Formation Sequence of Different Spinel Species in Megacrystalline Peridotites of the Udachnaya-East Kimberlite Pipe (Yakutia): Evidence for the Metasomatism of Depleted Mantle
Abstract
:1. Introduction
2. Methods and Samples
- (1)
- (2)
- Spinel from the kelyphitic rims around pyrope [19]: Rim1 (Al-orthopyroxene + spinel ± phlogopite ± Al-clinopyroxene ± amphibole ± sodalite, calcite, potash feldspar, magnetite and sulfides, including djerfisherite) between garnet and olivine, but closer to garnet; Rim2 (phlogopite border of the Rim1 ± Al-orthopyroxene, spinel, and Al-clinopyroxene) between garnet and olivine, but closer to olivine; Rim3 (phlogopite + spinel + magnetite) between garnet and kimberlite (Figure 1f and Figure 2c,d ).
- (3)
- Inclusions of spinel in pentlandite, bordered with djerfisherite. The sulfides are observed in randomly throughout of olivine in megacrystalline dunite. The spinel inclusions are present in the central and marginal parts of sulfides (Figure 2e,f).
- (4)
- Spinel with a wide range of compositions from olivine microcracks, filled by low-iron serpentine in association with calcite, apatite, ilmenite, high-magnesia Ba-containing phlogopite, magnetite, perovskite, and sulfides (Figure 2b).
3. Results
3.1. Spinel Compositions
3.2. Spinel Thermometry and Barometry
4. Discussion
5. Conclusions
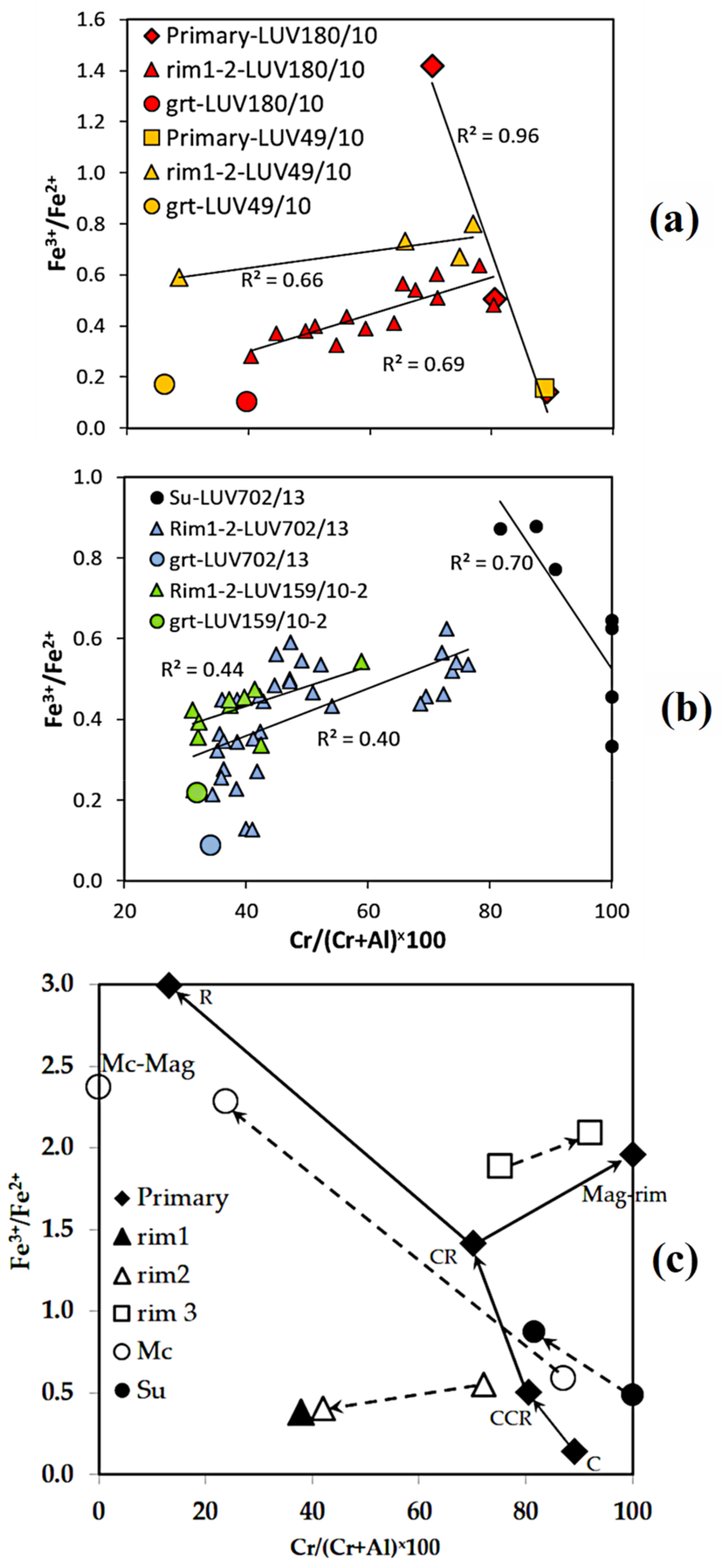
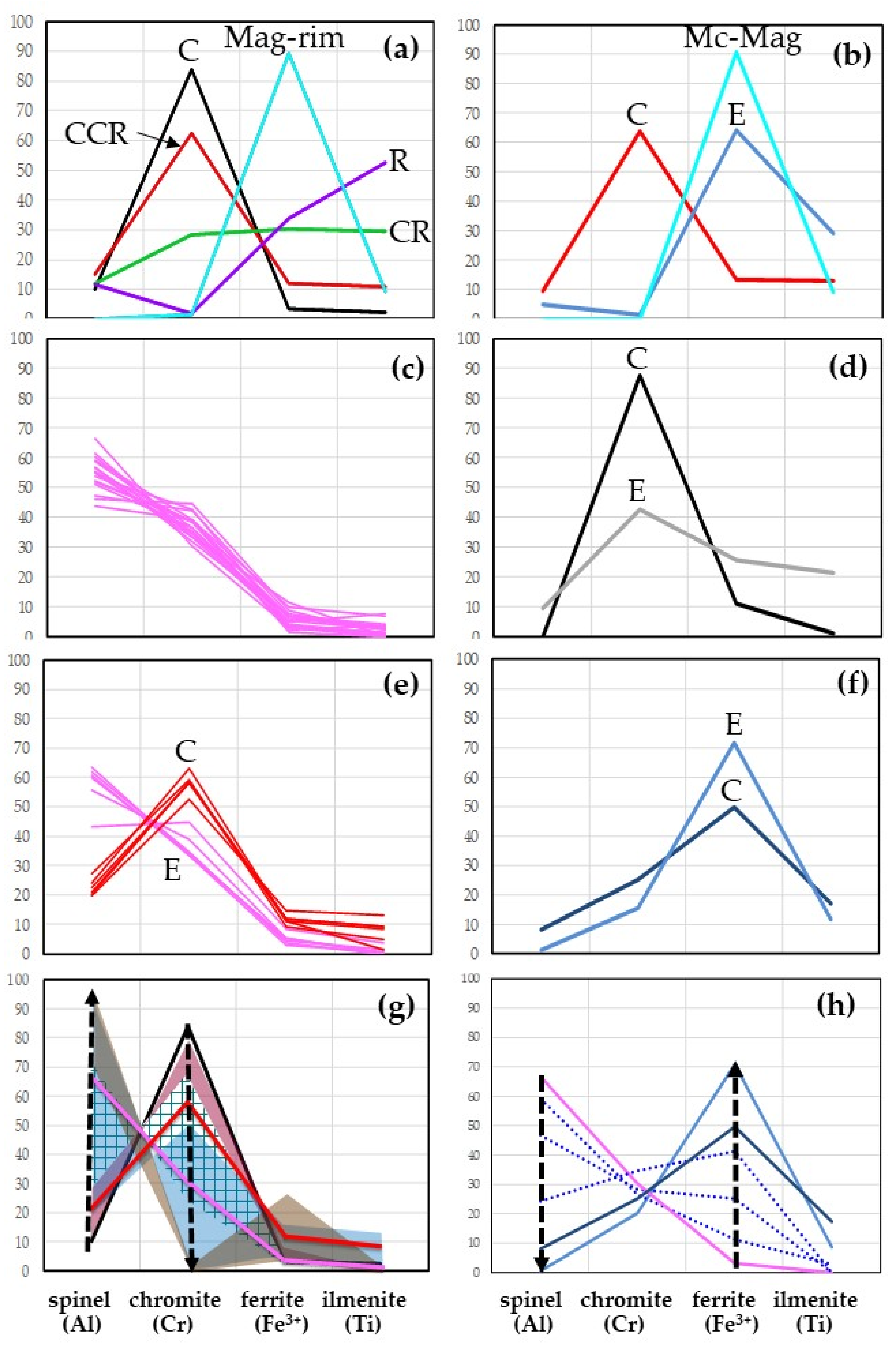
- In the depleted mantle, sulfides are formed under strongly reducing conditions; however, as the comparison of the Fe3+# ratio in chromite from the central part of sulfide and in the core of primary chromite from MHD shows, the latter is formed in an even more reduced environment.
- The enrichment of the depleted mantle rocks by Ti and Fe occurred more than once. The stages of enrichment had different length, intensity, and source. The first is most likely because of ancient mantle metasomatism, the last is associated with kimberlite activity.
- The formation of standard kelyphitic rims (Rim1–2) around the peridotitic garnets took place between these stages, before the xenoliths were captured by kimberlite magma. The transformation of Rim1 with the replacement of spinel by magnetite, and silicate minerals by phlogopite (Rim3) was influenced by kimberlite melts.
- Along with the traditional trends reflecting the change in the composition of spinel from chromite to magnetite (kimberlite and Fe–Ti trends) and from Chromite to Spinel (Cr–Al trend), for mantle xenoliths from kimberlite, the Al–Fe3+ trend should be taken into account as inherent in spinel compositions of eclogites, as well as, apparently, playing a key role in the transformation of the rims around the garnet (Rim1 → Rim3).
Funding
Acknowledgments
Conflicts of Interest
References
- Irvine, T.N. Chromian Spinel as a Petrogenetic Indicator: Part 1. Theory. Can. J. Earth Sci. 1965, 2, 648–672. [Google Scholar] [CrossRef]
- Irvine, T.N. Chromian Spinel as a Petrogenetic Indicator: Part 2. Petrologic applications. Can. J. Earth Sci. 1967, 4, 71–103. [Google Scholar] [CrossRef]
- Sack, R.O.; Ghiorso, M.S. Chromian Spinels as Petrogenetic Indicators Thermodynamics and Petrological Applications. Am. Mineral. 1991, 76, 827–847. [Google Scholar]
- Arai, S. Chemistry of chromian spinel in volcanic rocks as a potential guide to magma chemistry. Mineral. Magzine 1992, 56, 173–184. [Google Scholar] [CrossRef] [Green Version]
- Power, M.R.; Pirrie, D.; Andersen, J.C.Ø.; Wheeler, P.D. Testing the validity of chrome spinel chemistry as a provenance and petrogenetic indicator. Geology 2000, 28, 1027–1030. [Google Scholar] [CrossRef]
- Barnes, S.J.; Roeder, P.L. The range of spinel compositions in terrestrial mafic and ultramafic rocks. J. Petrol. 2001, 42, 2279–2302. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Sobolev, N.V.; Lavrent’ev, Y.G. Xenoliths of diamondiferous ultramafic rocks from Yakutian kimberlites. In Proceedings of the 2nd International Kimberlite Conference, Santa Fe, NM, USA, 3–7 October 1977. [Google Scholar]
- Sobolev, N.V.; Pokhilenko, N.P.; Efimova, E.S. Diamond-bearing peridotite xenoliths in kimberlites and the problem of the origin of diamonds. Geol. Geofiz. (Rus. Geol. Geophys.) 1984, 12, 63–80. [Google Scholar]
- Pokhilenko, N.P.; Pearson, D.G.; Boyd, F.R.; Sobolev, N.V. Megacrystalline Dunites and Peridotites: Hosts for Siberian Diamonds; Annual Report of Director Geophysical Laboratory; Carnegie Institution: Washington, DC, USA, 1991; pp. 11–18. [Google Scholar]
- Pokhilenko, N.P.; Sobolev, N.V. Xenoliths of diamondiferous peridotites from Udachnaya kimberlite pipe, Yakutia. In Proceedings of the of the 4th International Kimberlite Conference, Perth, Australia, 11–15 August 1986; pp. 309–311. [Google Scholar]
- Pokhilenko, N.P.; Sobolev, N.V.; Boyd, F.R.; Pearson, D.G.; Shimizu, N. Megacrystalline pyrope peridotites in the lithosphere of the Siberian Platform: Mineralogy, geochemical peculiarities and the problem of their origin. Russ. Geol. Geophys. 1993, 34, 71–84. [Google Scholar]
- Pokhilenko, L.N.; Mal’kovets, V.G.; Kuz’min, D.V.; Pokhilenko, N.P. New Data on the Mineralogy of Megacrystalline Pyrope Peridotite from the Udachnaya Kimberlite Pipe, Siberian Craton, Yakutian Diamondiferous Province. Dokl. Earth Sci. 2014, 454, 179–184. [Google Scholar] [CrossRef]
- McCammon, C.A.; Griffin, W.L.; Shee, S.R.; O’Neill, H.S.C. Oxidation during metasomatism in ultramafic xenoliths from the Wesselton kimberlite. South Africa: Implications for the survival of diamond. Contrib. Miner. Petrol. 2001, 141, 287–296. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Kadik, A.A.; Sobolev, N.V.; Zharkova, Y.V.; Pokhilenko, N.P. Redox conditions of formation of diamond-bearing peridotite xenoliths in the Udachnaya kimberlite pipe, Yakutia. Geochem. Int. 1990, 27, 41–53. [Google Scholar]
- Kadik, A.A. Evolution of Earth’s redox state during upwelling of carbon-bearing mantle. Phys. Earth Planet. Inter. 1997, 100, 157–166. [Google Scholar] [CrossRef]
- Pokhilenko, L.N. Features of the Fluid Regime of the Lithospheric Mantle of the Siberian Platform (on Xenoliths of Deep Rocks in Kimberlites). Ph.D. Thesis, Institute of the Earth’s Crust, SB RAS, Irkutsk, Russia, 3 October 2006. (In Russian). [Google Scholar]
- Pokhilenko, L.N.; Pokhilenko, N.P.; Fedorov, I.I.; Tomilenko, A.A.; Usova, L.V.; Fomina, L.N. Fluid regime peculiarities of the lithosphere mantle of the Siberian platform. In Deep-seated Magmatism, Its Sources and Plumes. In Proceedings of the VIII Interntional Conference, Vladivostok, Russia, 2–6 September 2008; Publishing House of the Institute of Geography SB RAS: Irkutsk, Russia, 2008; Volume 1, pp. 122–129, ISBN 978-5-94797-130-9. [Google Scholar]
- Pokhilenko, L.N. Particularities of the reaction rims around garnets from the mantle xenoliths of different parageneses (by the example of basic and ultrabasic rocks from Udachnaya kimberlite pipe, Yakutia). In Proceedings of the XXIX International Conference «Ore Potential of Alkaline, Kimberlite and Carbonatite Magmatism» School “Alkaline magmatism of the Earth”, Sudak-Moscow, Ukraine-Russia, 14–22 September 2012; pp. 79–82. [Google Scholar]
- Pokhilenko, L.N. Mantle metasomatism of megacrystalline peridotites: Chromspinelide and phlogopite from the xenoliths of Udachnaya kimberlite pipe (Yakutia). In Proceedings of the 6th Orogenic Lherzolite Conference, Marrakech, Morocco, 4–15 May 2014. [Google Scholar]
- Pokhilenko, L.N. Spinelide from the xenoliths of megacrystalline peridotites of Udachnaya kimberlite pipe (Yakutia). In Proceedings of the XXXIV International Conference “Magmatism of the Earth and related strategic metal deposits”, Miass, Russia, 4–9 August 2017; Zaitsev, V.A., Ermolaeva, V.N., Eds.; GEOKHI RAS: Moscow, Russia, 2017; pp. 182–184. [Google Scholar]
- Korolyuk, V.N.; Lavrent’ev, Y.G.; Usova, L.V.; Nigmatulina, E.N. JXA-8100 microanalyzer: Accuracy of analysis of rock-forming minerals. Russ. Geol. Geophys. 2008, 49, 165–168. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Korolyuk, V.N.; Usova, L.V.; Nigmatulina, E.N. Electron probe microanalysis of rock-forming minerals with JXA-8100 electron probe microanalyzer. Russ. Geol. Geophys. 2015, 56, 1428–1436. [Google Scholar] [CrossRef]
- Korolyuk, V.N.; Pokhilenko, L.N. Electron probe determination of trace elements in olivine. X-Ray Spectrom. 2014, 43, 353–358. [Google Scholar] [CrossRef]
- Roeder, P.L. Chromite: From the fiery rain of chondrules to the Kilauea Iki Lava lake. Can. Mineral. 1994, 32, 729–746. [Google Scholar]
- Arai, S. Characterization of spinel peridotites by olivine-spinel compositional relationships: Review and interpretation. Chem. Geol. 1994, 113, 191–204. [Google Scholar] [CrossRef]
- Wood, B.J.; Virgo, D. Upper mantle oxidation state: Ferric iron contents of lherzolite spinels by 57Fe Mössbauer spectroscopy and resultant oxygen fugacities. Geochim. Cosmochim. Acta 1989, 53, 1277–1291. [Google Scholar] [CrossRef]
- Wood, B.J. An experimental test of the spinel peridotite oxygen barometer. J. Geophys. Res.-Solid Earth 1990, 95, 15845–15851. [Google Scholar] [CrossRef]
- Wood, B.J.; Bryndzia, L.T.; Johnson, K.E. Mantle oxidation state and its relationship to tectonic environment and fluid speciation. Science 1990, 248, 337–345. [Google Scholar] [CrossRef]
- Carmichael, I.S. The Redox States of Basic and Silicic Magmas: A Reflection of Their Source Regions? Contrib. Mineral. Petrol. 1989, 106, 129–141. [Google Scholar] [CrossRef]
- O’Neill, H.S.C.; Wood, B.J. An experimental study of Fe-Mg partitioning between garnet and olivine and its calibration as a geothermometer. Contrib. Mineral. Petrol. 1979, 70, 59–70. [Google Scholar] [CrossRef]
- MacGregor, I.D. The system MgO-Al2O3-SiO2: Solubility of Al2O3 in enstatite for spinel and garnet peridotite compositions. Am. Mineral. 1974, 59, 110–119. [Google Scholar]
- Finnerty, A.A.; Rigden, S.M. Olivine barometry: Application to pressure estimation for terrestrial and lunar rocks. Lunar Planet. Sci. Conf. 1981, 12, 279–281. [Google Scholar]
- Ryan, C.G.; Griffin, W.L.; Pearson, N.J. Garnet geotherms: Pressure-temperature data from Cr-pyrope garnet xenocrysts in volcanic rocks. J. Geophys. Res. 1996, 101, 5611–5625. [Google Scholar] [CrossRef]
- Canil, D. The Ni-in-garnet geothermometer: Calibration at natural abundances. Contrib. Mineral. Petrol. 1999, 136, 240–246. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Malinovsky, I.Y.; Doroshev, A.M.; Ran, E.N. Stability of chrome-containing garnets of the pyrope-knorringite series. Experimental studies on mineralogy (1974–1975). Bull. Inst. Geol. Geophys. Novosib. Russ. 1975, 110–115. [Google Scholar]
- Brey, G.P.; Doroshev, A.M.; Kogarko, L.N. The Join Pyrope-Knorringite: Experimental Constraints for a New Geothermobarometer for Coexisting Garnet and Spinel. Contrib. Mineral. Petrol. 1999, 136, 240–246. [Google Scholar]
- Grütter, H.S.; Latti, D.; Menzies, A. Cr-saturation arrays in concentrate garnet compositions from kimberlite and their use in mantle barometry. J. Petrol. 2006, 47, 801–820. [Google Scholar] [CrossRef]
- Wan, Z.; Coogan, L.A.; Canil, D. Experimental calibration of aluminum partitioning between olivine and spinel as a geothermometer. Am. Mineral. 2008, 93, 1142–1147. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The Equilibrium Boundary Between Graphite and Diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef]
- Howarth, G.H.; Barry, P.H.; Pernet-Fisher, J.F.; Baziotis, I.P.; Pokhilenko, N.P.; Pokhilenko, L.N.; Bodnar, R.J.; Taylor, L.A.; Agashev, A.M. Superplume metasomatism: Evidence from Siberian mantle xenoliths. Lithos 2014, 184–185, 209–224. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Agashev, A.M.; Litasov, K.D.; Pokhilenko, L.N. Carbonatite metasomatism of peridotite lithospheric mantle: Implications for diamond formation and carbonatite-kimberlite magmatism. Russ. Geol. Geophys. 2015, 56, 280–295. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Sobolev, N.V.; Kuligin, S.S.; Shimizu, N. Peculiarities of distribution of pyroxenite paragenesis garnets in Yakutian kimberlite and some aspects of the Evolution of the Siberian Craton lithospheric mantle. In Proceedings of the 7th International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; Gurney, J.J., et al., Eds.; Red Roof Design: Cape Town, South Africa, 1999; Volume 2, pp. 689–698. [Google Scholar]
- Yudin, D.S.; Tomilenko, A.A.; Alifirova, T.A.; Travin, A.V.; Murzintsev, N.G.; Pokhilenko, N.P. Results of 40Ar/39Ar Dating of Phlogopites from Kelyphitic Rims around Garnet Grains (Udachnaya-Vostochnaya Kimberlite Pipe). Dokl. Earth Sci. 2016, 469, 728–731. [Google Scholar] [CrossRef]




| Sample | 2/09 * | 49/10 * | 164/09 * | 43/01 * | 588/11 * | 180/10 | 180/10 | 180/10 | 180/10 | 180/10 |
|---|---|---|---|---|---|---|---|---|---|---|
| C | C | C | C | C | C | CCR | CR | R | Mag-rim | |
| TiO2 | 0.25 | 0.08 | 0.11 | 0.36 | 0.11 | 0.92 | 4.46 | 13.4 | 22.24 | 3.55 |
| Al2O3 | 5.06 | 5.64 | 8.02 | 4.13 | 4.89 | 5.21 | 8.03 | 6.94 | 6.27 | 0.00 |
| Cr2O3 | 65.1 | 65.78 | 63.2 | 64.2 | 65.3 | 63.7 | 49.5 | 24.4 | 1.42 | 1.00 |
| FeO tot | 18.1 | 16.46 | 15.4 | 17.2 | 18.0 | 19.8 | 26.7 | 42.2 | 51.6 | 93.1 |
| MnO | 0.27 | 0.212 | 0.24 | 0.28 | 0.28 | 0.00 | 0.00 | 0.00 | 1.86 | 1.33 |
| MgO | 11.5 | 12.21 | 13.0 | 11.9 | 11.4 | 10.4 | 11.1 | 13.1 | 16.62 | 1.0 |
| Total | 100.0 | 100.4 | 99.9 | 98.1 | 100.0 | 100.0 | 99.7 | 100.0 | 100.0 | 100.0 |
| Fe3+/Fe2+ | 0.19 | 0.16 | 0.16 | 0.25 | 0.19 | 0.14 | 0.50 | 1.42 | 3.00 | 1.96 |
| Cr/(Cr + Al) | 89.61 | 88.67 | 84.10 | 91.26 | 89.96 | 89.13 | 80.51 | 70.22 | 13.19 | 100.0 |
| Fe2+/(Fe2+ + Mg) | 0.43 | 0.40 | 0.36 | 0.39 | 0.43 | 0.48 | 0.47 | 0.43 | 0.30 | 0.95 |
| Fe3+/(Fe3+ + Al + Cr) | 0.04 | 0.03 | 0.03 | 0.05 | 0.04 | 0.04 | 0.13 | 0.43 | 0.79 | 0.98 |
| Sample | avg 21 * | avg 15 * | avg 24 * | 702/13 | 702/13 | 702/13 | 702/13 | 702/13 | 702/13 | 702/13 |
| Rim1 | Rim2-C | Rim2-E | Rim3-C | Rim3-E | Su-C | Su-E | Mc-C | Mc-E | Mc-Mag | |
| TiO2 | 0.38 | 3.38 | 0.52 | 7.24 | 4.64 | 0.44 | 9.05 | 5.46 | 12.72 | 3.58 |
| Al2O3 | 37.95 | 12.12 | 32.62 | 4.37 | 0.66 | 0.00 | 5.15 | 5.08 | 2.78 | 0.00 |
| Cr2O3 | 27.38 | 46.52 | 32.08 | 19.94 | 11.72 | 64.08 | 34.04 | 50.67 | 1.3 | 0.00 |
| FeO tot | 14.79 | 23.43 | 16.17 | 57.28 | 75.61 | 23.52 | 41.7 | 27.18 | 72.45 | 91.15 |
| MnO | 0.37 | 0.48 | 0.39 | 1.71 | 1.58 | 0.00 | 1.42 | 0.00 | 1.69 | 0.6 |
| MgO | 17.69 | 12.39 | 16.47 | 9.06 | 5.42 | 10.48 | 7.78 | 11.42 | 8.65 | 4.27 |
| Total | 98.56 | 98.31 | 98.24 | 99.60 | 99.63 | 98.52 | 99.14 | 99.81 | 99.59 | 99.60 |
| Fe3+/Fe2+ | 0.43 | 0.54 | 0.43 | 1.89 | 2.09 | 0.48 | 0.87 | 0.59 | 2.28 | 2.37 |
| Cr/(Cr + Al) | 0.33 | 0.72 | 0.40 | 0.75 | 0.92 | 1.00 | 0.82 | 0.87 | 0.24 | 0.00 |
| Fe2+/(Fe2+ + Mg) | 0.25 | 0.41 | 0.28 | 0.55 | 0.72 | 0.46 | 0.62 | 0.46 | 0.59 | 0.78 |
| Fe3+/(Fe3+ + Al + Cr) | 0.05 | 0.12 | 0.06 | 0.60 | 0.81 | 0.11 | 0.33 | 0.15 | 0.91 | 1.00 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokhilenko, L. Formation Sequence of Different Spinel Species in Megacrystalline Peridotites of the Udachnaya-East Kimberlite Pipe (Yakutia): Evidence for the Metasomatism of Depleted Mantle. Minerals 2019, 9, 607. https://doi.org/10.3390/min9100607
Pokhilenko L. Formation Sequence of Different Spinel Species in Megacrystalline Peridotites of the Udachnaya-East Kimberlite Pipe (Yakutia): Evidence for the Metasomatism of Depleted Mantle. Minerals. 2019; 9(10):607. https://doi.org/10.3390/min9100607
Chicago/Turabian StylePokhilenko, Lyudmila. 2019. "Formation Sequence of Different Spinel Species in Megacrystalline Peridotites of the Udachnaya-East Kimberlite Pipe (Yakutia): Evidence for the Metasomatism of Depleted Mantle" Minerals 9, no. 10: 607. https://doi.org/10.3390/min9100607





