Mixed-Habit Type Ib-IaA Diamond from an Udachnaya Eclogite
Abstract
:1. Introduction
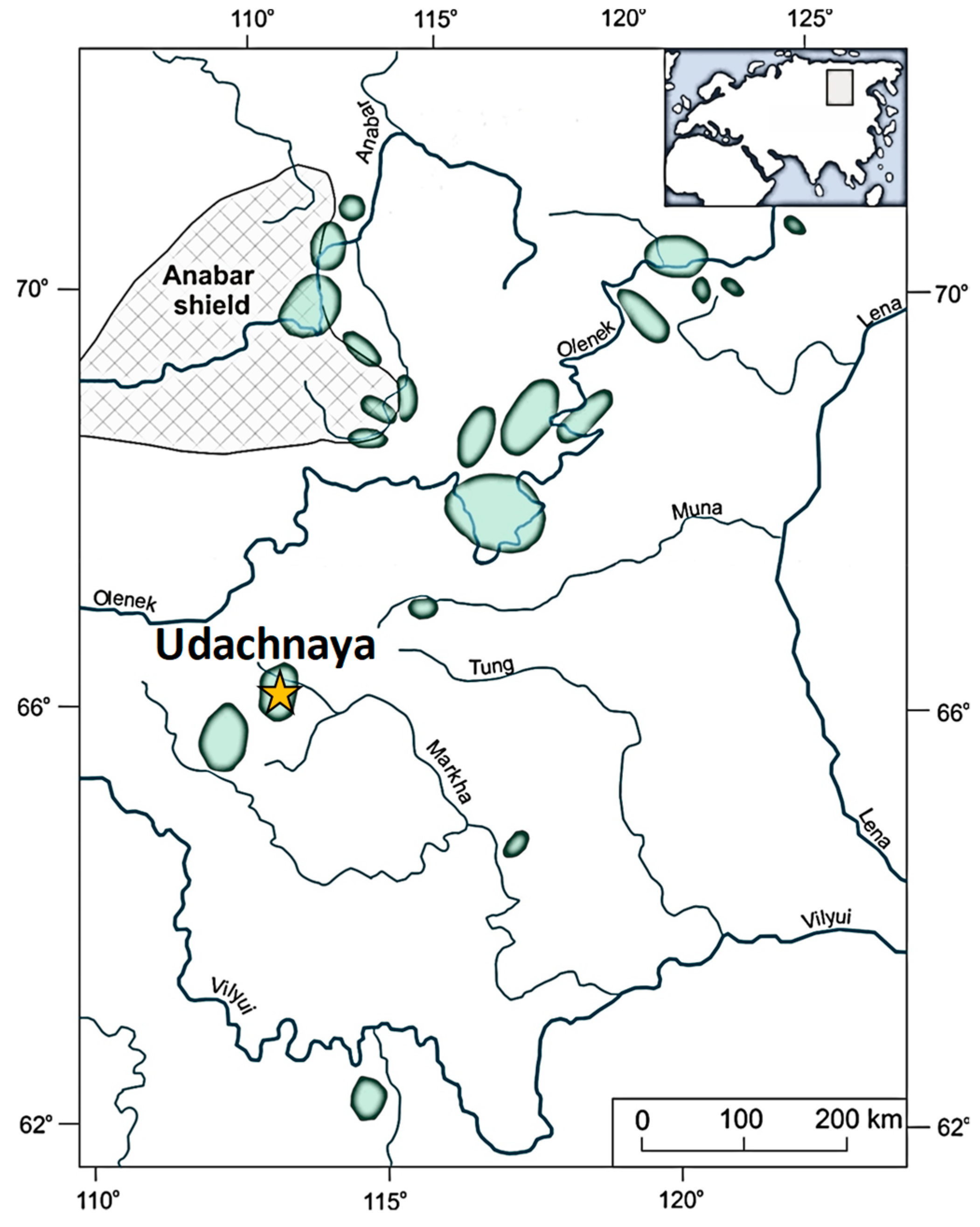
2. Samples and Methods
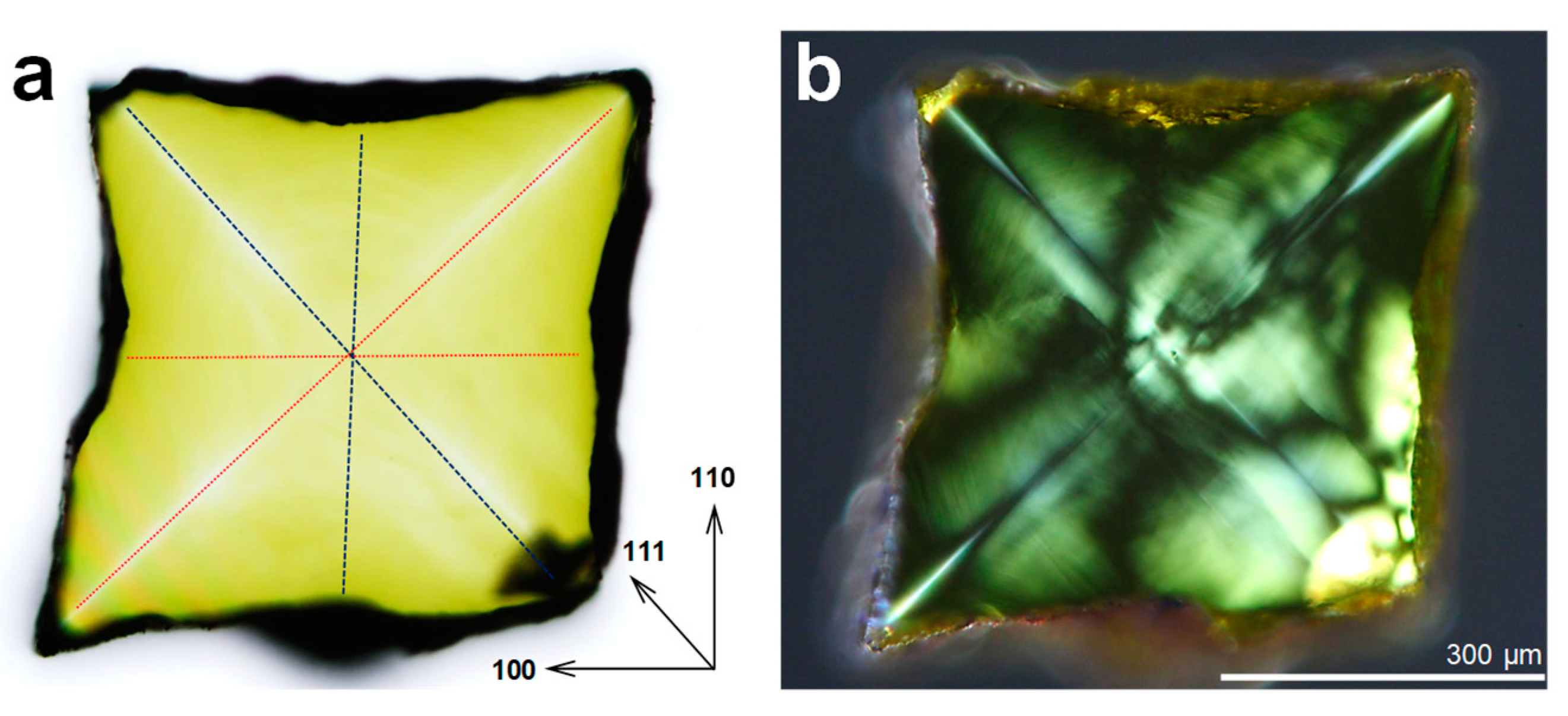
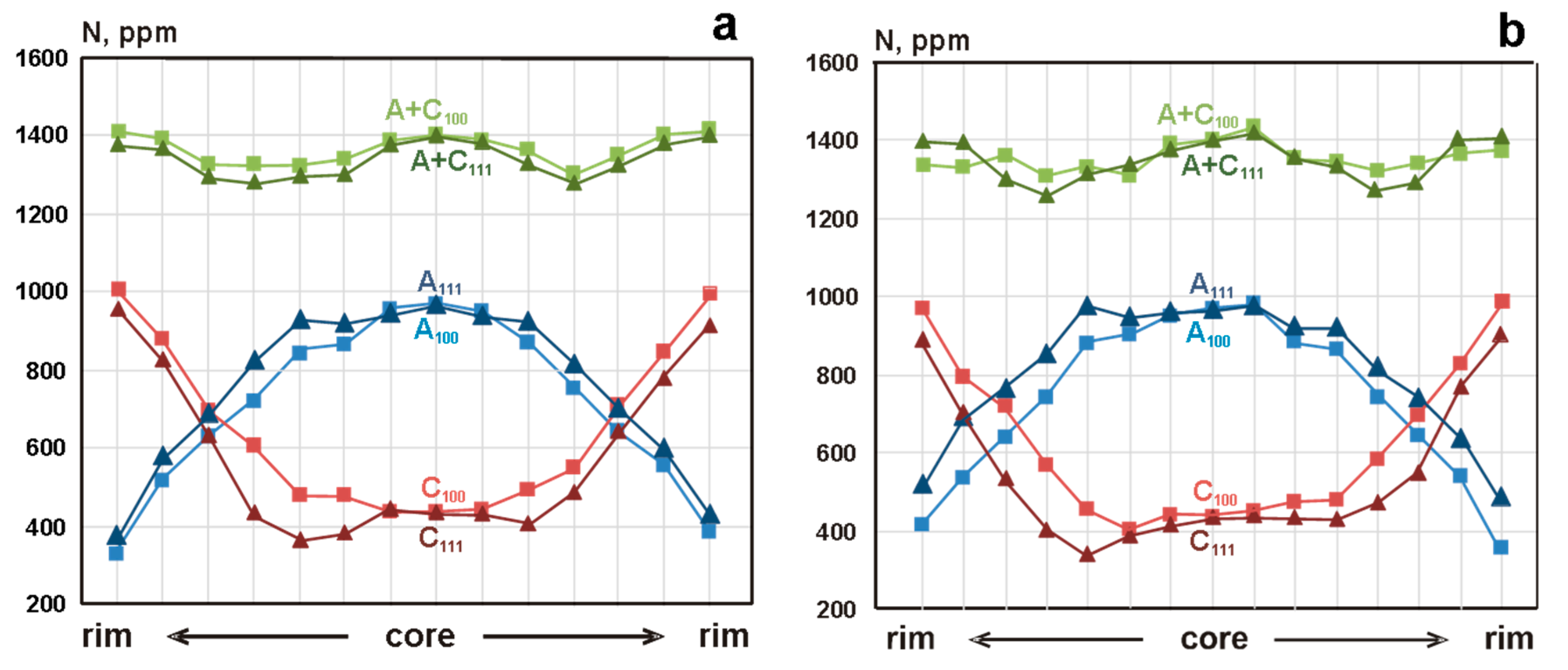
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Cpx (n = 15) | σ | Grt (n = 16) | σ | |
|---|---|---|---|---|
| wt. % | ||||
| SiO2 | 55.2 | 0.30 | 38.9 | 0.10 |
| TiO2 | 0.16 | 0.02 | 0.15 | 0.02 |
| Al2O3 | 8.42 | 0.12 | 21.1 | 0.09 |
| Cr2O3 | 0.03 | 0.01 | 0.03 | 0.01 |
| FeO | 6.57 | 0.11 | 22.9 | 0.18 |
| MnO | 0.04 | 0.01 | 0.45 | 0.01 |
| MgO | 8.89 | 0.11 | 7.42 | 0.13 |
| CaO | 14.8 | 0.10 | 8.90 | 0.20 |
| Na2O | 5.39 | 0.07 | 0.11 | 0.01 |
| K2O | 0.06 | 0.00 | - | - |
| Total | 99.6 | 0.3 | 100.0 | 0.2 |
| f.u. | ||||
| Si | 1.998 | 2.999 | ||
| Ti | 0.004 | 0.009 | ||
| Al | 0.359 | 1.920 | ||
| Cr | 0.001 | 0.002 | ||
| Fe | 0.199 | 1.475 | ||
| Mn | 0.001 | 0.030 | ||
| Mg | 0.480 | 0.853 | ||
| Ca | 0.576 | 0.737 | ||
| Na | 0.379 | 0.016 | ||
| K | 0.003 | - | ||
| Total | 4.005 | 8.040 |
| No. | Sample | δ13C, ‰ |
|---|---|---|
| 1 | Ud-208-02 dia1 | −6.7 |
| 2 | Ud-208-02 dia2 | −6.4 |
| 3 | Ud-208-02 dia3 | −7.2 |
| 4 | Ud-208-02 dia5 | −6.5 |
| 5 | Ud-208-02 dia6 | −6.3 |
| 6 | Ud-208-02 dia8 | −6.9 |
| 7 | Ud-208-02 dia9 | −7.1 |
References
- Sobolev, N.V. Deep-Seated Inclusions in Kimberlites and the Problem of the Composition of the Upper Mantle; AGU: Washington, DC, USA, 1977; p. 279. [Google Scholar]
- Efimova, E.S.; Sobolev, N.V. Abundance of crystalline inclusions in Yakutian diamonds. Dokl. Akad. Nauk SSSR 1977, 237, 1475–1478. [Google Scholar]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Taylor, L.A.; Anand, M. Diamonds: Time capsules from Siberian mantle. Geochemistry 2004, 64, 1–74. [Google Scholar] [CrossRef]
- Anderson, D.L. Theory of the Earth; Blackwell Scientific Publications: Boston, MA, USA, 1989. [Google Scholar]
- Ringwood, A.E.; Green, D.H. An experimental investigation of the gabbro–eclogite transformation and some geophysical implications. Tectonophys 1966, 3, 383–427. [Google Scholar] [CrossRef]
- MacGregor, I.D.; Manton, W.I. Roberts Victor eclogites: Ancient oceanic crust. J. Geophys. Res. 1986, 91, 14063–14079. [Google Scholar] [CrossRef]
- Jacob, D.; Jagoutz, E.; Lowry, D.; Mattey, D.; Kudrjavtseva, G. Diamondiferous eclogites from Siberia: Remnants of Archean oceanic crust. Geochim. Cosmochim. Acta 1994, 58, 5191–5207. [Google Scholar] [CrossRef]
- Pokhilenko, N.P.; Sobolev, N.V.; Yefimova, Y.S. Xenolith of deformed diamond-bearing kyanite eclogite from the Udachnaya pipe, Yakutia. Dokl. Akad. Nauk SSSR 1982, 266, 212–216. [Google Scholar]
- Spetsius, Z.V. Diamondiferous Eclogites from Yakutia: Evidence for a Late and Multistage Formation of Diamonds. In Proceedings of the 6th International Kimberlite Conference, Novosibirsk, Russia, 30 July–19 August 1995; pp. 572–574. [Google Scholar]
- Harlow, G.E. The Nature of Diamonds; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Sobolev, N.V.; Taylor, L.A.; Zuev, V.M.; Bezborodov, S.M.; Snyder, G.A.; Sobolev, V.N.; Yefimova, E.S. The specific features of eclogitic paragenesis of diamonds from Mir and Udachnaya kimberlite pipes (Yakutia). Russ. Geol. Geophys. 1998, 39, 1653–1663. [Google Scholar]
- Shatsky, V.S.; Zedgenizov, D.A.; Ragozin, A.L. Evidence for a subduction component in the diamond-bearing mantle of the Siberian craton. Russ. Geol. Geophys. 2016, 57, 111–126. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Ragozin, A.L.; Zedgenizov, D.A.; Mityukhin, S.I. Evidence for multistage evolution in a xenolith of diamond-bearing eclogite from the Udachnaya kimberlite pipe. Lithos 2008, 105, 289–300. [Google Scholar] [CrossRef]
- Javoy, M.; Pineau, F.; Delorme, H. Carbon and nitrogen isotopes in the mantle. Chem. Geol. 1986, 57, 41–62. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Zedgenizov, D.A.; Ragozin, A.L.; Kalinina, V.V. Carbon isotopes and nitrogen contents in placer diamonds from the NE Siberian craton: Implications for diamond origins. Eur. J. Mineral. 2014, 26, 41–52. [Google Scholar] [CrossRef]
- Galimov, E.M. Isotope fractionation related to kimberlite magmatism and diamond formation. Geochim. Cosmochim. Acta 1991, 55, 1697–1708. [Google Scholar] [CrossRef]
- Cartigny, P. Stable isotopes and origin of diamond. Elements 2005, 1, 79–84. [Google Scholar] [CrossRef]
- Zaitsev, A.M. Optical Properties of Diamond: A Data Handbook; Springer: Berlin/Heidelberg, Germany, 2001; p. 502. [Google Scholar]
- De Weerdt, F.; Pal’yanov, Y.N.; Collins, A.T. Absorption spectra of hydrogen in 13C diamond produced by high-pressure, high-temperature synthesis. J. Phys. Condens. Matter 2003, 15, 3163–3170. [Google Scholar] [CrossRef]
- Evans, T.; Qi, Z.; Maguire, J. The stages of nitrogen aggregation in diamond. J. Phys. C Solid State Phys. 1981, 14, 379. [Google Scholar] [CrossRef]
- Taylor, W.R.; Bulanova, G.; Milledge, H.J. Quantitative nitrogen aggregation study of some Yakutian diamonds: Constraints on the growth, thermal and deformation history of peridotitic and eclogitic diamonds. Int. Kimberl. Conf. Ext. Abstr. 1995, 6, 608–610. [Google Scholar]
- Boyd, F.R.; Pokhilenko, N.P.; Pearson, D.G.; Mertzman, S.A.; Sobolev, N.V.; Finger, L.W. Composition of the Siberian cratonic mantle: Evidence from Udachnaya peridotite xenoliths. Contrib. Mineral. Petrol. 1997, 128, 228–246. [Google Scholar] [CrossRef]
- Tychkov, N.S.; Agashev, A.M.; Malygina, E.V.; Nikolenko, E.I.; Pokhilenko, N.P. Thermal perturbations in the lithospheric mantle as evidenced from P–T equilibrium conditions of xenoliths from the Udachnaya kimberlite pipe. Dokl. Earth Sci. 2014, 454, 84–88. [Google Scholar] [CrossRef]
- Misra, K.C.; Anand, M.; Taylor, L.A.; Sobolev, N.V. Multi-stage metasomatism of diamondiferous eclogite xenoliths from the Udachanay kimberlite pipe, Yakutia, Siberia. Lithos 2004, 146, 696–714. [Google Scholar]
- Coleman, R.G.; Lee, D.E.; Brannock, W.W. Eclogites and eclogites, their difference and similarities. Bull. Geol. Soc. Am. 1965, 76, 483–508. [Google Scholar] [CrossRef]
- Taylor, L.A.; Neal, C.R. Eclogites with oceanic crustal and mantle signature from Bellsbank kimberlite, South Africa, Part I: Mineralogy, petrography, and whole rock chemistry. J. Geol. 1989, 97, 551–567. [Google Scholar] [CrossRef]
- Sobolev, V.N.; Taylor, L.A.; Snyder, G.A.; Sobolev, N.V. Diamondiferous eclogites from the Udachnaya kimberlite pipe, Yakutia. Int. Geol. Rev. 1994, 36, 42–64. [Google Scholar] [CrossRef]
- Ellis, D.J.; Green, D.H. An experimental study of the effect of Ca upon garnet clinopyroxene Fe–Mg exchange equilibria. Contrib. Mineral. Petrol. 1979, 71, 13–22. [Google Scholar] [CrossRef]
- Beyer, C.; Frost, D.J.; Miyajima, N. Experimental calibration of a garnet-clinopyroxene geobarometer for mantle eclogites. Contrib. Mineral. Petrol. 2015, 169, 18. [Google Scholar] [CrossRef]
- Reutsky, V.N.; Borzdov, Y.M.; Palyanov, Y.N. Effect of diamond growth rate on carbon isotope fractionation in Fe–Ni–C system. Diam. Relat. Mater. 2012, 21, 7–10. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Muehlenbachs, K. Sources of carbon in inclusion bearing diamonds. Lithos 2009, 112, 625–637. [Google Scholar] [CrossRef]
- Howell, D. Strain-induced birefringence in natural diamond: A review. Eur. J. Mineral. 2012, 24, 575–585. [Google Scholar] [CrossRef]
- Walmsley, J.C.; Lang, A.R.; Rooney, M.-L.T.; Welbourn, C.M. Newly observed microscopic planar defects on {111} in natural diamond. Philos. Mag. Lett. 1987, 55, 209–213. [Google Scholar] [CrossRef]
- Boyd, S.R.; Kiflawi, I.; Woods, G.S. The relationship between infrared absorption and the A defect concentration in diamond. Philos. Mag. B 1994, 69, 1149–1153. [Google Scholar] [CrossRef]
- Kiflawi, I.A.; Mayer, E.; Spear, P.M.; Woods, G.S. Infrared absorption by the single nitrogen and A defect centres in diamond. Philos. Mag. B 1994, 69, 1141–1147. [Google Scholar] [CrossRef]
- Goss, J.P.; Briddon, P.R.; Hill, V.; Jones, R.; Rayson, M.J. Identification of the structure of the 3107 cm−1 H-related defect in diamond. J. Phys. Condens. Matter 2014, 26, 145801. [Google Scholar] [CrossRef] [PubMed]
- Jerde, E.A.; Taylor, L.A.; Crozaz, G.; Sobolev, N.V.; Sobolev, V.N. Diamondiferous eclogites from Yakutia, Siberia: Evidence for a diversity of protoliths. Contrib. Mineral. Petrol. 1993, 114, 189–202. [Google Scholar] [CrossRef]
- Snyder, G.A.; Jerde, E.A.; Taylor, L.A.; Halliday, A.N.; Sobolev, V.N.; Sobolev, N.V. Nd and Sr isotopes from diamondiferous eclogites, Udachnaya kimberlite pipe, Yakutia, Siberia: Evidence of differentiation in the early Earth? Earth Planet. Sci. Lett. 1993, 118, 91–100. [Google Scholar] [CrossRef]
- Snyder, G.A.; Taylor, L.A.; Jerde, E.A.; Clayton, R.N.; Mayeda, T.K.; Deines, P.; Rossman, G.; Sobolev, N.V. Archean mantle heterogeneity and the origin of diamondiferous eclogites, Siberia: Evidence from stable isotopes and hydroxyl in garnet. Am. Mineral. 1995, 80, 799–809. [Google Scholar] [CrossRef]
- Taylor, L.A.; Keller, R.A.; Snyder, A.; Wang, W.; Carlson, W.D.; Hauri, E.H.; McCandless, T.; Kim, K.; Sobolev, N.V.; Bezborodov, S.M. Diamonds and their mineral inclusions, and what they tell us: A detailed “Pull-Apart”of a diamondiferous eclogite. Int. Geol. Rev. 2000, 42, 959–983. [Google Scholar] [CrossRef]
- Pearson, D.G.; Snyder, G.A.; Shirey, S.B.; Taylor, L.A.; Carlson, R.W.; Sobolev, N.V. Archean Re–Os age for Siberian eclogites and constraints on Archean tectonics. Nature 1995, 374, 711–713. [Google Scholar] [CrossRef]
- Jacob, D.E.; Foley, S.F. Evidence for Archean ocean crust with low high field strength element signature from diamondiferous eclogite xenoliths. Lithos 1999, 48, 317–336. [Google Scholar] [CrossRef]
- Jacob, D.E. Nature and origin of eclogite xenoliths from kimberlites. Lithos 2004, 77, 295–316. [Google Scholar] [CrossRef]
- Ickert, R.B.; Stachel, T.; Stern, R.A.; Harris, J.W. Diamond from recycled crustal carbon documented by coupled δ18O–δ13C measurements of diamonds and theirinclusions. Earth Planet. Sci. Lett. 2013, 364, 85–97. [Google Scholar] [CrossRef]
- Davis, G.L.; Sobolev, N.V.; Kharkiv, A.D. New data on the age of Yakutian kimberlites obtained by the U–Pb method on zircons. Dokl. Akad. Nauk SSSR 1980, 254, 175–179. [Google Scholar]
- Kinny, P.D.; Griffin, B.; Heaman, L.M.; Brakhfogel, F.F.; Spetsius, Z.V. SHRIMP U–Pb ages of perovskite from Yakutian kimberlites. Russ. Geol. Geophys. 1997, 38, 91–99. [Google Scholar]
- Moore, M.; Lang, A.R. On the internal structure of natural diamonds of cubic habit. Philos. Mag. 1972, 26, 1313–1325. [Google Scholar] [CrossRef]
- Sunagawa, I. Growth and morphology of diamond crystals under stable and metastable conditions. J. Cryst. Growth 1990, 99, 1156–1161. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Harte, B. Microscale variotions of δ13C and N content in diamonds with mixed-habit growth. Chem. Geol. 2004, 205, 169–175. [Google Scholar] [CrossRef]
- Howell, D.; Griffin, W.L.; Piazolo, S.; Say, J.M.; Stern, R.A.; Stachel, T.; Nasdala, L.; Rabeau, J.R.; Pearson, N.J.; O’Reilly, S.Y. A spectroscopic and carbon-isotope study of mixed-habit diamonds: Impurity characteristics and growth environment. Am. Mineral. 2013, 98, 66–77. [Google Scholar] [CrossRef]
- Smit, K.V.; Shirey, S.B.; Stern, R.A.; Steele, A.; Wang, W. Diamond growth from C–H–N–O recycled fluids in the lithosphere: Evidence from CH4 micro-inclusions and δ13C–δ15N–N content in Marange mixed-habit diamonds. Lithos 2016, 265, 68–81. [Google Scholar] [CrossRef]
- Skuzovatov, S.Y.; Zedgenizov, D.A.; Rakevich, A.L. Spectroscopic constraints on growth of Siberian mixed-habit diamonds. Contrib. Mineral. Petrol. 2017, 172, 46. [Google Scholar] [CrossRef]
- Smit, K.V.; Shirey, S.B.; Wang, W. Type Ib diamond formation and preservation in the West African lithospheric mantle: Re–Os age constraints from sulphide inclusions in Zimmi diamonds. Precambrian Res. 2016, 286, 152–166. [Google Scholar] [CrossRef]
- Anand, M.; Taylor, L.A.; Misra, K.C.; Carlson, W.D.; Sobolev, N.V. Nature of diamonds in Yakutian eclogites: Views from eclogite tomography and mineral inclusions in diamonds. Lithos 2004, 77, 333–348. [Google Scholar] [CrossRef]
- Taylor, W.R.; Canil, D.; Millendge, H.J. Kinetics of Ib to IaA nitrogen aggregation in diamond. Geochim. Cosmochim. Acta 1996, 60, 4725–4733. [Google Scholar] [CrossRef]
- Evans, T. Changes produced by high temperature treatment of diamond. In The properties of Diamond; Evans, T., Field, J.E., Eds.; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Stepanov, A.; Shatsky, V.; Zedgenizov, D.; Sobolev, N. Causes of variations in morphology and impurities of diamonds from the Udachnaya Pipe eclogite. Russ. Geol. Geophys. 2007, 48, 758–769. [Google Scholar] [CrossRef]
- Collins, A.T. Vacancy enhanced aggregation of nitrogen in diamond. J. Phys. C Solid State Phys. 1980, 13, 2641. [Google Scholar] [CrossRef]
- Smit, K.V.; D’Haenens-Johansson, U.F.S.; Howell, D.; Loudin, L.C.; Wang, W. Deformation-related spectroscopic features in natural Type Ib-IaA diamonds from Zimmi (West African craton). Mineral. Petrol. 2018, 112, 243–257. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedgenizov, D.; Bogush, I.; Shatsky, V.; Kovalchuk, O.; Ragozin, A.; Kalinina, V. Mixed-Habit Type Ib-IaA Diamond from an Udachnaya Eclogite. Minerals 2019, 9, 741. https://doi.org/10.3390/min9120741
Zedgenizov D, Bogush I, Shatsky V, Kovalchuk O, Ragozin A, Kalinina V. Mixed-Habit Type Ib-IaA Diamond from an Udachnaya Eclogite. Minerals. 2019; 9(12):741. https://doi.org/10.3390/min9120741
Chicago/Turabian StyleZedgenizov, Dmitry, Irina Bogush, Vladislav Shatsky, Oleg Kovalchuk, Alexey Ragozin, and Viktoriya Kalinina. 2019. "Mixed-Habit Type Ib-IaA Diamond from an Udachnaya Eclogite" Minerals 9, no. 12: 741. https://doi.org/10.3390/min9120741





