Genesis of the Koka Gold Deposit in Northwest Eritrea, NE Africa: Constraints from Fluid Inclusions and C–H–O–S Isotopes
Abstract
:1. Introduction
2. Regional Geology
3. Geology of the Koka Deposit
4. Sampling and Analytical Method
4.1. Fluid Inclusions (FIs)
4.2. Stable Isotope Analytical Methods
5. Fluid Inclusions
5.1. Fluid Inclusion Petrography
5.2. Fluid Inclusion Microthermometry
5.3. Laser Raman Spectroscopy
5.4. Ion and Gas Chromatography
6. C–H–O–S Isotopes
7. Discussion
7.1. Nature and Evolution of Ore-Forming Fluid
7.2. Source of Ore-Forming Fluids and Metal
7.3. Genetic Model for Ore Deposition
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kröner, A.; Eyal, M.; Eyal, Y. Early Pan-African evolution of the basement around Elat, Israel and the Sinai Peninsula revealed by single-zircon evaporation dating and implication for crustal accretion rates. Geology 1990, 18, 545–548. [Google Scholar] [CrossRef]
- Stern, R.J. Neoproterozoic (900–550 ma) arc assembly and continental collision in the east Africa orogen: Implications for the consolidation of Gondwanaland. Annu. Rev. Earth & Planet. Sci. 1994, 22, 319–351. [Google Scholar]
- Stern, R.J. Neoproterozoic crustal growth: The solid Earth system during a critical episode of Earth history. Gondwana Res. 2008, 14, 33–50. [Google Scholar] [CrossRef]
- Johnson, P.R.; Zoheir, B.A.; Ghebreab, W.; Stern, R.J.; Barrie, C.T.; Hamer, R.D. Gold-bearing volcanogenic massive sulfides and orogenic-gold deposits in the Nubian Shield. S. Afr. J. Geol. 2017, 120, 63–76. [Google Scholar] [CrossRef]
- Ghebreab, W.; Greiling, R.O.; Solomon, S. Structural setting of Neoproterozoic mineralization, Asmara district, Eritrea. J. Afr. Earth Sci. 2009, 55, 219–235. [Google Scholar] [CrossRef]
- Barrie, C.T.; Nielsen, F.W.; Aussant, C.H. The Bisha volcanic-associated massive sulfide deposit, western Nakfa terrane, Eritrea. Econ. Geol. 2007, 102, 717–738. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Duan, H.C.; Wang, F.X. General characteristics of geology and mineral resources in Eritrea and exploration progress. Miner. Explor. 2012, 5, 707–714. (In Chinese) [Google Scholar]
- Xiang, P.; Wang, J.X. Ore geology character and type of Koka gold deposit, Eritrea. Acta Mineral. Sin. 2013, s2, 1067–1068. (In Chinese) [Google Scholar]
- Goldfarb, R.J.; Groves, D.I.; Gardoll, S. Orogenic gold and geologic time: A global synthesis. Ore Geol. Rev. 2001, 18, 12–75. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Robert, F. Gold deposits in metamorphic belts: Overview of current understanding, outstanding problems, future research, and exploration significance. Econ. Geol. 2003, 98, 1–29. [Google Scholar]
- Goldfarb, R.J.; Groves, D.I. Orogenic gold: Common or evolving fluid and metal sources through time. Lithos. 2015, 233, 2–26. [Google Scholar] [CrossRef]
- Abdelsalam, M.; Stern, R. Sutures and shear zones in the Arabian-Nubian Shield. J. Afr. Earth Sci. 1996, 23, 289–310. [Google Scholar] [CrossRef]
- Stern, R.J.; Johnson, P.R.; Kröner, A.; Yibas, B. Neoproterozoic ophiolites of the Arabian-Nubian shield. Dev. Precambrian Geol. 2004, 13, 95–128. [Google Scholar]
- Johnson, P.R.; Woldehaimanot, B. Development of the Arabian–Nubian Shield: Perspectives on Accretion and Deformation in the Northern East African Orogen and the Assembly of Gondwana; Special Publication: London, UK, 2003; pp. 290–325. [Google Scholar]
- Johnson, P.R.; Andresen, A.; Collins, A.S.; Fowler, A.R.; Fritz, H.; Ghebreab, W.; Kusky, T.; Stern, R.J. Late Cryogenian–Ediacaran history of the Arabian–Nubian shield: A review of depositional, plutonic, structural, and tectonic events in the closing stages of the northern East African Orogen. J. Afr. Earth Sci. 2011, 61, 167–232. [Google Scholar] [CrossRef]
- Drury, S.A.; Berhe, S.M. Accretion tectonics in Northern Eritrea revealed by remotely sensed imagery. Geol. Mag. 1993, 130, 177–190. [Google Scholar] [CrossRef]
- Teklay, M. Petrology, Geochemistry and Geochronology of Neoproterozoic Magmatic Arc Rocks from Eritrea: Implications for Crustal Evolution in the Southern Nubian Shield; Department of Mines-Ministry of Energy Mines and Water Resources-State of Eritrea: Asmara, Eritrea, 1997; Volume 1, pp. 1–125. [Google Scholar]
- Drury, S.A.; De Souza Filho, C.R. Neoproterozoic terrane assemblages in Eritrea: Review and prospects. J. Afr. Earth Sci. 1998, 27, 331–348. [Google Scholar] [CrossRef]
- Zhao, K.; Yao, H.Z.; Wang, J.X.; Ghebsha, F.G.; Xiang, W.S.; Yang, Z. Zircon U-Pb geochronology and geochemistry of Koka granite and its geological significances, Eritrea. Earth Sci. in press. (In Chinese).
- Dean, C.; David, L.; David, G. Technical Report on the Koka Gold Deposit, Eritea; Chalice Gold Mine Limited: Asmara, Eritrea, 2010; pp. 1–111. [Google Scholar]
- Bozzo, A.T.; Chen, H.S.; Kass, J.R.; Barduhn, A.J. The properties of the hydrates of chlorine and carbon dioxide. Desalination 1975, 16, 303–320. [Google Scholar] [CrossRef]
- Lu, H.Z.; Fan, H.R.; Ni, P.; Ou, G.X.; Shen, K.; Zhang, W.H. Fluid Inclusions; Science Press: Beijing, China, 2004; pp. 406–419. (In Chinese) [Google Scholar]
- Clayton, J.; Tretiak, D.N. Amine-citrate buffers for pH control in starch gel electrophoresis. J. Fish. Board Can. 1972, 29, 1169–1172. [Google Scholar] [CrossRef]
- Roedder, E. Fluid Inclusions. Review in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; pp. 1–644. [Google Scholar]
- Chen, H.Y.; Chen, Y.J.; Baker, M.J. Evolution of ore-forming fluids in the Sawayaerdun gold deposit in the Southwestern Chinese Tianshan metallogenic belt. J. Asian Earth Sci. 2012, 49, 131–144. [Google Scholar] [CrossRef]
- Taylor, H.P. The application of oxygen and hydrogen isotope studies to problems of hydrothermal alteration and ore deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Yang, L.Q.; Deng, J.; Guo, L.N.; Wang, Z.L.; Li, X.Z.; Li, J.L. Origin and evolution of ore fluid, and gold-deposition processes at the giant Taishang gold deposit, Jiaodong Peninsula, eastern China. Ore Geol. Rev. 2016, 72, 585–602. [Google Scholar] [CrossRef]
- Yang, L.Q.; Guo, L.N.; Wang, Z.L.; Zhao, R.X.; Song, M.C.; Zheng, X.L. Timing and mechanism of gold mineralization at the Wang’ershan gold deposit, Jiaodong Peninsula, eastern China. Ore Geol. Rev. 2017, 88, 491–510. [Google Scholar] [CrossRef]
- Qiu, K.F.; Taylor, R.D.; Song, Y.H.; Yu, H.C.; Song, K.R.; Li, N. Geologic and geochemical insights into the formation of the Taiyangshan porphyry copper–molybdenum deposit, Western Qinling Orogenic Belt, China. Gondwana Res. 2016, 35, 40–58. [Google Scholar] [CrossRef]
- Yang, L.Q.; Deng, J.; Wang, Z.L.; Guo, L.N.; Li, R.H.; Groves, D.I.; Danyushevsky, L.V.; Zhang, C.; Zheng, X.L.; Zhao, H. Relationships between gold and pyrite at the Xincheng gold deposit, Jiaodong Peninsula, China: Implications for gold source and deposition in a brittle epizonal environment. Econ. Geol. 2016, 111, 105–126. [Google Scholar] [CrossRef]
- Yang, L.Q.; Deng, J.; Li, R.P.; Guo, L.N.; Wang, Z.L.; Chen, B.H.; Wang, X.D. World-class Xincheng gold deposit: An example from the giant Jiaodong gold province. Geosci. Front. 2016, 7, 419–430. [Google Scholar] [CrossRef]
- Groves, D.I.; Goldfarb, R.J.; Gebre-Mariam, M.; Hagemann, S.G.; Robert, F. Orogenic gold deposits: A proposed classification in the context of their crustal distribution and relationship to other gold deposit types. Ore Geol. Rev. 1998, 13, 7–27. [Google Scholar] [CrossRef]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Chapman & Hall: Abingdon, UK, 1985; pp. 1–239. [Google Scholar]
- Naden, J.; Shepherd, T.J. Role of methane and carbon dioxide in gold deposition. Nature 1989, 342, 793–795. [Google Scholar] [CrossRef]
- Brown, P.E. FLINCOR: A microcomputer program for the reduction and investigation of fluid-inclusion data. Am. Mineral. 1989, 74, 1390–1393. [Google Scholar]
- Roedder, E.; Bodnar, R.J. Geologic pressure determinations from fluid inclusion studies. Annu. Rev. Earth Planet. Sci. 1980, 8, 263–301. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Shao, Y.J.; Zhou, H.D.; Wu, Q.H.; Liu, J.P.; Wei, H.T.; Zhao, R.C.; Cao, J.Y. Ore-forming mechanism of quartz-vein-type W-Sn deposits of the Xitian district in SE China: Implications from the trace element analysis of wolframite and investigation of fluid inclusions. Ore Geol. Rev. 2017, 83, 152–173. [Google Scholar] [CrossRef]
- Xiong, S.; He, M.; Yao, S.; Cui, Y.; Shi, G.; Ding, Z.; Hu, X. Fluid evolution of the Chalukou giant Mo deposit in the northern Great Xing’an Range, NE China. Geol. J. 2015, 50, 720–738. [Google Scholar] [CrossRef]
- Van den Kerkhof, A.; Thiéry, R. Carbonic inclusions. Lithos 2001, 55, 49–68. [Google Scholar] [CrossRef]
- Qiu, K.F.; Marsh, E.; Yu, H.C.; Pfaff, K.; Gulbransen, C.; Gou, Z.Y.; Li, N. Fluid and metal sources of the Wenquan porphyry molybdenum deposit, Western Qinling, NW China. Ore Geol. Rev. 2017, 86, 459–473. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 130–135. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 1997; pp. 1–201. [Google Scholar]
- Schidowski, M.; Hayes, J.M.; Kaplan, I.R. Isotopic inferences of ancient biochemistry: Carbon, sulfur, hydrogen and nitrogen. In Earth’s Earliest Biosphere; Schopf, J.W., Ed.; Princeton University Press: Princeton, NJ, USA, 1983; pp. 149–186. [Google Scholar]
- Faure, G. Principles of Isotope Geology, 2nd ed.; Wiley: New York, NY, USA, 1977; p. 589. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; John Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Chaussidon, M.; Lorand, J.P. Sulphur isotope composition of orogenic spinel lherzolite massifs from Ariege (North-Eastern Pyrenees, France): An ion microprobe study. Geochim. Cosmochim. Acta. 1990, 54, 2835–2846. [Google Scholar] [CrossRef]
- Xiong, Y.Q.; Shao, Y.J.; Mao, J.W.; Wu, S.C.; Zhou, H.D.; Zheng, M.H. The polymetallic magmatic-hydrothermal Xiangdong and Dalong systems in the W–Sn–Cu–Pb–Zn–Ag Dengfuxian orefield, SE China: Constraints from geology, fluid inclusions, H–O–S–Pb isotopes, and sphalerite Rb–Sr geochronology. Miner. Depos. 2019. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Xu, B.L.; Zhou, G.T. Geochemical studies of stable isotopes in minerals. Earth Sci. Front. 2000, 7, 299–320. (In Chinese) [Google Scholar]
- Deng, J.; Liu, X.F.; Wang, Q.F.; Pan, R.G. Origin of the Jiaodong-type Xinli gold deposit, Jiaodong peninsula, China: Constraints from fluid inclusion and C–D–O–S–Sr isotope compositions. Ore Geol. Rev. 2015, 65, 674–686. [Google Scholar] [CrossRef]
- Yang, L.Q.; Deng, J.; Li, N.; Zhang, C.; Yu, J.Y. Isotopic characteristics of gold deposits in the Yangshan Gold Belt, West Qinling, central China: Implications for fluid and metal sources and ore genesis. J. Geochem. Explor. 2016, 168, 103–118. [Google Scholar] [CrossRef]
- Ghebreab, W. Tectono-metamorphic history of Neoproterozoic rocks in eastern Eritrea. Precambrian Res. 1999, 98, 83–105. [Google Scholar] [CrossRef]
- Albino, G.V.; Jalal, S.; Christensen, K. Neoproterozoic mesothermal gold mineralization at Sukhaybarat East mine. Trans. Inst. Min. Metall. (Sect. B Appl. Earth Sci.) 1995, 104, 157–170. [Google Scholar]
- Walker, B.M.; Lewis, R.S.; Al Otaibi, R.; Ben Talib, M.; Christian, R.; Gabriel, B.R. An Najadi Gold Prospect, Kingdom of Saudi Arabia; Geology and Gold-Resource Assessment; Saudi Arabian Deputy Ministry for Mineral Resources Technical Report, USGS-TR-94-5; Reston Publishing Service Center: Reston, VA, USA, 1994; pp. 1–89. [Google Scholar]
- Doebrich, J.L.; Zahony, S.G.; Leavitt, J.D.; Portacio, J.S., Jr.; Siddiqui, A.A.; Wooden, J.L.; Fleck, R.J.; Stein, H.J. Ad Duwayhi, Saudi Arabia: Geology and geochronology of a Neoproterozoic intrusion-related gold system in the Arabian shield. Econ. Geol. 2004, 99, 713–741. [Google Scholar] [CrossRef]
- Billay, A.Y.; Kisters, A.F.M.; Meyer, F.M.; Schneider, J. The geology of the Lega Dembi gold deposit, southern Ethiopia: Implications for Pan-African gold exploration. Miner. Depos. 1997, 32, 491–504. [Google Scholar] [CrossRef]
- Andersson, U.B.; Ghebreab, W.; Teklay, M. Crustal evolution and metamorphism in east-central Eritrea, south-east Arabian-Nubian Shield. J. Afr. Earth Sci. 2006, 44, 45–65. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ni, P.; Fan, H.R.; Prajno, F.; Nai, Y.; Su, W.C.; Zhang, H. Diagnostic fluid inclusions of different types hydrothermal gold deposits. Acta Petrol. Sin. 2007, 23, 2085–2108. (In Chinese) [Google Scholar]



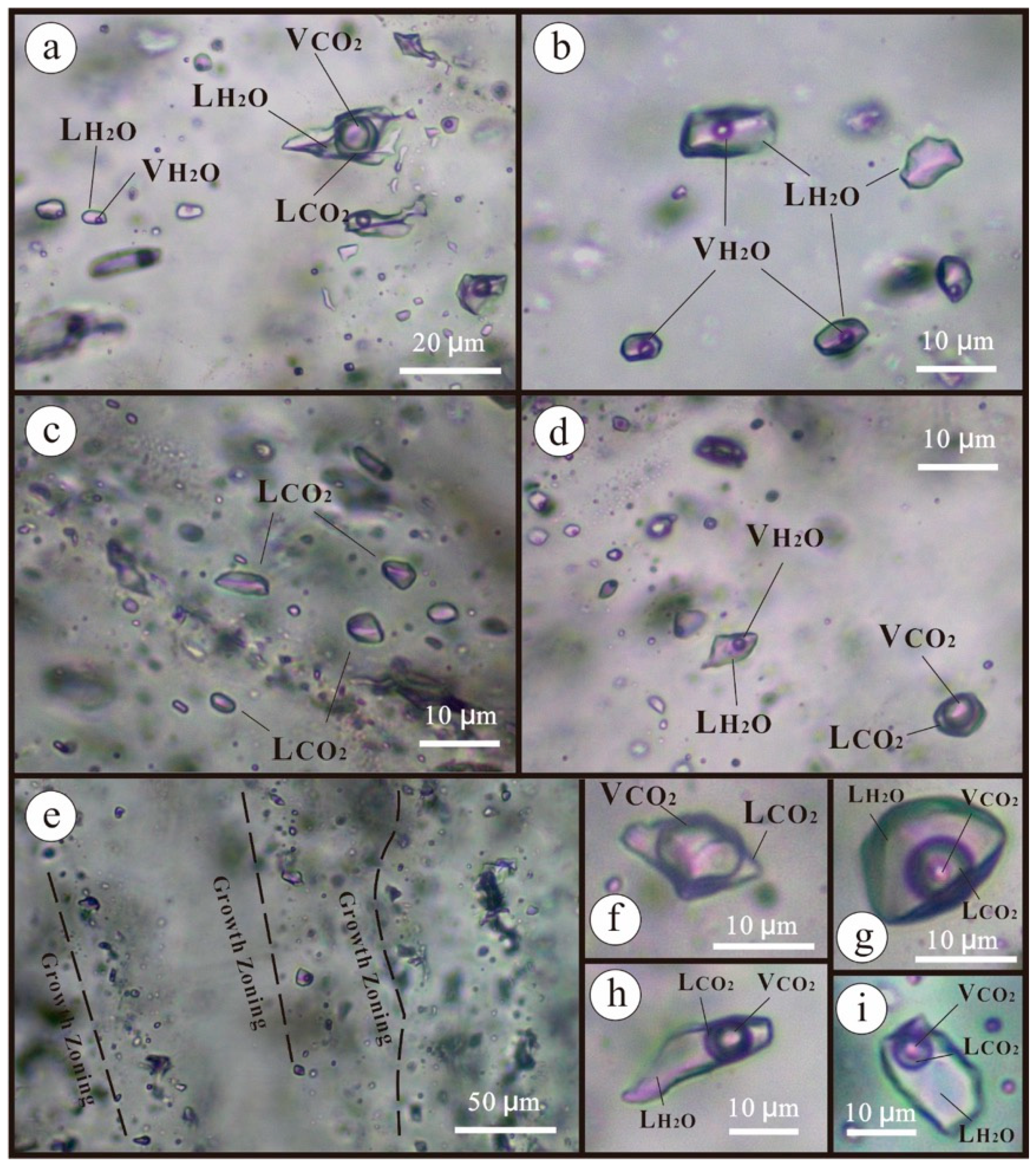







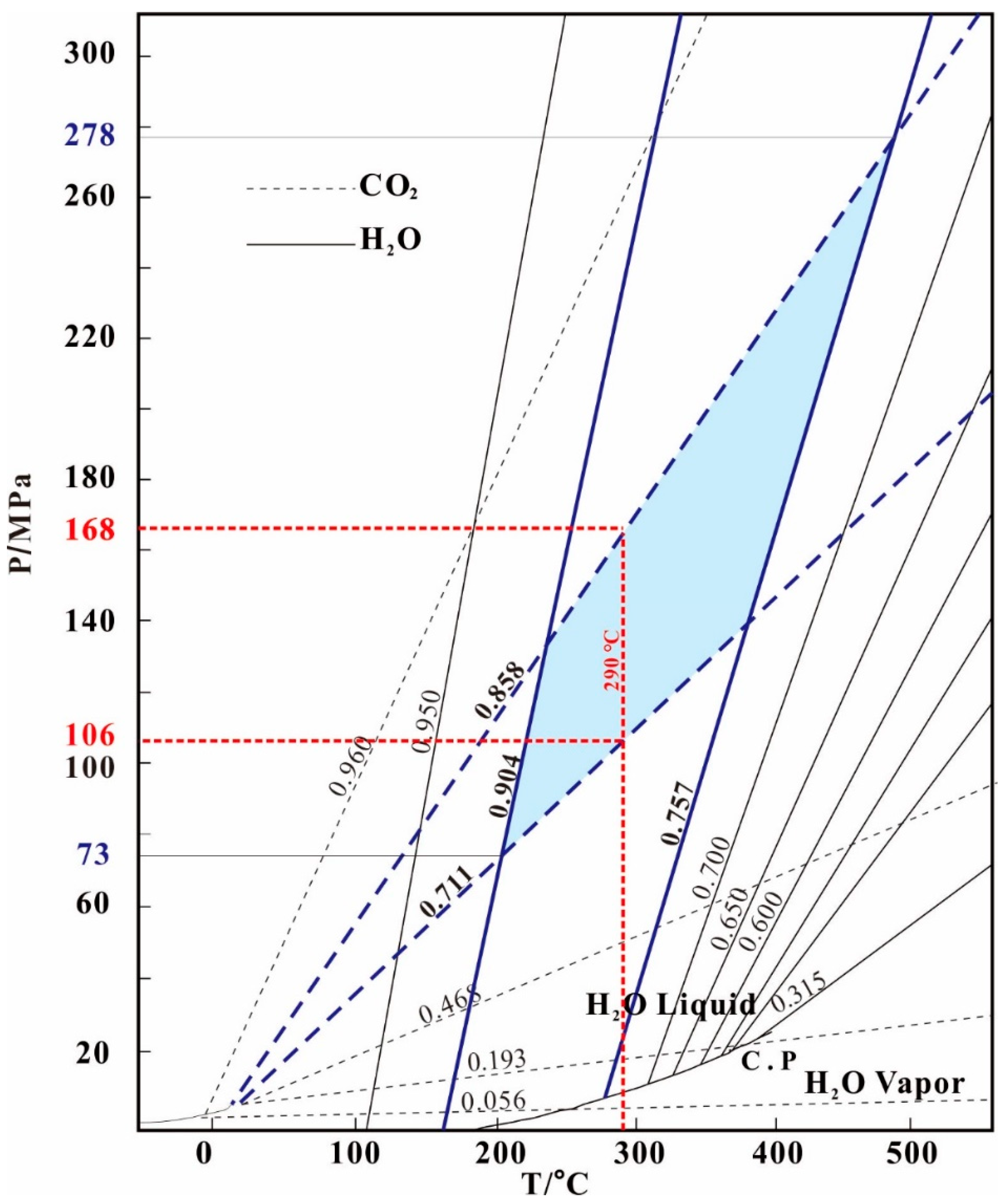

| Sample No. | Stage | FI type | Size (μm) | Number | Tm, CO2 (°C) | Tm, ice (°C) | Tm, Cl (°C) | Th, CO2 (°C) | Th, (°C) | Salinity (wt% NaCl) | Density (g/cm3) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| KO-15 | Stage I | Type I, minor | 6~12 | 4 | –4.3~–2.4 | 254~341 (V) | 4.0~6.9 | 0.757~0.822 | |||
| Type II, abundant | 8~16 | 9 | –56.9~–58.0 | –2.3~17.2 | 0.801~0.904 | ||||||
| Type III, abundant | 8~16 | 11 | –56.7~–57.9 | 5.7~7.8 | 19.2~30.1 | 280~356 (V) | 4.3~7.9 | 0.576~0.793 | |||
| KO-16 | Type I, minor | 6~12 | 5 | –2.6~–1.9 | 243~288 (V) | 3.2~4.3 | 0.711~0.831 | ||||
| Type II, abundant | 6~12 | 10 | –56.7~–58.2 | –4.9~18.9 | 0.757~0.898 | ||||||
| Type III, minor | 6~20 | 14 | –56.8~–58.1 | 5.8~7.9 | 19.9~29.6 | 235~295 (V) | 4.1~7.7 | 0.608~0.783 | |||
| KO-17 | Type I, minor | 6~12 | 6 | –3.2~–1.8 | 223~275 (V) | 3.1~5.3 | 0.783~0.858 | ||||
| Type II, abundant | 6~14 | 13 | –56.8~–58.0 | –4.7~16.4 | 0.812~0.901 | ||||||
| Type III, abundant | 10~24 | 14 | –56.6~–58.0 | 5.3~8.0 | 10.6~29.4 | 238~305 (V) | 3.3~8.5 | 0.613~0.862 | |||
| KO-3 | Stage II | Type I, abundant | 8~16 | 6 | –2.6~–1.5 | 221~288 (V) | 2.6~4.3 | 0.765~0.872 | |||
| Type III, abundant | 8~20 | 11 | –57.0~–57.9 | 6.2~8.2 | 19.7~29.7 | 228~295 (V) | 3.5~7.1 | 0.613~0.787 | |||
| KO-7 | Type I, abundant | 8~12 | 6 | –2.8~–1.3 | 212~280 (V) | 2.2~4.7 | 0.763~0.871 | ||||
| Type III, abundant | 8~24 | 11 | –56.9~–57.4 | 6.6~8.5 | 18.5~29.7 | 232~318 (V) | 3.0~7.5 | 0.597~0.793 | |||
| KO-14 | Type I, minor | 8~14 | 5 | –2.6~–0.9 | 235~267 (V) | 2.2~4.3 | 0.786~0.842 | ||||
| Type III, abundant | 10~22 | 10 | –56.7~–57.3 | 5.6~8.0 | 18.2~25.7 | 259~309 (V) | 3.9~8.0 | 0.672~0.701 |
| Sample No. | Stage | H2O | CO2 | CO | CH4 | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− |
|---|---|---|---|---|---|---|---|---|---|---|---|
| mol% | mol% | mol% | mol% | mg/L | mg/L | mg/L | mg/L | mg/L | mg/L | ||
| KO-15 | Stage I | 0.595 | 0.405 | <0.01 | <0.01 | 0.18 | 4.74 | 0.09 | 0.02 | 5.99 | 0.52 |
| KO-16 | 0.526 | 0.474 | <0.01 | 0.11 | 0.31 | 6.24 | 0.07 | <0.01 | 8.36 | 0.48 | |
| KO-17 | 0.484 | 0.516 | <0.01 | <0.01 | <0.01 | 5.22 | 0.06 | <0.01 | 6.52 | 0.56 | |
| KO-3 | Stage II | 0.741 | 0.259 | <0.01 | <0.01 | 0.47 | 8.06 | 0.08 | <0.01 | 9.62 | 0.50 |
| KO-7 | 0.765 | 0.235 | <0.01 | <0.01 | 0.88 | 11.22 | 0.10 | 0.02 | 15.63 | 0.50 |
| Sample NO. | Stage | Mineral | δDsmow (‰) | δ18Osmow (‰) | δ18OH2O (‰) | δ13Cv-PDB (‰) | Temperature (°C) |
|---|---|---|---|---|---|---|---|
| KO-15 | Stage I | Quartz | –52.5 | 9.7 | 2.4 | –5.0 | 290 |
| KO-16 | Quartz | –57.0 | 9.0 | 1.7 | –5.4 | 290 | |
| KO-17 | Quartz | –50.1 | 9.5 | 2.2 | –5.5 | 290 | |
| KO-3 | Stage II | Quartz | –53.1 | 10.8 | 2.7 | –4.4 | 270 |
| KO-7 | Quartz | –54.1 | 10.1 | 2.0 | –4.4 | 270 |
| Sample No. | Stage | Mineral | δ34SCDT (‰) |
|---|---|---|---|
| KO-11 | Stage I | Pyrite | 1.7 |
| KO-11 | Pyrite | −0.1 | |
| KO-15 | Pyrite | 1.3 | |
| KO-16 | Pyrite | 1.4 | |
| KO-17 | Pyrite | 1.6 | |
| KO-17 | Pyrite | 1.6 | |
| KO-17 | Chalcopyrite | 1.3 | |
| KO-17 | Galena | –1.3 | |
| KO-17 | Sphalerite | 1.2 | |
| KO-3 | Stage II | Pyrite | 1.9 |
| KO-3 | Pyrite | 1.9 | |
| KO-7 | Pyrite | 2.7 | |
| KO-14 | Pyrite | 1.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, K.; Yao, H.; Wang, J.; Ghebretnsae, G.F.; Xiang, W.; Xiong, Y.-Q. Genesis of the Koka Gold Deposit in Northwest Eritrea, NE Africa: Constraints from Fluid Inclusions and C–H–O–S Isotopes. Minerals 2019, 9, 201. https://doi.org/10.3390/min9040201
Zhao K, Yao H, Wang J, Ghebretnsae GF, Xiang W, Xiong Y-Q. Genesis of the Koka Gold Deposit in Northwest Eritrea, NE Africa: Constraints from Fluid Inclusions and C–H–O–S Isotopes. Minerals. 2019; 9(4):201. https://doi.org/10.3390/min9040201
Chicago/Turabian StyleZhao, Kai, Huazhou Yao, Jianxiong Wang, Ghebsha Fitwi Ghebretnsae, Wenshuai Xiang, and Yi-Qu Xiong. 2019. "Genesis of the Koka Gold Deposit in Northwest Eritrea, NE Africa: Constraints from Fluid Inclusions and C–H–O–S Isotopes" Minerals 9, no. 4: 201. https://doi.org/10.3390/min9040201
APA StyleZhao, K., Yao, H., Wang, J., Ghebretnsae, G. F., Xiang, W., & Xiong, Y.-Q. (2019). Genesis of the Koka Gold Deposit in Northwest Eritrea, NE Africa: Constraints from Fluid Inclusions and C–H–O–S Isotopes. Minerals, 9(4), 201. https://doi.org/10.3390/min9040201





