A > C > D | ||||
| FST (h) | k1 | 116 | 93 | 112 | 109 |
| k2 | 106 | 106 | 107 | 104 | |
| k3 | 105 | 109 | 104 | 107 | |
| k4 | 99 | 118 | 103 | 105 | |
| Rj | 17 | 25 | 9 | 5 | |
| Ranking | B > A > C > D | ||||
| Bleeding rate (%) | k1 | 41.05 | 35.97 | 33.64 | 35.62 |
| k2 | 40.13 | 34.41 | 34.19 | 34.34 | |
| k3 | 35.61 | 38.28 | 38.13 | 37.99 | |
| k4 | 27.76 | 35.88 | 38.59 | 36.60 | |
| Rj | 13.29 | 3.87 | 4.95 | 3.65 | |
| Ranking | A > C > B > D |
| Evaluation Index | Value Name | A | B | C | D |
|---|---|---|---|---|---|
| F− (mg/L) | k1 | 315 | 286 | 384 | 373 |
| k2 | 363 | 299 | 376 | 371 | |
| k3 | 383 | 357 | 364 | 370 | |
| k4 | 418 | 537 | 356 | 365 | |
| Rj | 103 | 251 | 28 | 8 | |
| Ranking | B > A > C > D | ||||
| SO42− (mg/L) | k1 | 2443 | 2300 | 2610 | 2574 |
| k2 | 2456 | 2332 | 2574 | 2559 | |
| k3 | 2591 | 2472 | 2530 | 2530 | |
| k4 | 2717 | 3102 | 2492 | 2543 | |
| Rj | 274 | 802 | 118 | 44 | |
| Ranking | B > A > C > D | ||||
| PO43− (mg/L) | k1 | 2.96 | 2.61 | 2.81 | 2.71 |
| k2 | 2.30 | 2.50 | 2.49 | 2.34 | |
| k3 | 2.76 | 3.29 | 3.06 | 3.50 | |
| k4 | 2.98 | 2.60 | 2.64 | 2.45 | |
| Rj | 0.68 | 0.79 | 0.58 | 1.16 | |
| Ranking | D > B > A > C |
| Trial No. | pH | |||||||
|---|---|---|---|---|---|---|---|---|
| Period 1 (0.25 d) | Period 2 (1 d) | Period 3 (2.25 d) | Period 4 (4 d) | Period 5 (9 d) | Period 6 (16 d) | Period 7 (36 d) | Period 8 (64 d) | |
| 1 | 8.81 | 9.87 | 10.28 | 10.19 | 10.80 | 11.60 | 11.27 | 10.62 |
| 2 | 9.20 | 10.17 | 10.27 | 10.61 | 10.89 | 11.74 | 11.40 | 10.75 |
| 3 | 9.44 | 9.97 | 10.57 | 10.42 | 10.63 | 11.43 | 11.12 | 10.46 |
| 4 | 9.49 | 9.44 | 9.88 | 9.87 | 10.48 | 11.08 | 10.75 | 10.13 |
| 5 | 10.47 | 10.06 | 10.54 | 10.45 | 10.85 | 11.82 | 11.64 | 11.18 |
| 6 | 8.98 | 9.27 | 10.63 | 10.60 | 10.64 | 11.64 | 11.59 | 11.26 |
| 7 | 8.77 | 8.91 | 9.95 | 8.82 | 10.29 | 11.27 | 10.92 | 10.71 |
| 8 | 8.65 | 7.84 | 9.05 | 8.61 | 9.58 | 10.17 | 10.18 | 10.08 |
| 9 | 9.27 | 9.63 | 10.33 | 10.03 | 10.68 | 11.68 | 11.60 | 11.40 |
| 10 | 9.02 | 9.93 | 10.19 | 10.22 | 10.64 | 11.70 | 11.42 | 11.02 |
| 11 | 8.87 | 8.66 | 9.21 | 9.26 | 10.33 | 11.27 | 10.86 | 10.51 |
| 12 | 8.40 | 9.53 | 9.06 | 8.71 | 10.03 | 10.76 | 10.46 | 10.35 |
| 13 | 9.88 | 9.32 | 9.83 | 10.37 | 10.60 | 11.75 | 11.47 | 11.52 |
| 14 | 8.08 | 9.46 | 9.55 | 9.28 | 10.50 | 11.37 | 11.13 | 11.09 |
| 15 | 8.01 | 8.29 | 9.25 | 9.28 | 10.37 | 11.01 | 10.87 | 10.71 |
| 16 | 8.94 | 8.48 | 9.10 | 8.85 | 9.77 | 10.42 | 10.34 | 10.28 |
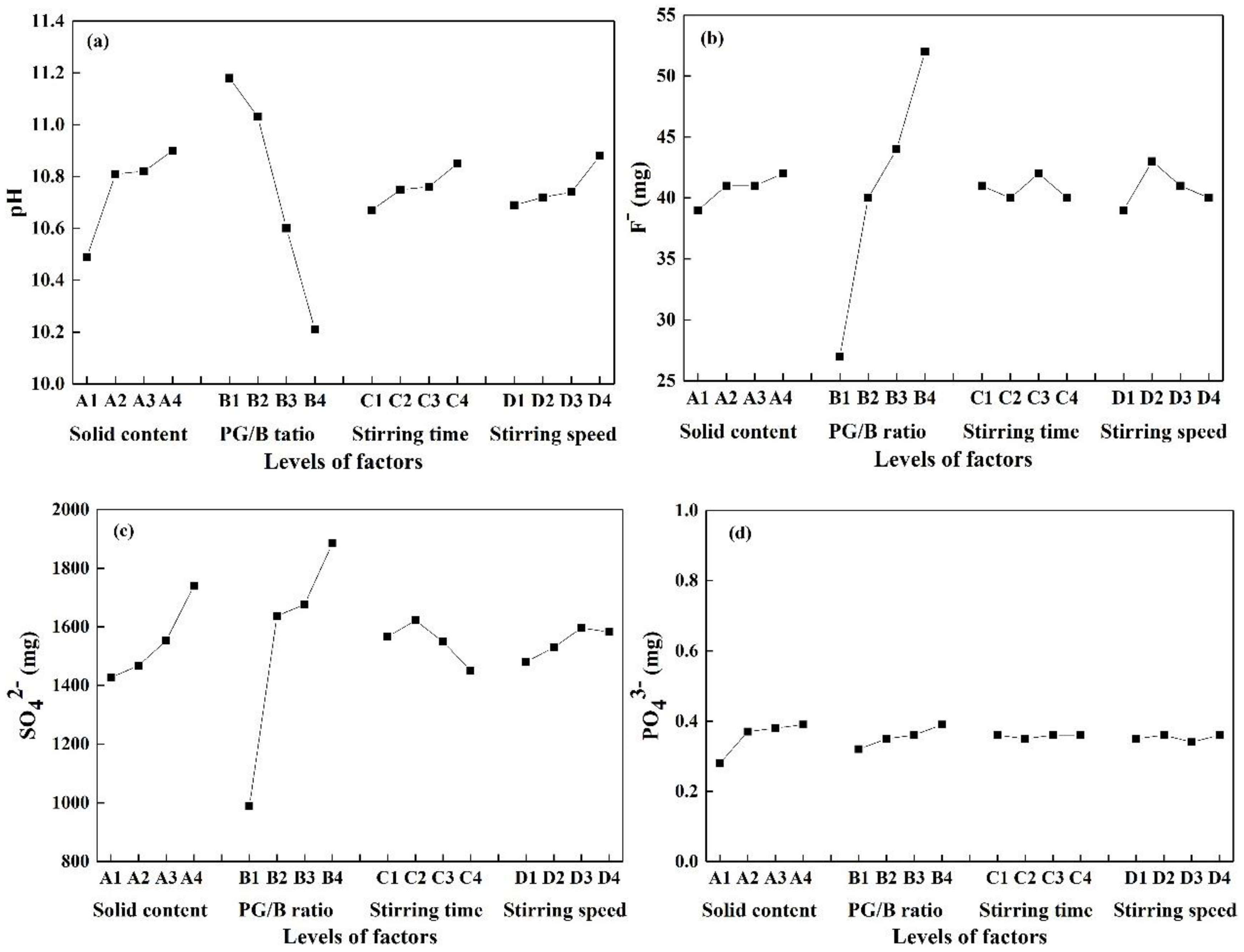
| Evaluation Index | Value Name | A | B | C | D |
|---|---|---|---|---|---|
| pH | k1 | 10.49 | 11.18 | 10.67 | 10.69 |
| k2 | 10.81 | 11.03 | 10.75 | 10.72 | |
| k3 | 10.82 | 10.60 | 10.76 | 10.74 | |
| k4 | 10.90 | 10.21 | 10.85 | 10.88 | |
| Rj | 0.41 | 0.97 | 0.18 | 0.18 | |
| Ranking | B > A > C > D | ||||
| F− (mg) | k1 | 39 | 27 | 41 | 39 |
| k2 | 41 | 40 | 40 | 43 | |
| k3 | 41 | 44 | 42 | 41 | |
| k4 | 42 | 52 | 40 | 40 | |
| Rj | 3 | 25 | 2 | 4 | |
| Ranking | B > D > A > C | ||||
| SO42− (mg) | k1 | 1427 | 989 | 1566 | 1480 |
| k2 | 1468 | 1637 | 1622 | 1530 | |
| k3 | 1553 | 1677 | 1549 | 1596 | |
| k4 | 1741 | 1885 | 1451 | 1582 | |
| Rj | 314 | 896 | 171 | 116 | |
| Ranking | B > A > C > D | ||||
| PO43− (mg) | k1 | 0.28 | 0.32 | 0.36 | 0.35 |
| k2 | 0.37 | 0.35 | 0.35 | 0.36 | |
| k3 | 0.38 | 0.36 | 0.36 | 0.34 | |
| k4 | 0.39 | 0.39 | 0.36 | 0.36 | |
| Rj | 0.11 | 0.07 | 0.01 | 0.02 | |
| Ranking | A > B > D > C |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, Y.; Zhu, Q.; Zhou, S.; Min, C.; Shi, Y. Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment. Minerals 2019, 9, 31. https://doi.org/10.3390/min9010031
Li X, Zhou Y, Zhu Q, Zhou S, Min C, Shi Y. Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment. Minerals. 2019; 9(1):31. https://doi.org/10.3390/min9010031
Chicago/Turabian StyleLi, Xibing, Yanan Zhou, Quanqi Zhu, Shitong Zhou, Chendi Min, and Ying Shi. 2019. "Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment" Minerals 9, no. 1: 31. https://doi.org/10.3390/min9010031
APA StyleLi, X., Zhou, Y., Zhu, Q., Zhou, S., Min, C., & Shi, Y. (2019). Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment. Minerals, 9(1), 31. https://doi.org/10.3390/min9010031