Corrosion Behavior of a Pyrite and Arsenopyrite Galvanic Pair in the Presence of Sulfuric Acid, Ferric Ions and HQ0211 Bacterial Strain
Abstract
:1. Introduction
2. Experimental Methods
2.1. Electrochemical Methods
2.2. DFT Calculational Details
3. Results and Discussion
3.1. Surface Activity
3.2. Dissolution Behavior of the Galvanic Pair
3.3. DFT Calculations
4. Conclusions
- After a detailed comparison, the corrosion process of the pyrite/arsenopyrite galvanic pair is similar to that of arsenopyrite, which is clearly supported by electrochemical data.
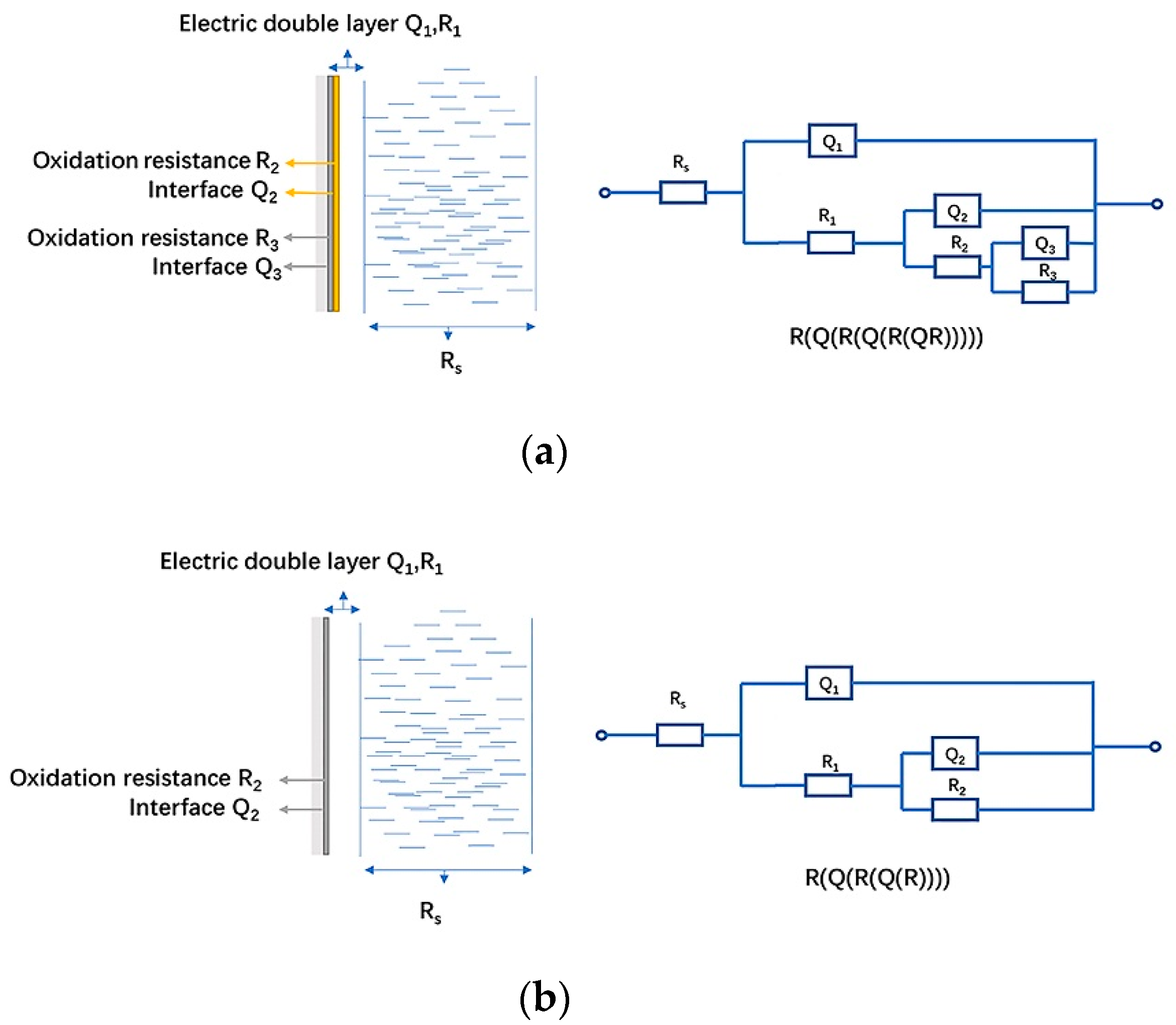
- EIS models of pyrite/arsenopyrite galvanic pair were established as R(Q(R(Q(R(QR))))) and R(Q(R(Q(R)))) and the physical meaning was clearly indicated.
- The mechanism of the galvanic effect that accelerates arsenopyrite corrosion by increasing the cathode and anode currents as well as the charge transfer resistance during the oxidation of the surface layer of the reaction was proposed.
- The quantum mechanics perspective of pyrite/arsenopyrite galvanic effect was provided. The calculated results show that the oxidation behavior of the galvanic pair is characterized by arsenopyrite oxidation behavior, and the presence of pyrite accelerates the oxidation of arsenopyrite in the galvanic pair.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, S.N.; Ni, P.; Bao, T.; Li, C.Z.; Xiang, H.L.; Wang, G.G.; Huang, B.; Chi, Z.; Dai, B.Z.; Ding, J.Y. Geology, fluid inclusion, and stable isotope systematics of the Dongyang epithermal gold deposit, Fujian Province, southeast China: Implications for ore genesis and mineral exploration. J. Geochem. Explor. 2018, 195, 16–30. [Google Scholar] [CrossRef]
- Mansurov, Y.N.; Miklin, Y.A.; Miklin, N.A.; Nikol’Skii, A.V. Methods and Equipment for Breaking Down Gold-Containing Concentrates from Lean Ores and Mining Industry Waste. Metallurgist 2018, 62, 169–175. [Google Scholar] [CrossRef]
- Kravtsova, R.G.; Tauson, V.L.; Nikitenko, E.M. Modes of Au, Pt, and Pd occurrence in arsenopyrite from the Natalkinskoe deposit, NE Russia. Geochem. Int. 2015, 53, 964–972. [Google Scholar] [CrossRef]
- Khishgee, C.; Akasaka, M.; Ohira, H.; Sereenen, J. Gold Mineralization of the Gatsuurt Deposit in the North Khentei Gold Belt, Central Northern Mongolia. Resour. Geol. 2014, 64, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Komnitsas, C.; Pooley, F. Mineralogical characteristics and treatment of refractory gold ores. Miner. Eng. 1989, 2, 449–457. [Google Scholar] [CrossRef]
- Rajasekar, A. Bio-oxidation and bio-cyanidation of refractory mineral ores for gold extraction: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1611–1643. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Perrot, F.; Morris, C.; Rea, S.; Benvie, B.; Austin, P.; Hackl, R. Evaluation of submerged bio-oxidation concept for refractory gold ores. Hydrometallurgy 2014, 141, 117–125. [Google Scholar] [CrossRef]
- Anjum, F.; Shahid, M.; Akcil, A. Biohydrometallurgy techniques of low grade ores: A review on black shale. Hydrometallurgy 2012, 117–118, 1–12. [Google Scholar] [CrossRef]
- Komnitsas, C.; Pooley, F. Bacterial oxidation of an arsenical gold sulphide concentrate from Olympias, Greece. Miner. Eng. 1990, 3, 295–306. [Google Scholar] [CrossRef]
- Smith, E.E.; Shumate, K.S.; Singer, P.C.; Stumm, W. Direct Oxidation by Adsorbed Oxygen during Acidic Mine Drainage. Science 1970, 169, 98. [Google Scholar] [CrossRef]
- Breed, A.W.; Glatz, A.; Hansfor, G.S.; Harrison, S.T.L. The effect of As(III) and As(V) on the batch bioleaching of a pyrite-arsenopyrite concentrate. Miner. Eng. 1996, 9, 1235–1252. [Google Scholar] [CrossRef]
- Yu, Y.; Zhu, Y.; Gao, Z.; Gammons, C.H.; Li, D. Rates of arsenopyrite oxidation by oxygen and Fe(III) at pH 1.8–12.6 and 15–45 °C. Environ. Sci. Technol. 2007, 41, 6460–6464. [Google Scholar] [CrossRef] [PubMed]
- Moses, C.O.; Nordstrom, D.K.; Herman, J.S.; Aaron, L. Aqueous pyrite oxidation by dissolved oxygen and by ferric iron. Geochim. Cosmochim. Acta 1987, 51, 1561–1571. [Google Scholar] [CrossRef]
- Mehta, A.P.; Murr, L.E. Fundamental studies of the contribution of galvanic interaction to acid-bacterial leaching of mixed metal sulfides. Hydrometallurgy 1983, 9, 235–256. [Google Scholar] [CrossRef]
- Urbano, G.; Reyes, V.E.; Veloz, M.A.; GonzáLez, I. Pyrite−Arsenopyrite Galvanic Interaction and Electrochemical Reactivity. J. Phys. Chem. C 2008, 112, 10453–10461. [Google Scholar] [CrossRef]
- Saavedra, A.; García-Meza, J.V.; Cortón, E.; González, I. Understanding galvanic interactions between chalcopyrite and magnetite in acid medium to improve copper (Bio)Leaching. Electrochim. Acta 2018, 265, 569–576. [Google Scholar] [CrossRef]
- Santos, E.C.D.; Lourenço, M.P.; Pettersson, L.G.M.; Duarte, H.A. Stability, Structure, and Electronic Properties of the Pyrite/Arsenopyrite Solid-Solid Interface—A DFT Study. J. Phys. Chem. C 2017, 121, 8042–8051. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; He, G.; Li, L. Catalytic effect of pyrite on the leaching of arsenopyrite in sulfuric acid and acid culture medium. Electrochim. Acta 2018, 263, 8–16. [Google Scholar] [CrossRef]
- Huai, Y.; Plackowski, C.; Peng, Y. The galvanic interaction between gold and pyrite in the presence of ferric ions. Miner. Eng. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wang, X.J.; Li-Yuan, M.A.; Jiao, F.; Liu, R.Z.; Gao, K. Effects of galvanic interaction between galena and pyrite on their flotation in the presence of butyl xanthate. Trans. Nonferrous Met. Soc. China 2015, 25, 3111–3118. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Chevary, J.A.; Vosko, S.H.; Jackson, K.A.; Pederson, M.R.; Singh, D.J.; Fiolhais, C. Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 1993, 46, 6671–6687. [Google Scholar] [CrossRef]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892. [Google Scholar] [CrossRef]
- Bayliss, P. Crystal chemistry and crystallography of some minerals within the pyrite group. Am. Miner. 1989, 74, 1168–1176. [Google Scholar]
- Buerger, M. The symmetry and crystal structure of the minerals of the arsenopyrite group. Z. Krist. Cryst. Mater. 1936, 95, 83–113. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Gehrke, T. Extracellular polymeric substances mediate bioleaching/biocorrosion via interfacial processes involving iron(III) ions and acidophilic bacteria. Res. Microbiol. 2006, 157, 49–56. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G.; Xu, B.; Li, L.; Wu, B. Surface characterization of arsenopyrite during chemical and biological oxidation. Sci. Total Environ. 2018, 626, 349. [Google Scholar] [CrossRef]
- Corkhill, C.L.; Vaughan, D.J. Arsenopyrite oxidation—A review. Appl. Geochem. 2009, 24, 2342–2361. [Google Scholar] [CrossRef]
- Yang, H.Y.; Liu, Q.; Chen, G.B.; Tong, L.L.; Ali, A. Bio-dissolution of pyrite by Phanerochaete chrysosporium. Trans. Nonferrous Met. Soc. China 2018, 28, 766–774. [Google Scholar] [CrossRef]
- Deng, S.; Gu, G. An electrochemical impedance spectroscopy study of arsenopyrite oxidation in the presence of Sulfobacillus thermosulfidooxidans. Electrochim. Acta 2018, 287, 106–114. [Google Scholar] [CrossRef]
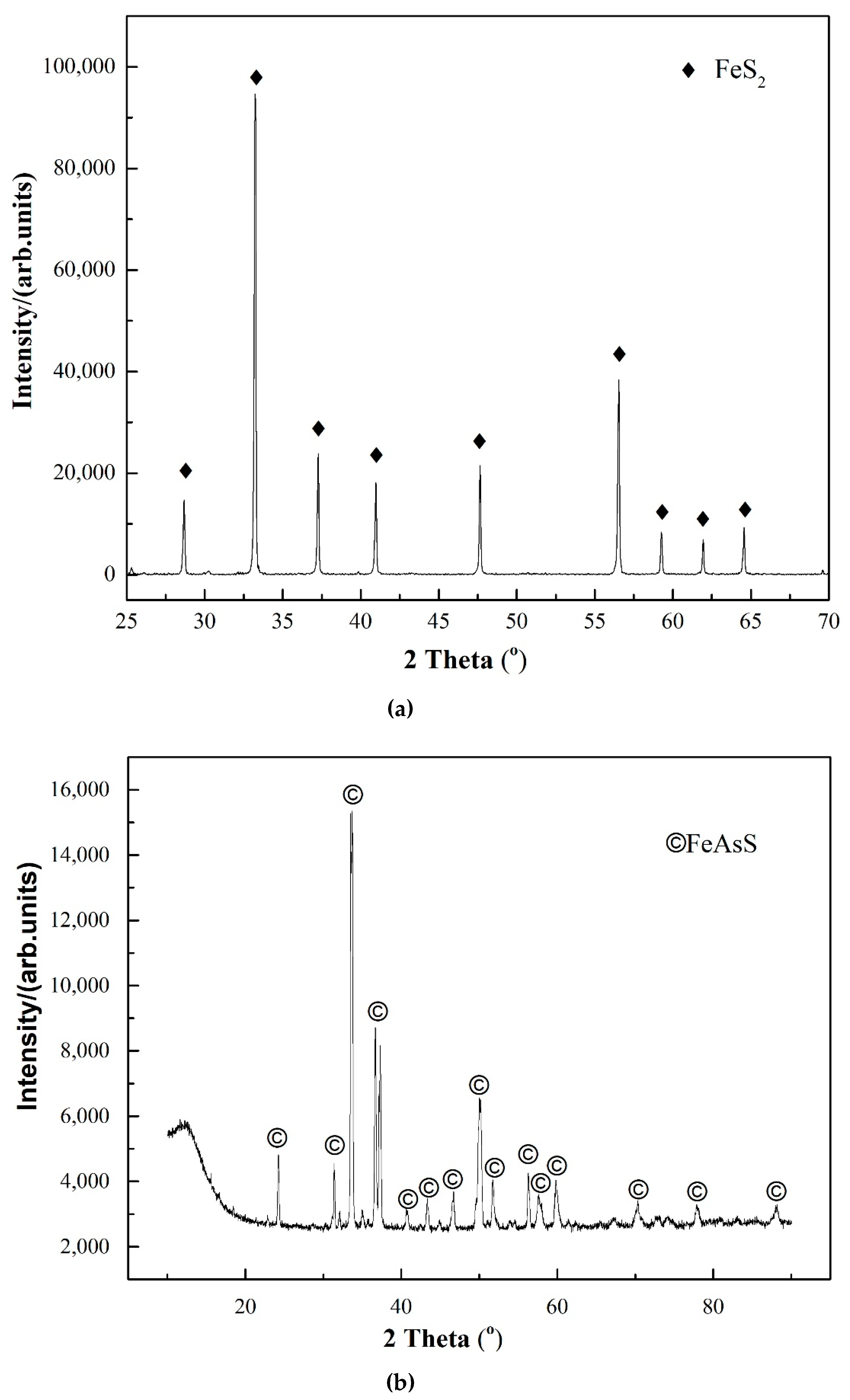
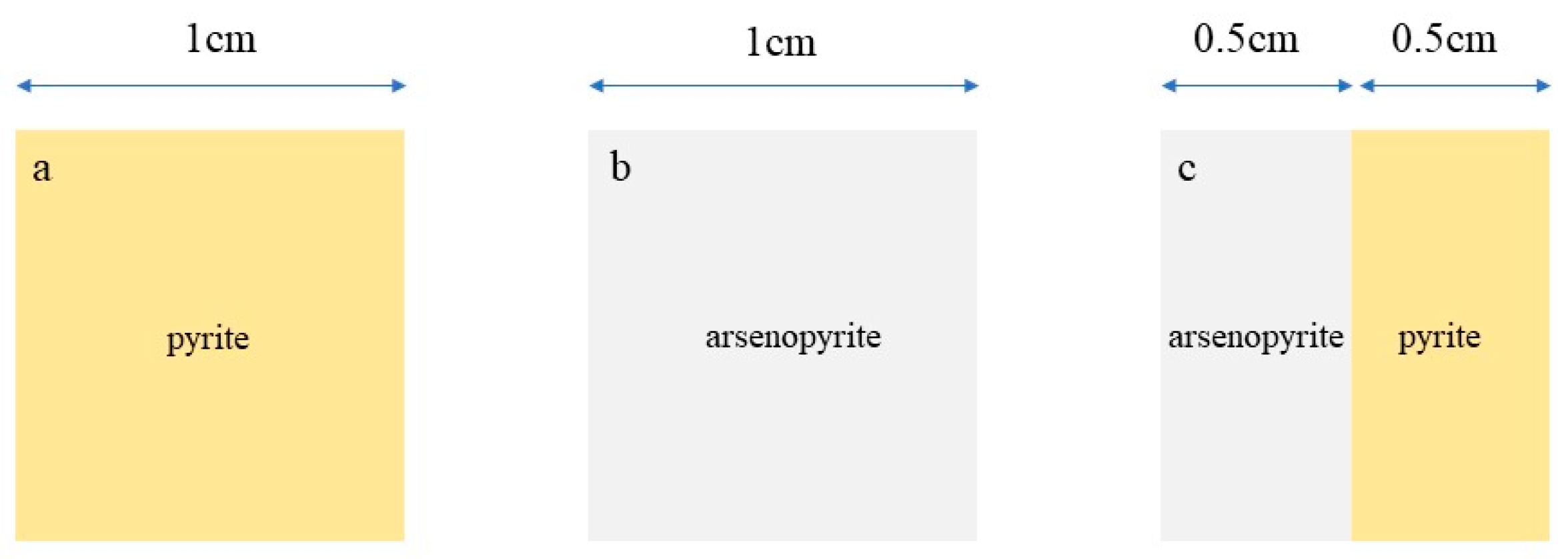
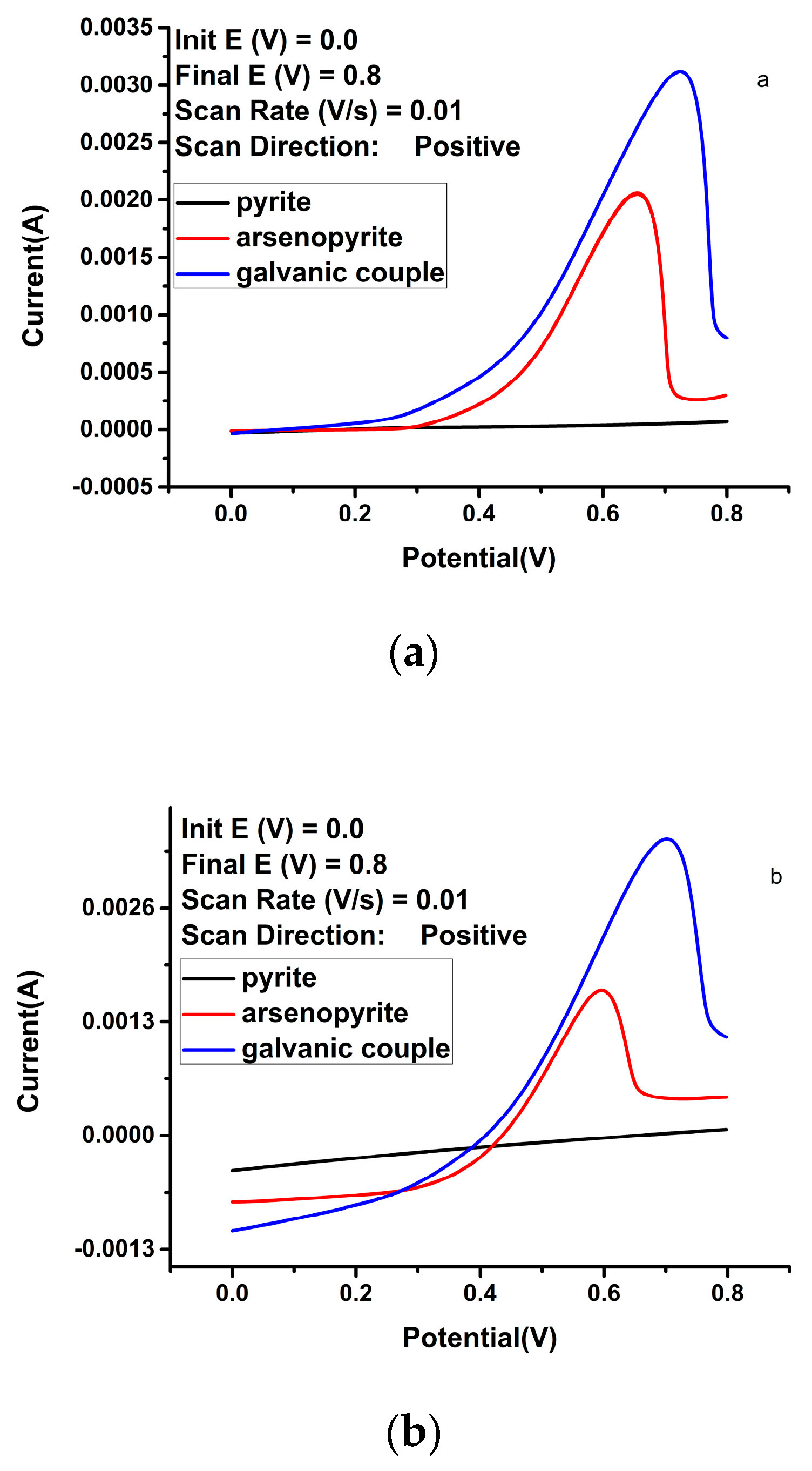
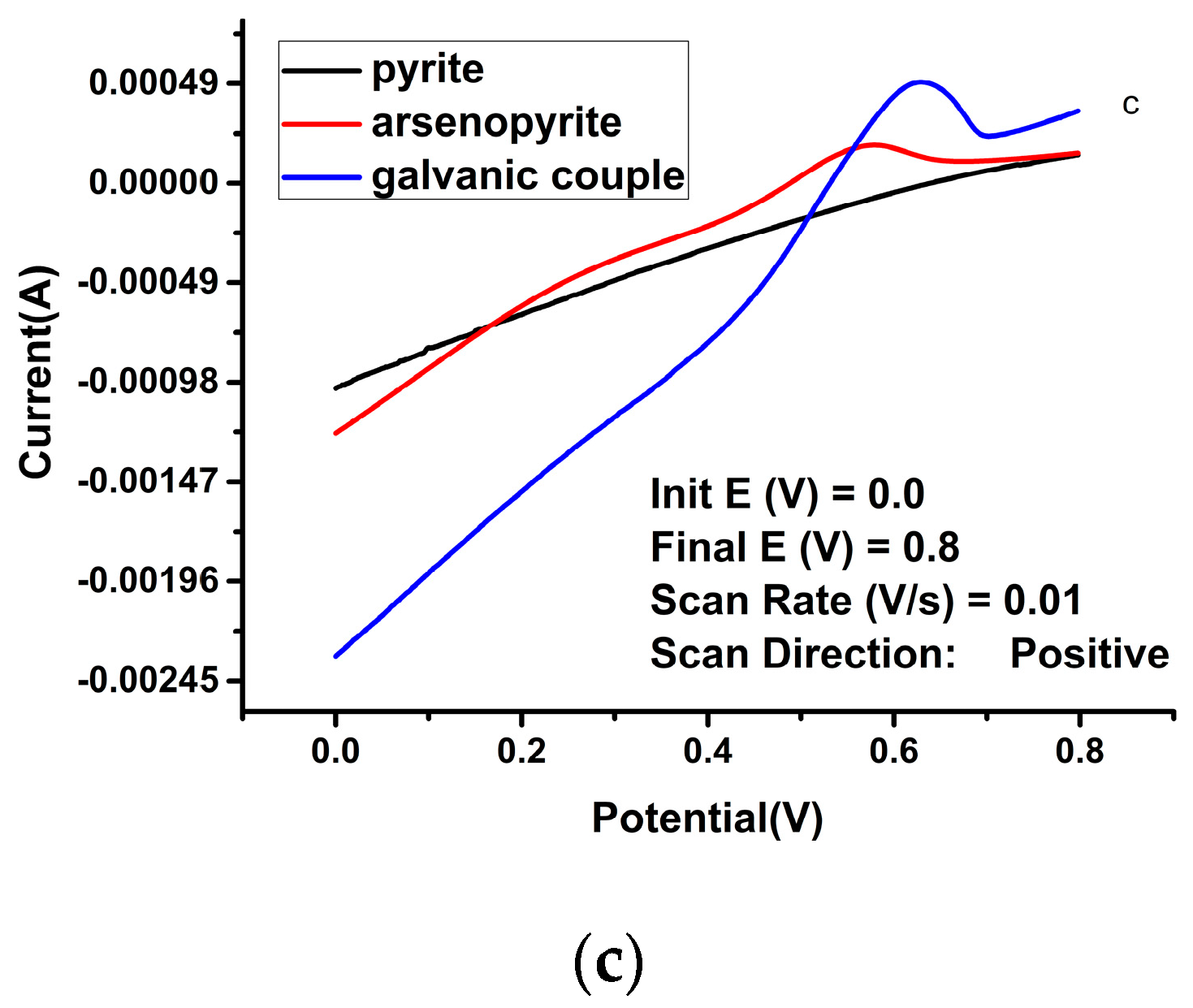
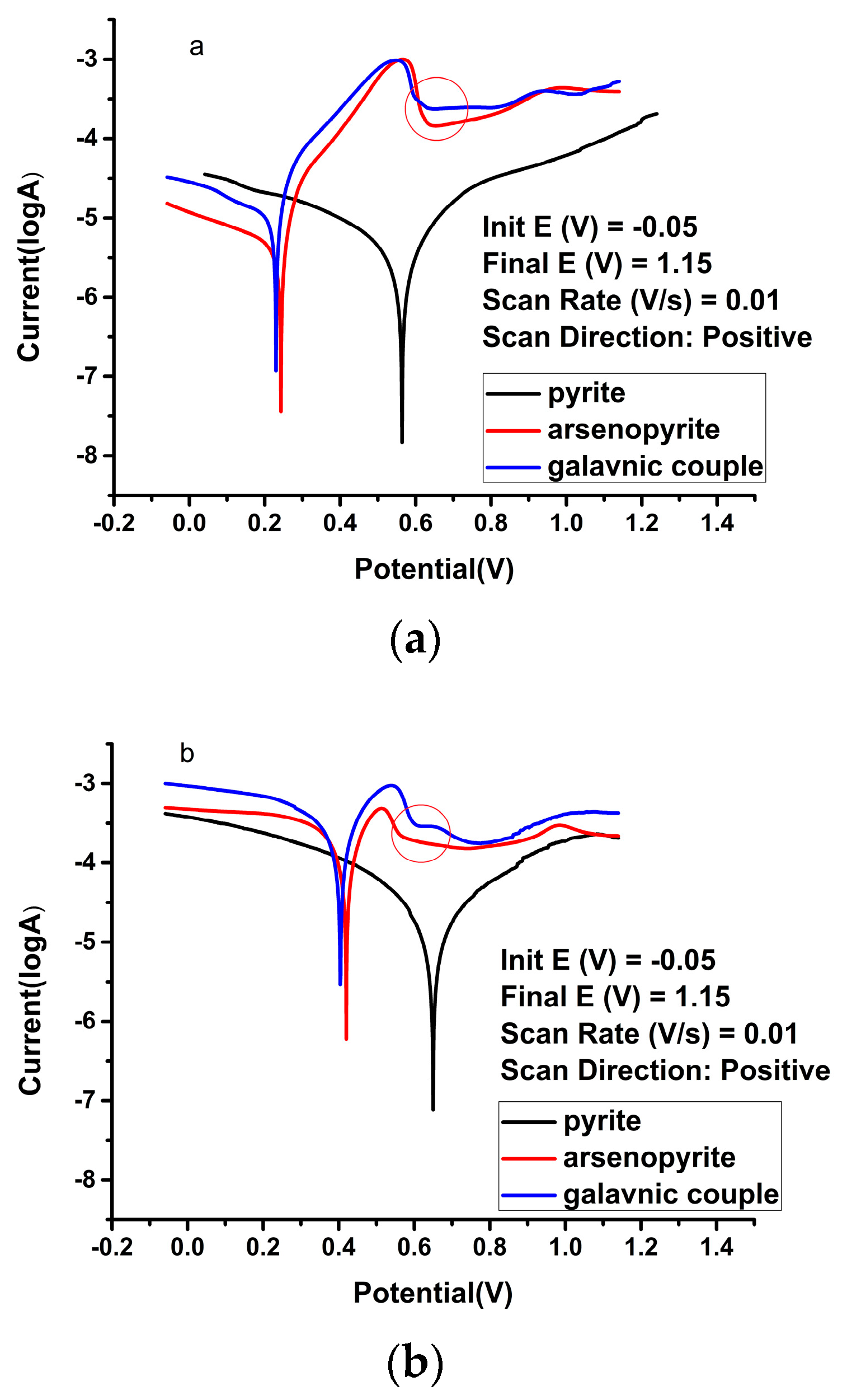
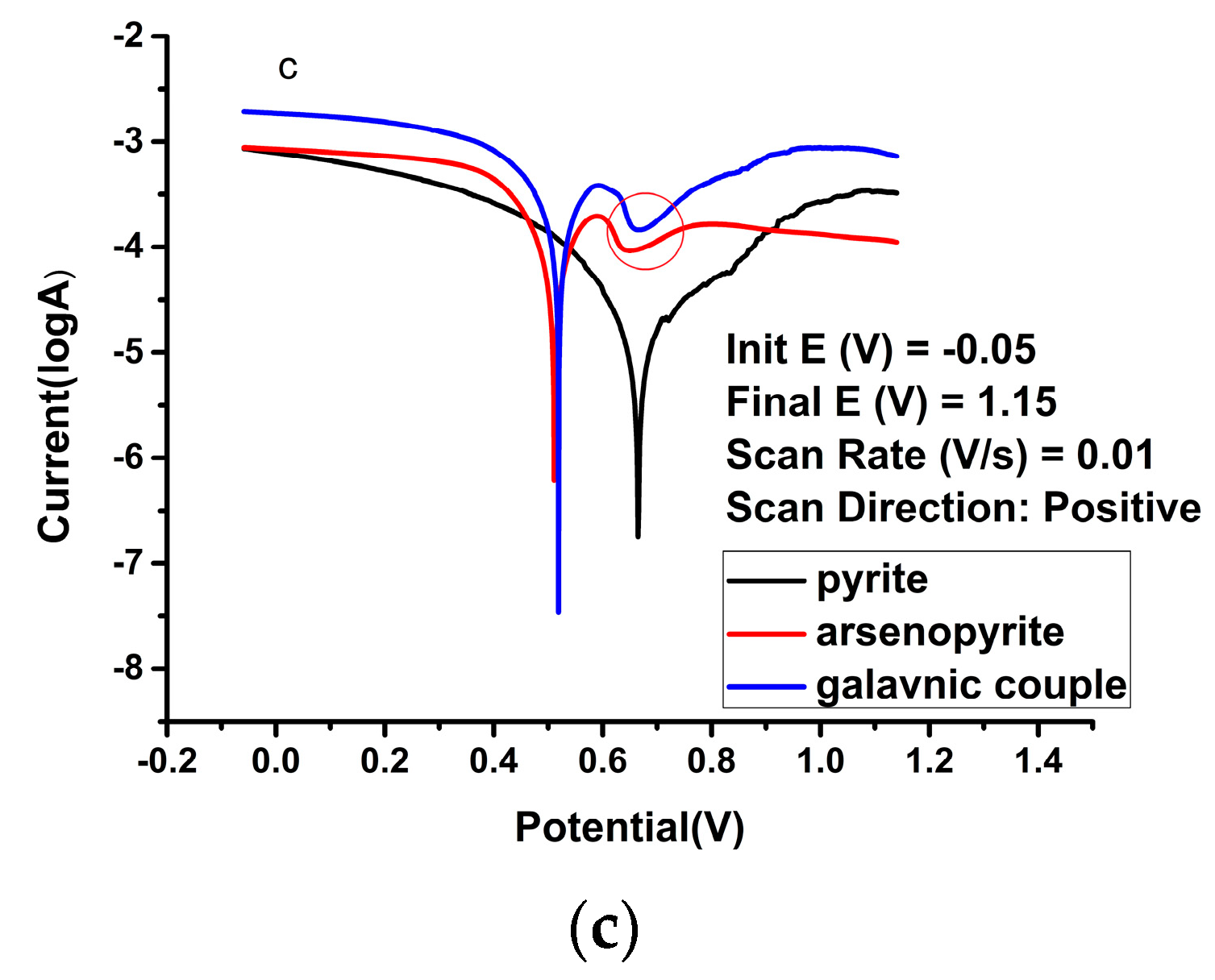
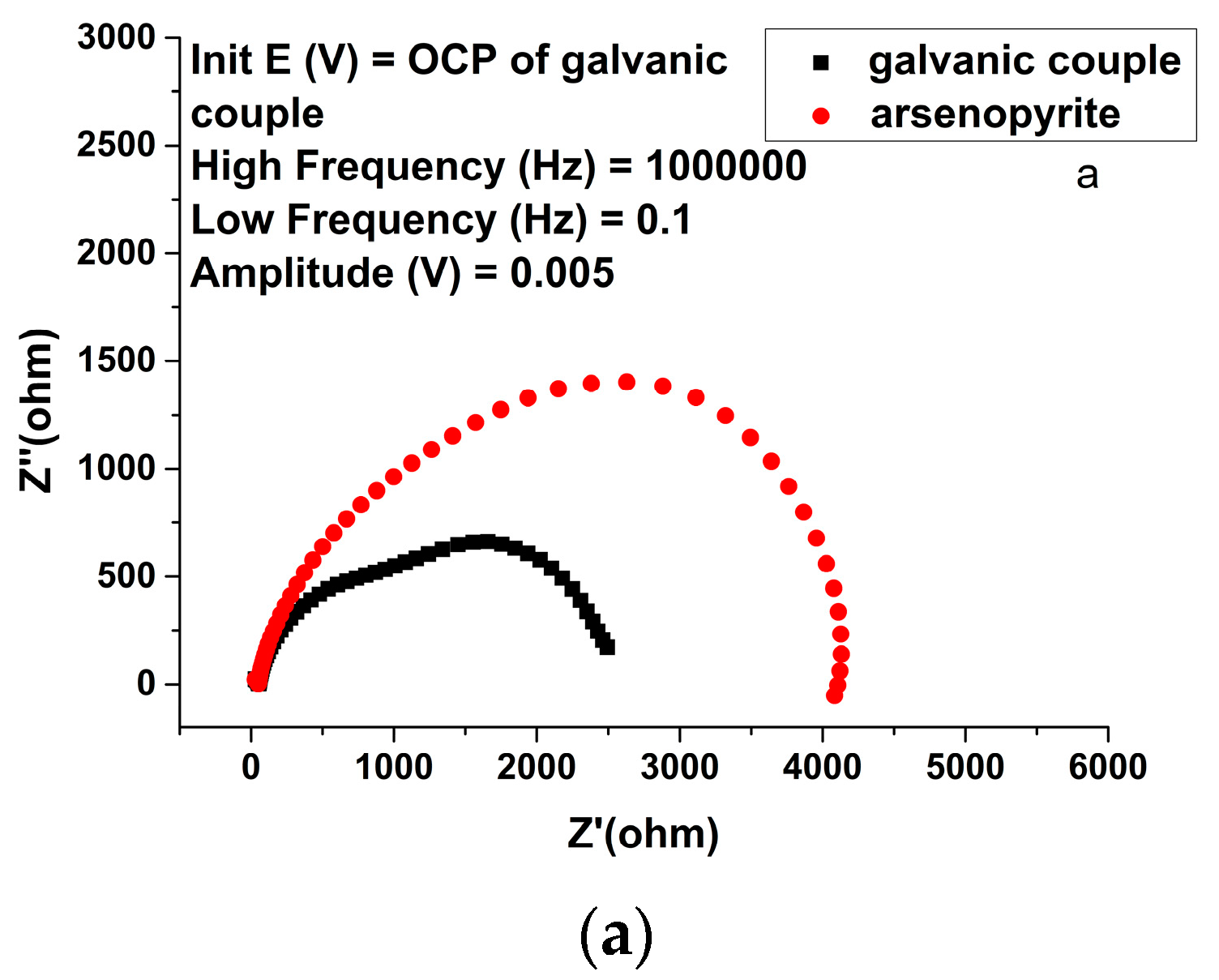
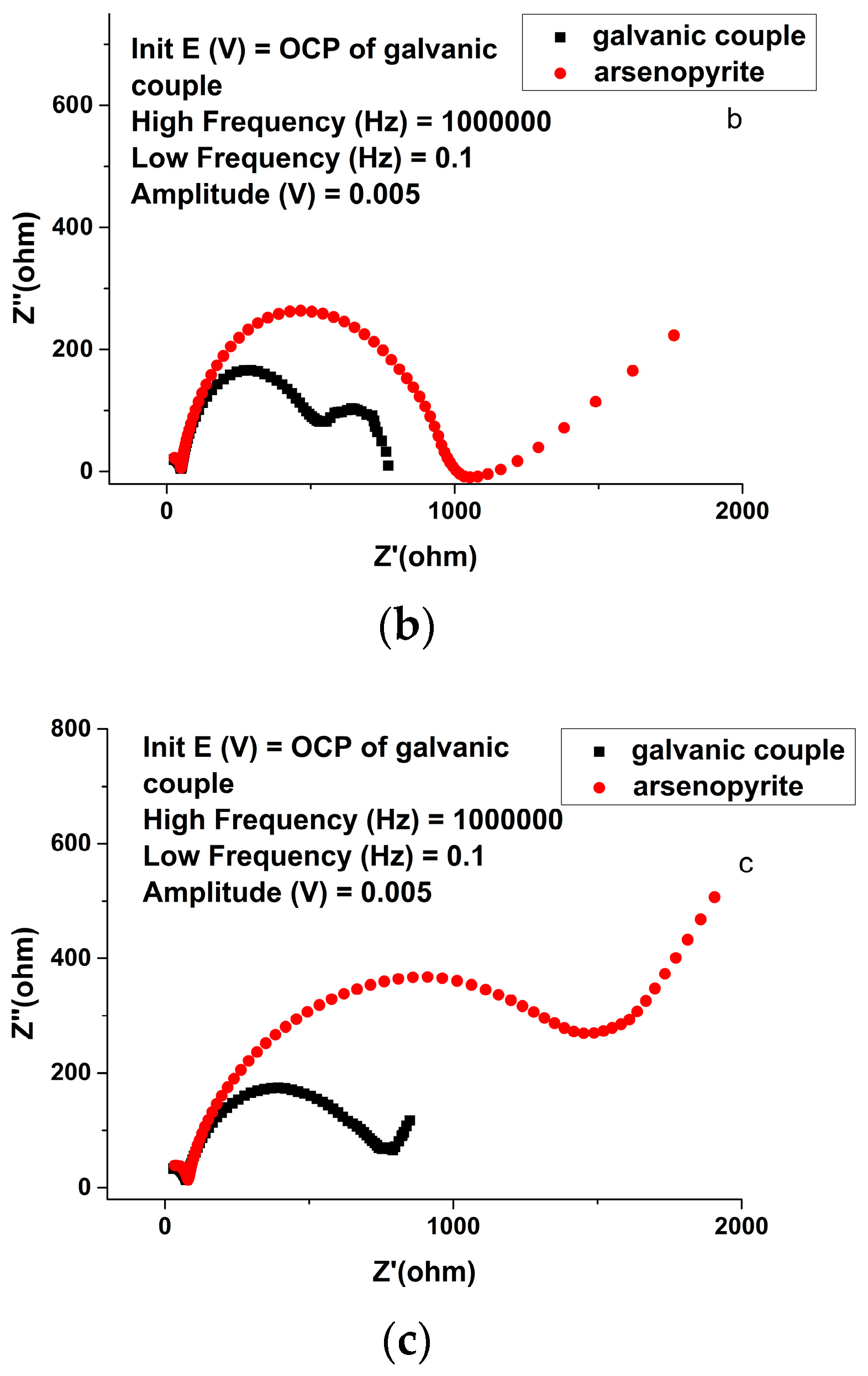

| Solution Type | Pyrite (mV) | Arsenopyrite (mV) | Galvanic Pair (mV) |
|---|---|---|---|
| Sulfuric acid | 704 | 316 | 354 |
| Ferric ion | 729 | 435 | 479 |
| HQ0211 strain | 768 | 446 | 614 |
| Solution | Pyrite | Arsenopyrite | Galvanic Pair | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C.C (lg i) | A.C (lg i) | icorr (10−6A) | Ecorr (V) | C.C (lg i) | A.C (lg i) | icorr (10−6A) | Ecorr (V) | C.C (lg i) | A.C (lg i) | icorr (10−6A) | Ecorr (V) | |
| Sulfuric acid | −5.61 | −5.59 | 3.83 | 0.56 | −5.37 | −4.81 | 14.1 | 0.22 | −5.00 | −4.54 | 33.8 | 0.20 |
| Ferric ion | −4.93 | −4.96 | 1.46 | 0.63 | −3.85 | −3.71 | 260 | 0.40 | −3.67 | −3.57 | 390 | 0.39 |
| HQ0211 strain | −4.83 | −4.91 | 18.8 | 0.64 | −3.92 | −3.40 | 160 | 0.46 | −3.40 | −3.23 | 467 | 0.45 |
| Solution | Electrode | Rs/Ω∙cm2 | Yo,1/10−9 S∙sn∙cm−2 (n) | R1/Ω∙cm2 | Yo,2/10−5 S∙sn∙cm−2 (n) | R2/Ω∙cm2 | Yo,3/105 S∙sn∙cm−2 | R3/Ω∙cm2 | Fitting Error χ2/10−3 |
|---|---|---|---|---|---|---|---|---|---|
| Sulfuric acid | Arsenopyrite | 7.958 | 5.33 | 42.44 | 1.059 | 1769 | 2.674 | 2398 | 0.23 |
| Galvanic pair | 6.408 | 8.49 | 48.21 | 1.635 | 1002 | 12.97 | 1475 | 0.69 | |
| Ferric ion | Arsenopyrite | 1.209 | 3.65 | 45.93 | 1.183 | 898 | - | - | 0.98 |
| Galvanic pair | 9.284 | 6.5 | 39.41 | 1.369 | 483 | - | - | 0.74 | |
| HQ0211 strain | Arsenopyrite | 2.337 | 2.69 | 75.23 | 1.25 | 1153 | - | - | 0.81 |
| Galvanic pair | 2.93 | 3.06 | 64.66 | 3.31 | 809 | - | - | 2.09 |
| Lattice Parameter | A (Angstrom) | B (Angstrom) | C (Angstrom) | α (°) | β (°) | γ (°) |
|---|---|---|---|---|---|---|
| Calculated pyrite lattice parameter | 5.411 | 5.411 | 5.411 | 90 | 90 | 90 |
| Calculation errors for pyrite | 0.01% | 0.01% | 0.01% | 0% | 0% | 0% |
| Calculated arsenopyrite lattice parameter | 9.46 | 5.63 | 6.39 | 89.9 | 89.7 | 89.9 |
| Calculation errors for arsenopyrite | 0.5% | 0.5% | 0.4% | 0.1% | 0.3% | 0.1% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.-N.; Shi, W.-G.; Ma, P.-C.; Lu, L.-S.; Chen, G.-M.; Yang, H.-Y. Corrosion Behavior of a Pyrite and Arsenopyrite Galvanic Pair in the Presence of Sulfuric Acid, Ferric Ions and HQ0211 Bacterial Strain. Minerals 2019, 9, 169. https://doi.org/10.3390/min9030169
Xu J-N, Shi W-G, Ma P-C, Lu L-S, Chen G-M, Yang H-Y. Corrosion Behavior of a Pyrite and Arsenopyrite Galvanic Pair in the Presence of Sulfuric Acid, Ferric Ions and HQ0211 Bacterial Strain. Minerals. 2019; 9(3):169. https://doi.org/10.3390/min9030169
Chicago/Turabian StyleXu, Jia-Ning, Wen-Ge Shi, Peng-Cheng Ma, Liang-Shan Lu, Gui-Min Chen, and Hong-Ying Yang. 2019. "Corrosion Behavior of a Pyrite and Arsenopyrite Galvanic Pair in the Presence of Sulfuric Acid, Ferric Ions and HQ0211 Bacterial Strain" Minerals 9, no. 3: 169. https://doi.org/10.3390/min9030169




