The Effect of Weathering on Salt Release from Coal Mine Spoils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Classification
2.2. Funnel Leaching Experiment
2.3. Chemical Analyses
2.4. Physical Analyses
2.5. Dynamics of Salt Release and Salt Production Rate
3. Results
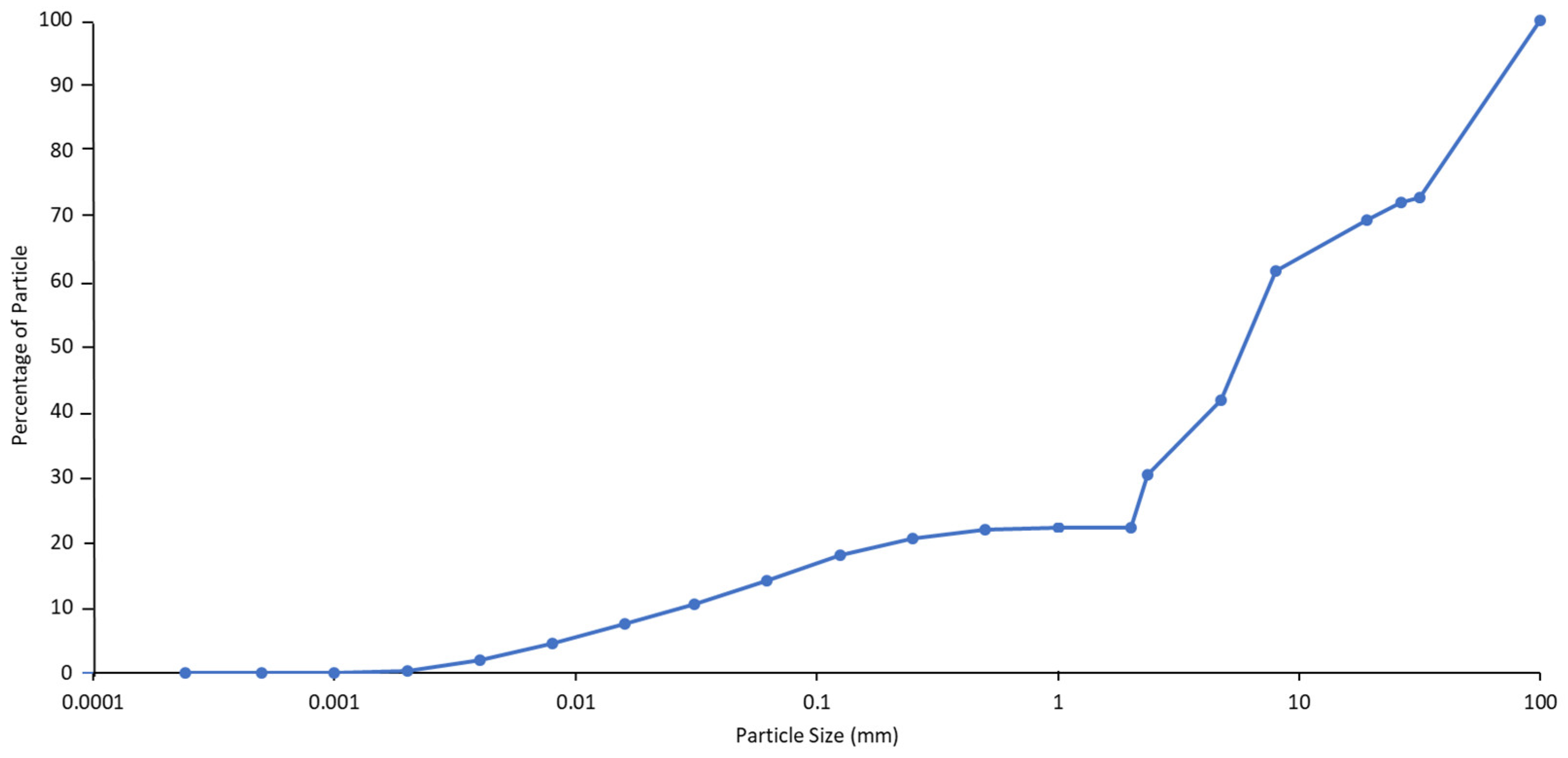
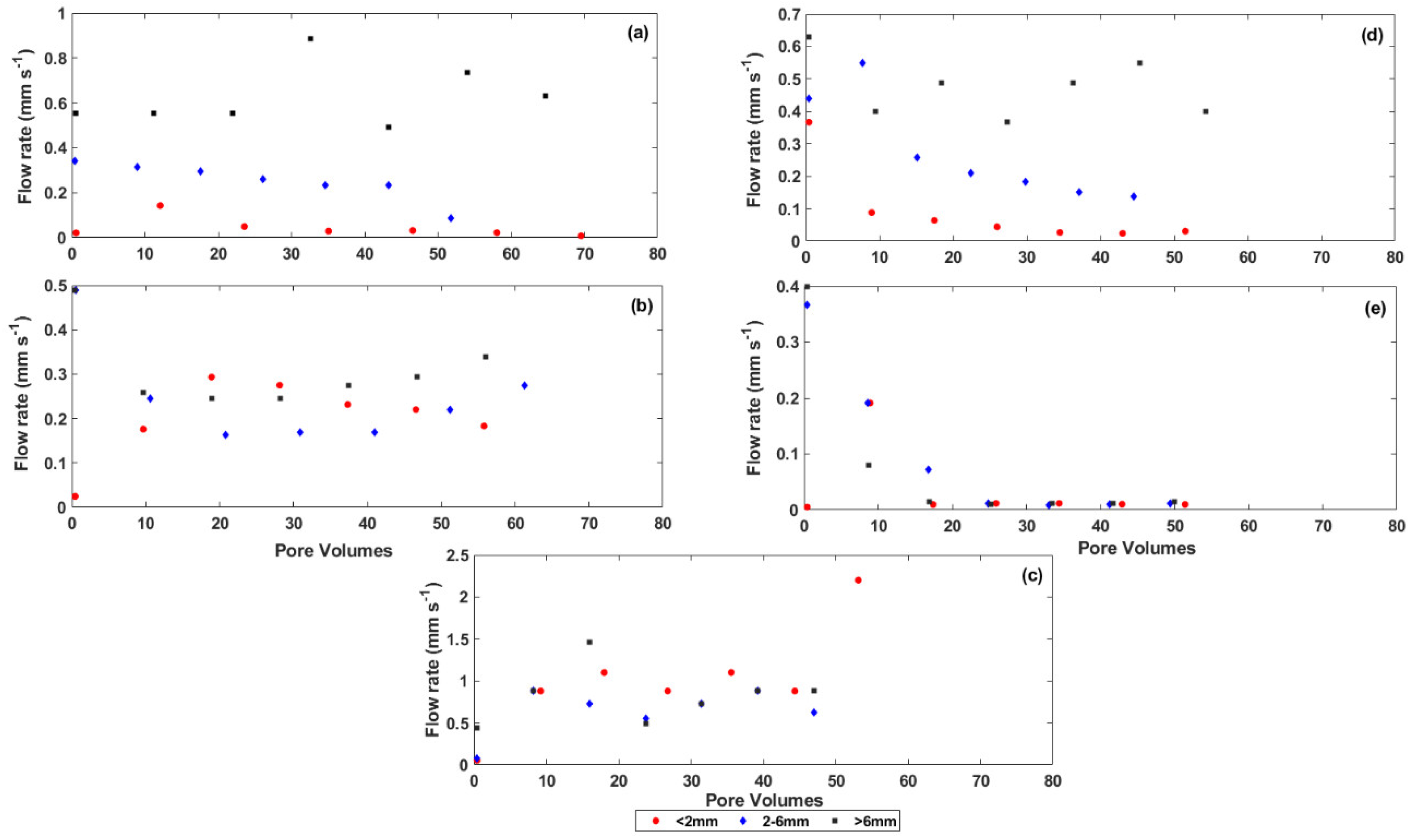
3.1. Changes in Physical Properties of the Coal Mine Spoils during Weathering
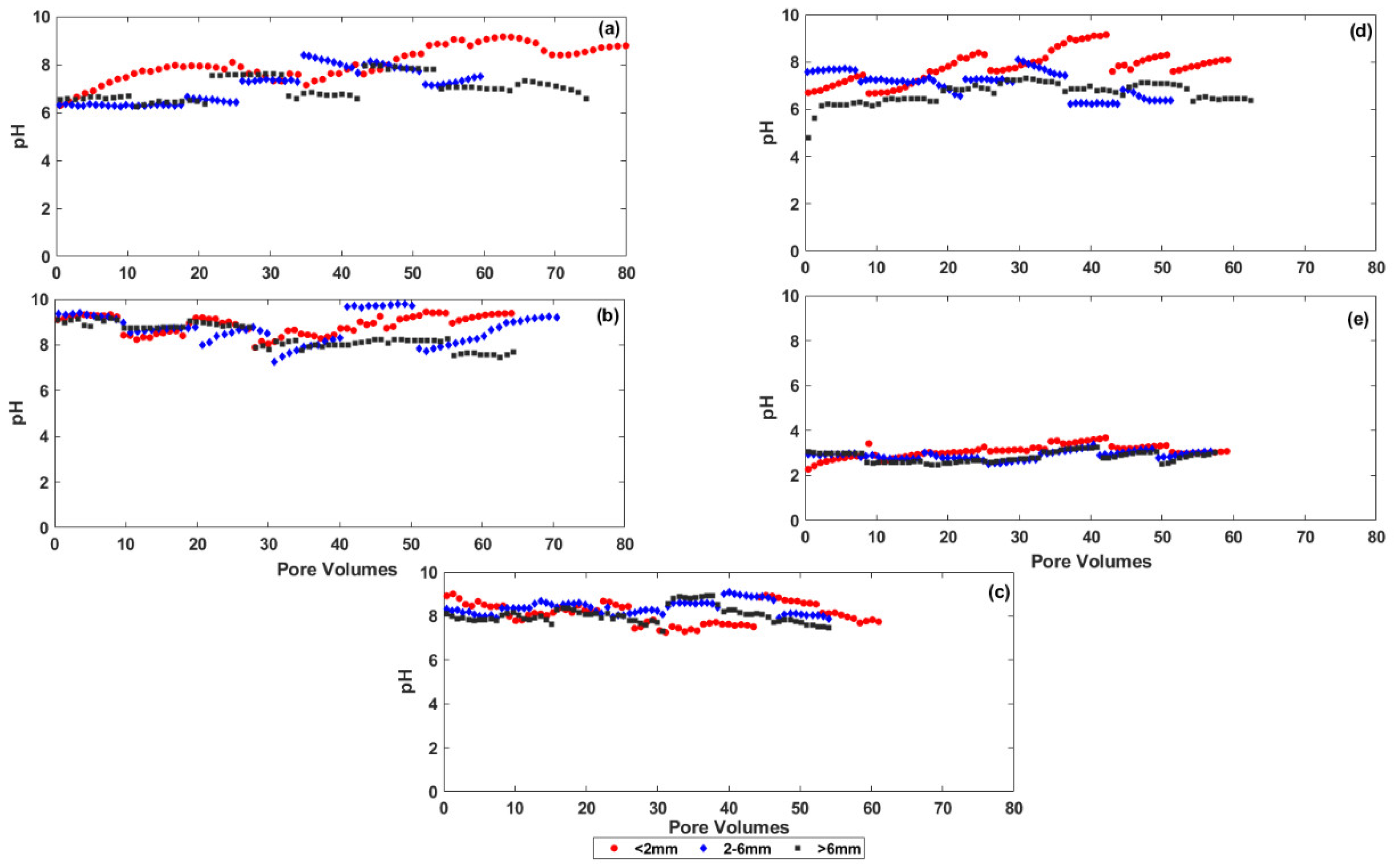
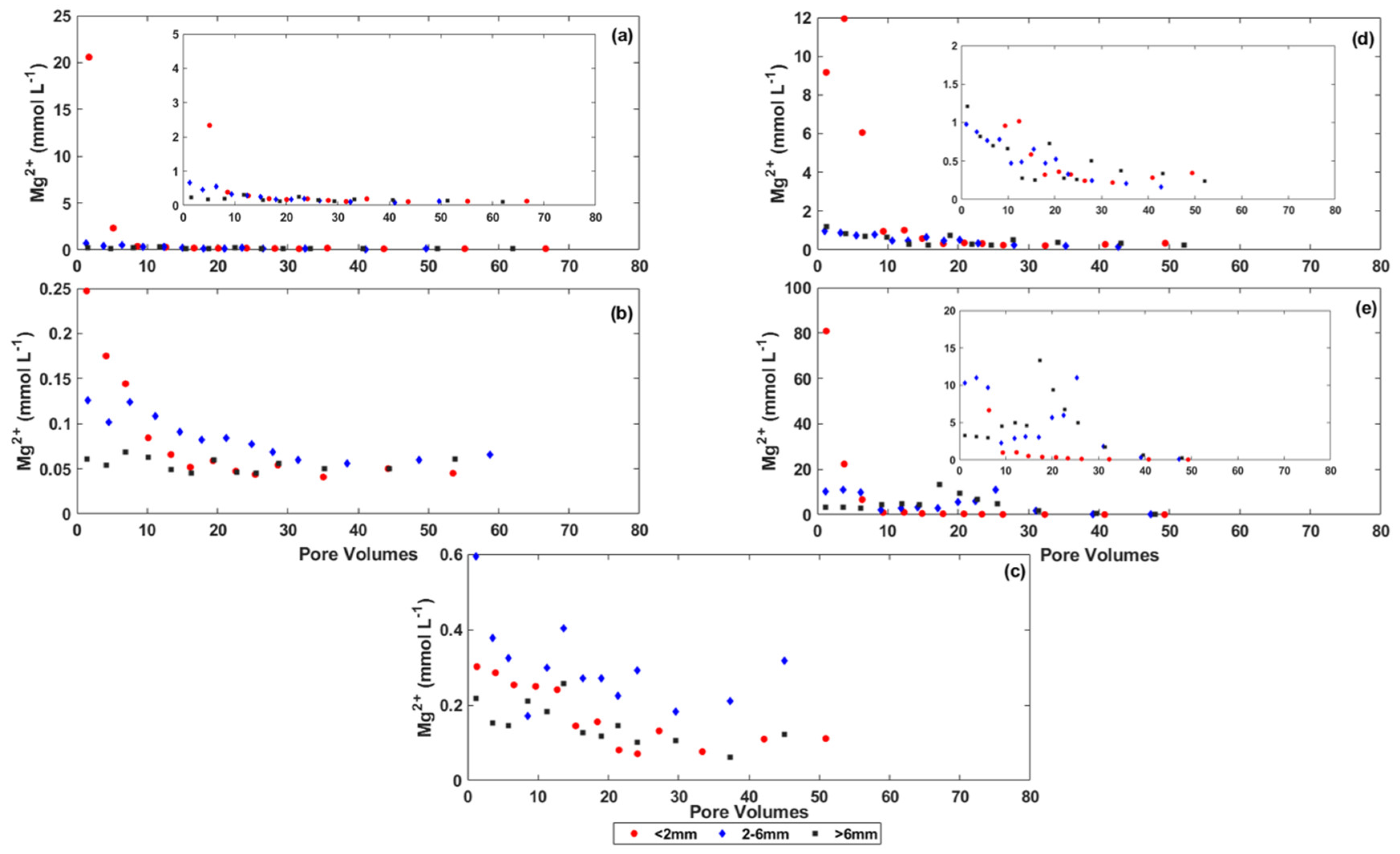
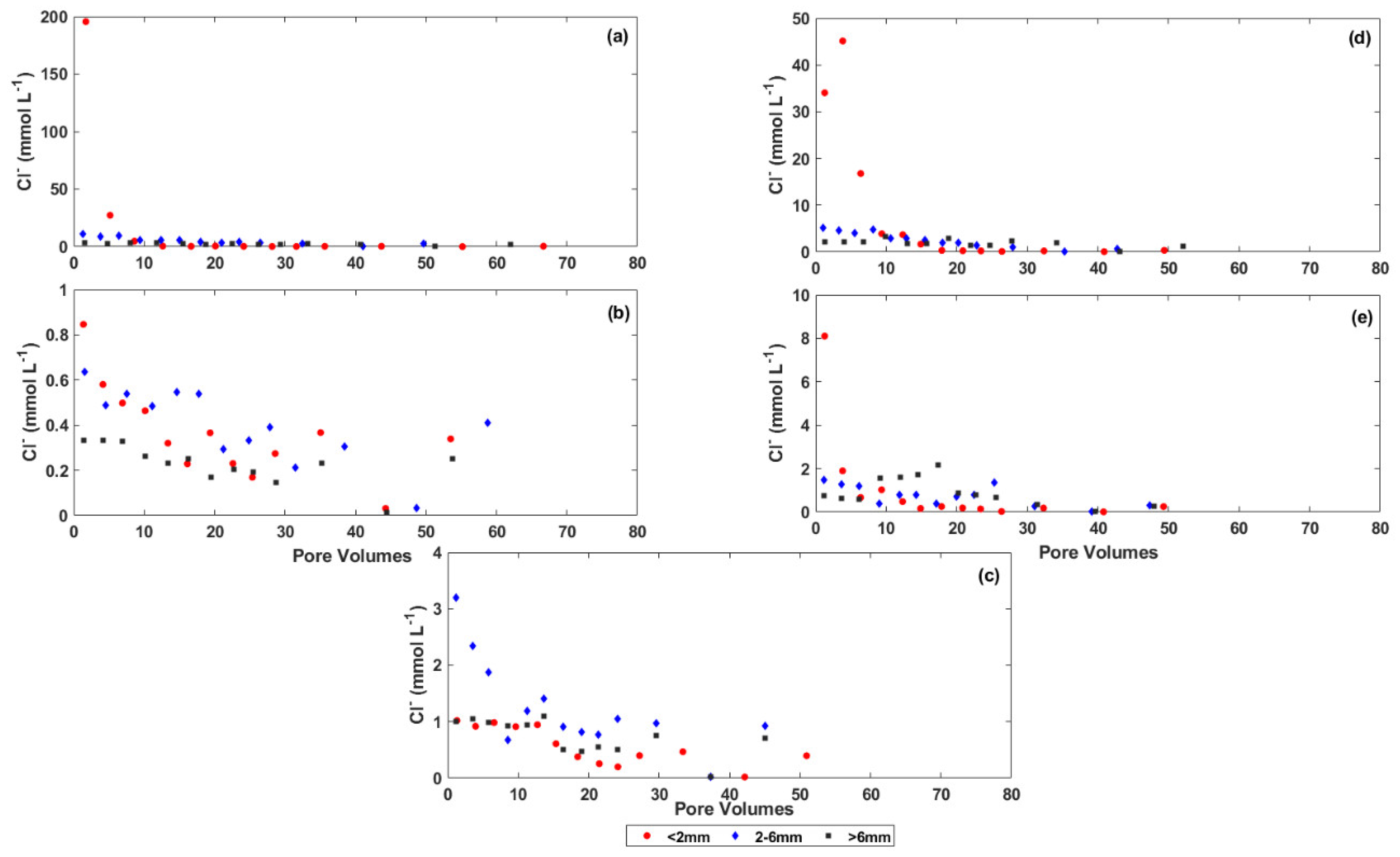
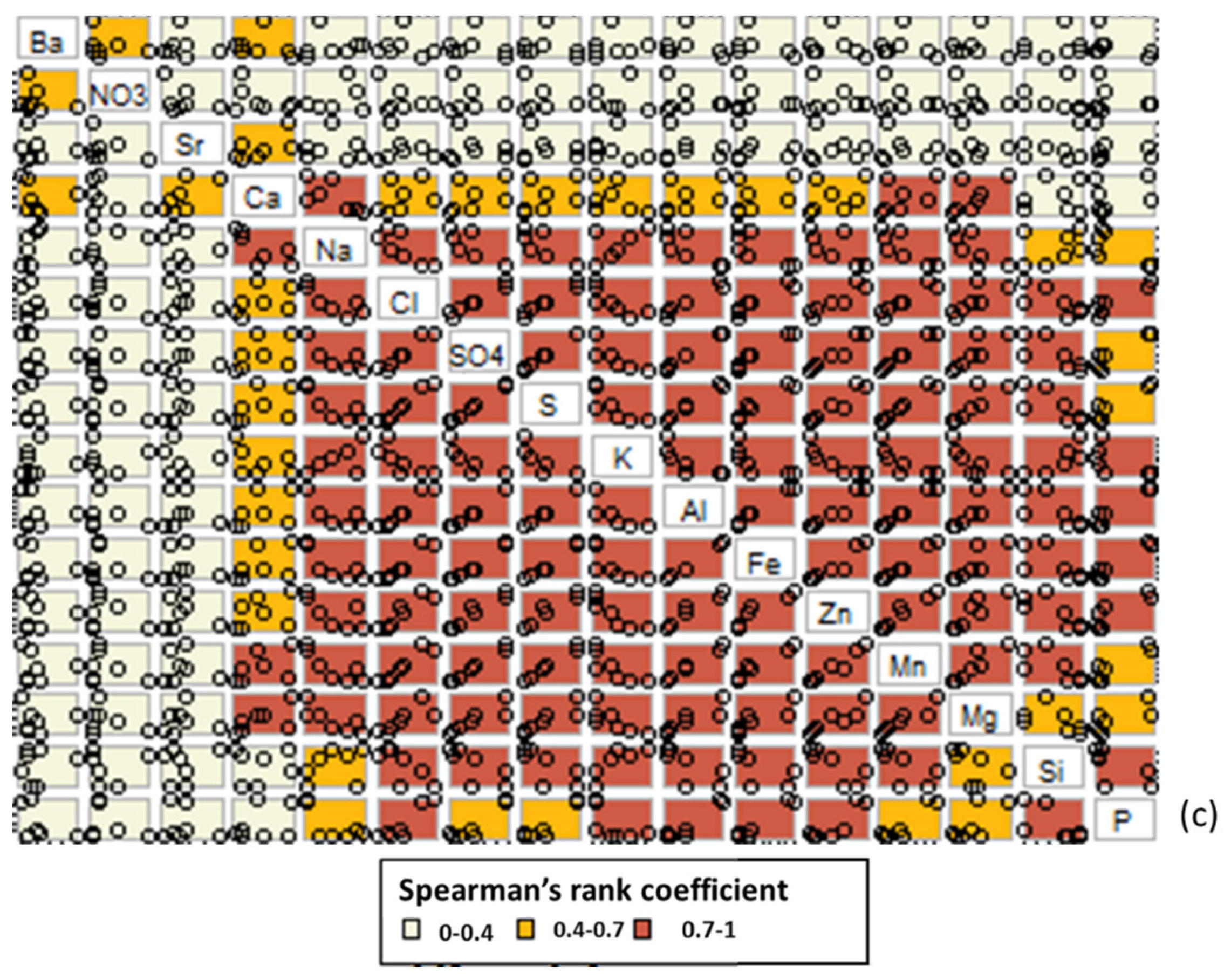
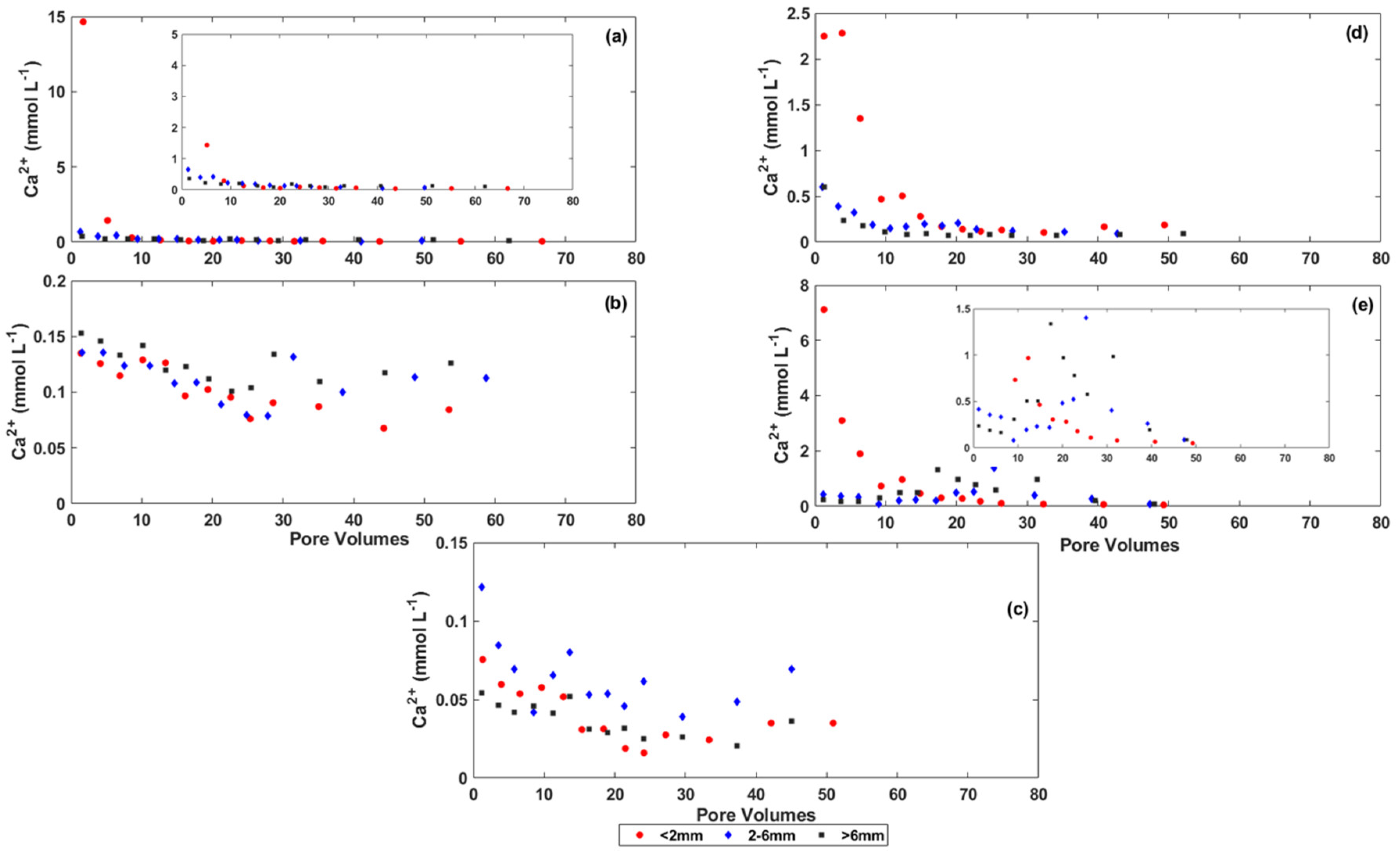
3.2. Changes in the Chemistry of Leachate Solutions during Weathering
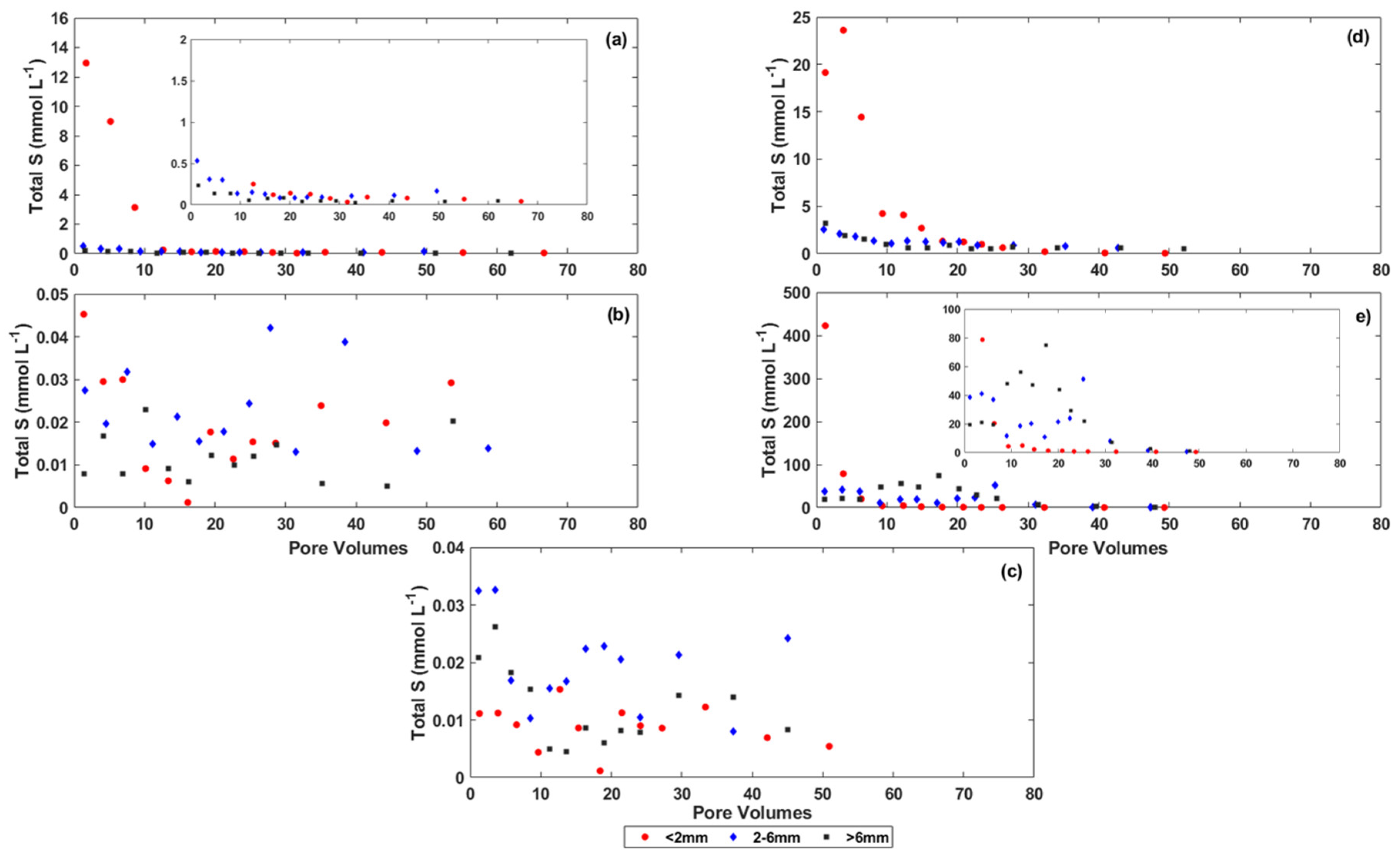
3.3. Rate of Salt Release from the Coal Mine Spoils
3.4. Changes in Chemical Properties of the Coal Mine Spoils after Weathering
4. Discussion
4.1. The Effect of Particle Size on Salt Release from the Coal Mine Spoils
4.2. The Effect of Physical and Chemical Weathering on Salt Release from the Coal Mine Spoils
4.3. Implications for Management of Coal Mine Spoils
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Szczepanska, J.; Twardowska, I. Distribution and environmental impact of coal-mining wastes in Upper Silesia, Poland. Environ. Geol. 1999, 38, 249–258. [Google Scholar] [CrossRef]
- Spargo, A.; Doley, D. Selective coal mine overburden treatment with topsoil and compost to optimise pasture or native vegetation establishment. J. Environ. Manag. 2016, 182, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, J.; Bai, Z.; Reading, L. Effects of surface coal mining and land reclamation on soil properties: A review. Earth Sci. Rev. 2019, 191, 12–25. [Google Scholar] [CrossRef]
- Li, X.; Park, J.H.; Edraki, M.; Baumgartl, T. Understanding the salinity issue of coal mine spoils in the context of salt cycle. Environ. Geochem. Health 2014, 36, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, R.K. Environmental impact of coal mining on water regime and its management. Water Air Soil Pollut. 2001, 132, 185–199. [Google Scholar] [CrossRef]
- Park, J.H.; Li, X.; Edraki, M.; Baumgartl, T.; Bernie, K. Geotechnical assessment and classification of f coal mine spoils for better understanding of potential salinity issues at closure. Environ. Sci. Process. Impacts 2013, 15, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Al-Sibai, M.; Adey, M.A.; Rose, D.A. Movement of solute through a porous medium under intermittent leaching. Eur. J. Soil Sci. 1997, 48, 711–725. [Google Scholar] [CrossRef]
- Reading, L.P.; Baumgartl, T.; Bristow, K.L.; Lockington, D.A. Hydraulic conductivity increases in a sodic clay soil in response to gypsum applications: Impacts of bulk density and cation exchange. Soil Sci. 2012, 177, 165–171. [Google Scholar] [CrossRef]
- Shaygan, M.; Reading, L.P.; Baumgartl, T. Effect of physical amendments on salt leaching characteristics for reclamation. Geoderma 2017, 292, 96–110. [Google Scholar] [CrossRef]
- David, R.; Dimitrios, P. Diffusion and cation exchange during the reclamation of saline-structured soils. Geoderma 2002, 107, 271–279. [Google Scholar] [CrossRef]
- Marwan, M.M.; Rowell, D.L. Cation exchange, hydrolysis and clay movement during the displacement of saline solutions from soils by water. Irrig. Sci. 1995, 16, 81–87. [Google Scholar] [CrossRef]
- Park, J.H.; Edraki, M.; Baumgartl, T. A practical testing approach to predict the geochemical hazards of in-pit coal mine tailings and rejects. Catena 2017, 148, 3–10. [Google Scholar] [CrossRef]
- Opitz, J.; Edraki, M.; Baumgartl, T. The effect of particle size and mineral liberation on the acid generating potential of sulphidic waste rock. Geochem. Explor. Environ. Anal. 2016, 16, 245–252. [Google Scholar] [CrossRef]
- Kohnke, H. The reclamation of coal mine spoils. In Advances in Agronomy; Norman, A.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1950; Volume 2, pp. 317–349. [Google Scholar]
- Hawkins, J.W.; Aljoe, W. Hydrologic characterization and modeling of a heterogeneous acid-producing surface coal mine spoil, Upshur County, West Virginia. In Proceedings of the Proceedings-National Symposium on Mining, Lexington, KY, USA, 14–18 May 1990; pp. 43–52. [Google Scholar]
- Sáinz, A.; Grande, J.A.; de la Torre, M.L.; Sánchez-Rodas, D. Characterisation of sequential leachate discharges of mining waste rock dumps in the Tinto and Odiel rivers. J. Environ. Manag. 2002, 64, 345–353. [Google Scholar] [CrossRef]
- Beven, K.; Germann, P. Macropores and water flow in soils. Water Resour. Res. 1982, 18, 1311–1325. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Gräsle, W.; Horn, R. Cation exchange processes in structured soils at various hydraulic properties. Soil Tillage Res. 1998, 47, 67–72. [Google Scholar] [CrossRef]
- Guebert, M.D.; Gardner, T.W. Macropore flow on a reclaimed surface mine: Infiltration and hillslope hydrology. Geomorphology 2001, 39, 151–169. [Google Scholar] [CrossRef]
- Adibee, N.; Osanloo, M.; Rahmanpour, M. Adverse effects of coal mine waste dumps on the environment and their management. Environ. Earth Sci. 2013, 70, 1581–1592. [Google Scholar] [CrossRef]
- Gerke, H.H.; Molson, J.W.; Frind, E.O. Modelling the effect of chemical heterogeneity on acidification and solute leaching in overburden mine spoils. J. Hydrol. 1998, 209, 166–185. [Google Scholar] [CrossRef]
- Gerke, H.H.; Molson, J.W.; Frind, E.O. Modelling the impact of physical and chemical heterogeneity on solute leaching in pyritic overburden mine spoils. Ecol. Eng. 2001, 17, 91–101. [Google Scholar] [CrossRef]
- Gerke, H.H. Preferential flow descriptions for structured soils. J. Plant Nutr. Soil Sci. 2006, 169, 382–400. [Google Scholar] [CrossRef]
- Sidle, R.C.; Kitahara, H.; Terajima, T.; Nakai, Y. Experimental studies on the effects of pipeflow on throughflow partitioning. J. Hydrol. 1995, 165, 207–219. [Google Scholar] [CrossRef]
- Paktunc, A.D. Characterization of mine wastes for prediction of acid mine drainage. In Environmental Impacts of Mining Activities; Springer: Berlin, Germany, 1999; pp. 19–40. [Google Scholar]
- Fookes, P.G.; Gourley, C.S.; Ohikere, C. Rock weathering in engineering time. Q. J. Eng. Geol. Hydrogeol. 1988, 21, 33–57. [Google Scholar] [CrossRef]
- Sumner, M. Sodic soils: New perspectives. Soil Res. 1993, 31, 683–750. [Google Scholar] [CrossRef]
- Price, D.G. Weathering and weathering processes. Q. J. Eng. Geol. Hydrogeol. 1995, 28, 243–252. [Google Scholar] [CrossRef]
- Ghafoor, A.; Hina, K.; Murtaza, G. Estimation of Gapon exchange coefficient for different textured soils and landforms of Punjab, Pakistan. Pak. J. Agric. Sci. 2004, 41, 25–28. [Google Scholar]
- Struthers, P.H. Chemical Weathering of Strip-Mine Spoils. Ohio Sci. J. 1964, 64, 125–131. [Google Scholar]
- Sollins, P.; Radulovich, R. Effects of soil physical structure on solute transport in a weathered tropical soil. Soil Sci. Soc. Am. J. 1988, 52, 1168–1173. [Google Scholar] [CrossRef]
- Orndorff, Z.; Daniels, W.L.; Beck, M.; Eick, M. Leaching potentials of coal spoil and refuse: Acid-base interactions and electrical conductivity. In Proceedings of the 27th National Meeting of the American Society of Mining and Reclamation (ASMR), Pittsburgh, PA, USA, 5–11 June 2010; pp. 736–766. [Google Scholar]
- Orndorff, Z.W.; Daniels, W.L.; Zipper, C.E.; Eick, M.; Beck, M. A column evaluation of Appalachian coal mine spoils’ temporal leaching behavior. Environ. Pollut. 2015, 204, 39–47. [Google Scholar] [CrossRef]
- Dang, Z.; Liu, C.; Haigh, M.J. Mobility of heavy metals associated with the natural weathering of coal mine spoils. Environ. Pollut. 2002, 118, 419–426. [Google Scholar] [CrossRef]
- Dutta, M.; Saikia, J.; Taffarel, S.R.; Waanders, F.B.; De Medeiros, D.; Cutruneo, C.M.; Silva, L.F.; Saikia, B.K. Environmental assessment and nano-mineralogical characterization of coal, overburden and sediment from Indian coal mining acid drainage. Geosci. Front. 2017, 8, 1285–1297. [Google Scholar] [CrossRef]
- Essilfie-Dughan, J.; Hendry, M.J.; Dynes, J.J.; Hu, Y.; Biswas, A.; Barbour, S.L.; Day, S. Geochemical and mineralogical characterization of sulfur and iron in coal waste rock, Elk Valley, British Columbia, Canada. Sci. Total Environ. 2017, 586, 753–769. [Google Scholar] [CrossRef] [PubMed]
- Edraki, M.; McIntyre, N.; Baumgartl, T.; Hilton, M. Prediction of Long-Term Salt Generation from Coal Spoils; The Australian Coal Industry’s Research Program: Brisbane, Australia, 2019; pp. 1–119. [Google Scholar]
- Nickmann, M.; Spaun, G.; Thuro, K. Engineering geological classification of weak rocks. In Proceedings of the 10th International IAEG Congress, Nottingham, UK, 6–10 September 2006. [Google Scholar]
- Simmons, J.; Fityus, S. The Stability of very High Spoil Piles; Australian Coal Association Research Program: Brisbane, Australia, 2016; pp. 1–205. [Google Scholar]
- Rayment, G.E.; Lyons, D.J. Soil Chemical Methods: Australasia; CSIRO: Collingwood, Victoria, Australia, 2011; Volume 3, 512p. [Google Scholar]
- Hillel, D. Introduction to Soil Physics; Elsevier: Amsterdam, The Netherlands, 1982; 392p. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Klute, A., Ed.; American Society of Agronomy-Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–382. [Google Scholar]
- Sheldrick, B.H.; Wang, C. Particle size distribution. In Soil Sampling and Methods of Analysis; Carter, M.R., Ed.; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 499–512. [Google Scholar]
- Šimůnek, J.; Šejna, M.; Saito, H.; Sakai, M.; van Genuchten, M.T. The Hydrus-1D Software Package for Simulating the Movement of Water, Heat, and Multiple Solutes in Variably Saturated Media; Department of Environmental Sciences, University of California Riverside: Riverside, CA, USA, 2013; 340p. [Google Scholar]
- Simunek, J.; Van Genuchten, M.T.; Sejna, M. Recent developments and applications of HYDRUS computer software packages. Vadose Zone J. 2016, 15. [Google Scholar] [CrossRef] [Green Version]
- Assouline, S.; Or, D. The concept of field capacity revisited: Defining intrinsic static and dynamic criteria for soil internal drainage dynamics. Water Resour. Res. 2014, 50, 4787–4802. [Google Scholar] [CrossRef]
- van Genuchten, M.T. A Closed-form equation for predicting the hydraulic conductivity of unsaturated soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef] [Green Version]
- Jalali, M. Effect of sodium and magnesium on kinetics of potassium release in some calcareous soils of western Iran. Geoderma 2008, 145, 207–215. [Google Scholar] [CrossRef]
- Forsyth, B. Understanding the Long-Term Seepage Geochemistry of Base Metal Mine Tailings in a Semiarid Subtropical Climate, Mount Isa, Australia. Ph.D. Thesis, The University of Queensland, Brisbane, Australia, 2014. [Google Scholar]
- Jurinak, J.; Whitmore, J.; Wagenet, R. Kinetics of salt release from a saline soil 1. Soil Sci. Soc. Am. J. 1977, 41, 721–724. [Google Scholar] [CrossRef]
- Molyneux, J. Some ecological aspects of colliery waste heaps around Wigan, South Lancashire. J. Ecol. 1963, 51, 315–321. [Google Scholar] [CrossRef]
- Down, C. Soil development on colliery waste tips in relation to age. I. Introduction and physical factors. J. Appl. Ecol. 1975, 12, 613–622. [Google Scholar] [CrossRef]
- Haering, K.C.; Daniels, W.L.; Roberts, J.A. Changes in mine soil properties resulting from overburden weathering. J. Environ. Qual. 1993, 22, 194–200. [Google Scholar] [CrossRef]
- Le Pera, E.; Sorriso-Valvo, M. Weathering and morphogenesis in a Mediterranean climate, Calabria, Italy. Geomorphology 2000, 34, 251–270. [Google Scholar] [CrossRef]
- Chamley, H. Clay formation through weathering. In Clay Sedimentology; Springer: Berlin, Germany, 1989; pp. 21–50. [Google Scholar]
- Jaynes, D.; Pionke, H.; Rogowski, A. Acid mine drainage from reclaimed coal strip mines 2. Simulation results of model. Water Resour. Res. 1984, 20, 243–250. [Google Scholar] [CrossRef]
- Rogowski, A.; Pionke, H.; Broyan, J. Modeling the Impact of Strip Mining and Reclamation Processes on Quality and Quantity of Water in Mined Areas: A Review. J. Environ. Qual. 1977, 6, 237–244. [Google Scholar] [CrossRef]
- Shaygan, M.; Mulligan, D.; Baumgartl, T. The potential of three halophytes (Tecticornia pergranulata, Sclerolaena longicuspis and Frankenia serpyllifolia) for the rehabilitation of brine-affected soils. Land Degrad. Dev. 2018, 29, 2002–2014. [Google Scholar] [CrossRef]
- Hannan, J.C. Mine Rehabilitation: A Handbook for the Coal Mining industry; New South Wales Coal Association: Sydney, Australia, 1995; 124p. [Google Scholar]
- Shaygan, M.; Reading, L.P.; Arnold, S.; Baumgartl, T. Modeling the effect of soil physical amendments on reclamation and revegetation success of a saline-sodic soil in a semi-arid environment. Arid Land Res. Manag. 2018, 32, 379–406. [Google Scholar] [CrossRef]
- Baumgartl, T.; Richards, B. Evaporation and salt transport under variable conditions. In Proceedings of the Life of Mine Conference, Maximising Rehabilitation Outcomes, Brisbane, Australia, 10–12 July 2012; pp. 179–186. [Google Scholar]
- Shaygan, M.; Baumgartl, T.; Arnold, S.; Reading, L.P. The effect of soil physical amendments on reclamation of a saline-sodic soil: Simulation of salt leaching using HYDRUS-1D. Soil Res. 2018, 56, 829–845. [Google Scholar] [CrossRef]



| Coal Mine Spoil | Lithology | Diagnostic Mineral | Degradation Degree | Geology | pH1:5 | EC1:5 (dS m−1) | Chemical Composition (mg kg−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Total S | Cl− | |||||||
| A10 | Sandstone | Carbonate and chlorite | Low (Rock-like spoil) | Rangal Formation | 6.90 | 2.60 | 7077 | 9367 | 1724 | 338 | 566 | 1979 |
| B8 | Sandstone and mudrock | Dolomite and calcite | Low (Rock-like spoil) | Rangal Formation | 7.17 | 0.46 | 4531 | 12,615 | 15,113 | 1159 | 555 | 121 |
| B9 | Sandstone and mudrock | Calcite | High (Soil-like spoil) | Rangal Formation | 6.04 | 0.51 | 5610 | 8360 | 1654 | 253 | 304 | 241 |
| C4 | Sandstone | Dolomite and calcite | Low (Rock-like spoil) | German Creek Formation | 7.26 | 1.70 | 10056 | 14,680 | 87,472 | 23,546 | 1049 | 556 |
| C6 | Sandstone | Pyrite and carbonate | High (Soil-like spoil) | German Creek Formation | 2.87 | 4.42 | 2935 | 18,431 | 320 | 1444 | 11,755 | 167 |
| Coal Mine Spoil | Bulk Density (g cm−3) | Particle Density (g cm−3) | Porosity (cm3 cm−3) | Average Flow Rate (mm s−1) (n = 7) |
|---|---|---|---|---|
| A10RL2 | 1.47 | 2.61 | 0.43 | 0.04 |
| A10RL26 | 1.08 | 2.60 | 0.58 | 0.25 |
| A10RL6 | 1.38 | 2.60 | 0.46 | 0.62 |
| B8RL2 | 1.21 | 2.64 | 0.54 | 0.20 |
| B8RL26 | 1.34 | 2.64 | 0.49 | 0.25 |
| B8RL6 | 1.21 | 2.64 | 0.53 | 0.30 |
| B9SL2 | 1.11 | 2.59 | 0.56 | 1.01 |
| B9SL26 | 0.90 | 2.53 | 0.64 | 0.64 |
| B9SL6 | 1.15 | 2.53 | 0.54 | 0.82 |
| C4RL2 | 1.14 | 2.76 | 0.58 | 0.09 |
| C4RL26 | 0.86 | 2.69 | 0.67 | 0.27 |
| C4RL6 | 1.19 | 2.69 | 0.55 | 0.47 |
| C6SL2 | 1.09 | 2.66 | 0.58 | 0.03 |
| C6SL26 | 0.98 | 2.54 | 0.61 | 0.09 |
| C6SL6 | 1.00 | 2.54 | 0.60 | 0.08 |
| Coal Mine Spoil | Gravel (%) | Coarse Sand (%) | Fine Sand (%) | Silt (%) | Clay (%) | Θs (cm3 cm−3) | Θr (cm3 cm−3) | α (1/cm) | n | Macropore Volume (cm3 cm−3) |
|---|---|---|---|---|---|---|---|---|---|---|
| C6SL2 | 0.0 | 8.2 | 52.4 | 11.6 | 27.2 | 0.55 | 0.08 | 0.023 | 1.36 | 0.22 |
| C6SL26 | 5.6 | 2.0 | 53.2 | 19.7 | 23.7 | 0.53 | 0.07 | 0.017 | 1.41 | 0.23 |
| C6SL6 | 5.6 | 4.0 | 52.1 | 15.8 | 23.7 | 0.54 | 0.07 | 0.019 | 1.38 | 0.24 |
| Coal Mine Spoil | Na+ | Ca2+ | K+ | Mg2+ | Cl− | Total S |
|---|---|---|---|---|---|---|
| A10RL2 | 7.400 | 0.962 | 0.210 | 0.856 | 11.544 | 1.181 |
| A10RL26 | 2.750 | 0.216 | 0.088 | 0.164 | 4.593 | 0.143 |
| A10RL6 | 1.005 | 0.124 | 0.018 | 0.085 | 1.686 | 0.049 |
| B8RL2 | 0.488 | 0.091 | 0.184 | 0.046 | 0.287 | 0.014 |
| B8RL26 | 0.406 | 0.089 | 0.156 | 0.041 | 0.286 | 0.014 |
| B8RL6 | 0.234 | 0.111 | 0.086 | 0.029 | 0.178 | 0.008 |
| B9SL2 | 0.424 | 0.038 | 0.241 | 0.100 | 0.497 | 0.006 |
| B9SL26 | 0.935 | 0.077 | 0.474 | 0.220 | 1.321 | 0.018 |
| B9SL6 | 0.499 | 0.034 | 0.177 | 0.085 | 0.608 | 0.009 |
| C4RL2 | 7.095 | 0.595 | 0.199 | 1.412 | 6.913 | 4.255 |
| C4RL26 | 2.757 | 0.277 | 0.070 | 0.408 | 2.933 | 1.333 |
| C4RL6 | 1.410 | 0.129 | 0.030 | 0.282 | 1.511 | 0.738 |
| C6SL2 | 0.138 | 1.167 | 0.076 | 5.247 | 0.899 | 32.79 |
| C6SL26 | 0.106 | 0.421 | 0.019 | 3.460 | 0.731 | 19.24 |
| C6SL6 | 0.102 | 0.568 | 0.018 | 3.057 | 0.880 | 26.04 |
| Experimental Conditions | Elements | B8RL | B9SL | C4RL | C6SL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before weathering experiment (n = 3) | Na+ (cmolc kg−1) | 1.050 ± 0.058 | 2.552 ± 0.025 | 0.917 ± 0.021 | 0.001 ± 0.0008 | ||||||||
| K+ (cmolc kg−1) | 0.146 ± 0.002 | 0.121 ± 0.010 | 0.104 ± 0.008 | 0.004 ± 0.0003 | |||||||||
| Mg2+ (cmolc kg−1) | 0.718 ± 0.022 | 2.026 ± 0.038 | 1.308 ± 0.069 | 0.813 ± 0.031 | |||||||||
| Ca2+ (cmolc kg−1) | 3.621 ± 0.102 | 1.964 ± 0.196 | 2.339 ± 0.232 | 0.480 ± 0.076 | |||||||||
| CEC (cmolc kg−1) | 5.537 ± 0.163 | 6.664 ± 0.219 | 4.668 ± 0.308 | 1.290 ± 0.102 | |||||||||
| EC1:5 (dS m−1) | 0.462 ± 0.020 | 0.519 ± 0.020 | 1.700 ± 0.120 | 4.420 ± 0.070 | |||||||||
| pH1:5 | 7.170 ± 0.300 | 6.040 ± 0.220 | 7.260 ± 0.070 | 2.870 ± 0.070 | |||||||||
| Elements | B8RL2 | B8RL26 | B8RL6 | B9SL2 | B9SL26 | B9SL6 | C4RL2 | C4RL26 | C4RL6 | C6SL2 | C6SL26 | C6SL6 | |
| After weathering experiment (n = 3) | Na+ (cmolc kg−1) | 0.970 ± 0.033 | 0.980 ± 0.005 | 1.009 ± 0.040 | 1.902 ± 0.032 | 1.808 ± 0.106 | 2.170 ± 0.670 | 0.021 ± 0.0004 | 0.020 ± 0.0008 | 0.410 ± 0.017 | 0.013 ± 0.0008 | 0.032 ± 0.019 | 0.016 ± 0.0006 |
| K+ (cmolc kg−1) | 0.170 ± 0.005 | 0.174 ± 0.004 | 0.172 ± 0.002 | 0.085 ± 0.002 | 0.082 ± 0.005 | 0.107 ± 0.043 | 0.066 ± 0.002 | 0.063 ± 0.003 | 0.080 ± 0.004 | 0.029 ± 0.001 | 0.107 ± 0.118 | 0.025 ± 0.001 | |
| Mg2+ (cmolc kg−1) | 0.797 ± 0.030 | 0.794 ± 0.030 | 0.787 ± 0.009 | 1.45 ± 0.030 | 1.350 ± 0.090 | 1.820 ± 0.540 | 0.984 ± 0.053 | 0.978 ± 0.031 | 1.107 ± 0.046 | 0.083 ± 0.001 | 0.115 ± 0.006 | 0.098 ± 0.002 | |
| Ca2+ (cmolc kg−1) | 3.730 ± 0.128 | 3.906 ± 0.115 | 4.340 ± 0.236 | 1.403 ± 0.107 | 1.103 ± 0.143 | 0.932 ± 0.138 | 3.080 ± 0.105 | 2.900 ± 0.264 | 2.106 ± 0.044 | 0.078 ± 0.003 | 0.150 ± 0.017 | 0.111 ± 0.007 | |
| CEC (cmolc kg−1) | 5.316 ± 0.108 | 5.859 ± 0.088 | 6.309 ± 0.214 | 4.841 ± 0.174 | 4.352 ± 0.178 | 5.030 ± 1.340 | 4.152 ± 0.160 | 3.965 ± 0.299 | 3.703 ± 0.099 | 0.205 ± 0.004 | 0.404 ± 0.157 | 0.253 ± 0.011 | |
| EC1:5 (dS m−1) | 0.335 ± 0.005 | 0.736 ± 0.010 | 0.337 ± 0.009 | 0.863 ± 0.409 | 0.895 ± 0.339 | 0.693 ± 0.273 | 0.092 ± 0.001 | 0.360 ± 0.190 | 0.808 ± 0.010 | 0.084 ± 0.009 | 0.082 ± 0.004 | 0.087 ± 0.003 | |
| pH1:5 | 9.690 ± 0.231 | 9.980 ± 0.055 | 9.990 ± 0.010 | 7.203 ± 0.430 | 8.140 ± 0.158 | 8.280 ± 0.160 | 7.600 ± 0.090 | 8.270 ± 0.200 | 8.200 ± 0.040 | 5.200 ± 0.110 | 4.780 ± 0.110 | 4.310 ± 0.120 | |
| Experimental Conditions | Major Ions | B8RL | B9SL | C4RL | C6SL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before weathering experiment (n = 3) | Na+ (mg kg−1) | 437.630 ± 29.998 | 485.426 ± 22.090 | 587.670 ± 214.950 | 1.160 ± 0.120 | ||||||||
| K+ (mg kg−1) | 8.518 ± 0.919 | 31.099 ± 19.182 | 24.240 ± 9.090 | 0.630 ± 0.150 | |||||||||
| Mg2+ (mg kg−1) | 1.137 ± 0.243 | 15.057 ± 9.817 | 47.130 ± 17.320 | 472.480 ± 80.400 | |||||||||
| Ca2+ (mg kg−1) | 4.187 ± 0.942 | 9.480 ± 0.450 | 58.250 ± 19.580 | 237.810 ± 15.680 | |||||||||
| Major ions | B8RL2 | B8RL26 | B8RL6 | B9SL2 | B9SL26 | B9SL6 | C4RL2 | C4RL26 | C4RL6 | C6SL2 | C6SL26 | C6SL6 | |
| After weathering experiment (n = 3) | Na+ (mg kg−1) | 368.530 ± 10.780 | 376.490 ± 12.890 | 407.900 ± 19.150 | 662.510 ± 11.206 | 505.700 ± 37.460 | 620.690 ± 35.820 | 17.480 ± 0.500 | 257.960 ± 11.690 | 16.960 ± 0.960 | 7.130 ± 0.290 | 6.560 ± 0.030 | 4.080 ± 0.110 |
| K+ (mg kg−1) | 4.795 ± 1.325 | 5.492 ± 0.822 | 4.149 ± 1.562 | 4.323 ± 0.330 | 9.283 ± 3.032 | 3.962 ± 0.299 | 11.090 ± 0.180 | 14.220 ± 1.690 | 10.740 ± 0.440 | 9.410 ± 0.150 | 8.320 ± 0.330 | 7.930 ± 0.250 | |
| Mg2+ (mg kg−1) | 3.869 ± 1.110 | 2.539 ± 0.727 | 0.875 ± 0.200 | 3.676 ± 0.102 | 5.530 ± 1.732 | 2.646 ± 0.172 | 25.900 ± 0.460 | 35.750 ± 4.690 | 25.980 ± 0.400 | 2.100 ± 0.110 | 3.100 ± 0.280 | 5.560 ± 0.200 | |
| Ca2+ (mg kg−1) | 4.790 ± 1.320 | 5.490 ± 0.820 | 4.140 ± 1.560 | 9.780 ± 0.250 | 4.740 ± 2.090 | 3.130 ± 0.380 | 22.980 ± 0.840 | 22.720 ± 2.640 | 22.700 ± 1.010 | 3.090 ± 0.360 | 5.700 ± 0.440 | 3.870 ± 0.220 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilton, M.; Shaygan, M.; McIntyre, N.; Baumgartl, T.; Edraki, M. The Effect of Weathering on Salt Release from Coal Mine Spoils. Minerals 2019, 9, 760. https://doi.org/10.3390/min9120760
Hilton M, Shaygan M, McIntyre N, Baumgartl T, Edraki M. The Effect of Weathering on Salt Release from Coal Mine Spoils. Minerals. 2019; 9(12):760. https://doi.org/10.3390/min9120760
Chicago/Turabian StyleHilton, Melinda, Mandana Shaygan, Neil McIntyre, Thomas Baumgartl, and Mansour Edraki. 2019. "The Effect of Weathering on Salt Release from Coal Mine Spoils" Minerals 9, no. 12: 760. https://doi.org/10.3390/min9120760





