Semi-Smelting Reduction and Magnetic Separation for the Recovery of Iron and Alumina Slag from Iron Rich Bauxite
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Analysis
2.2. Semi-Smelting Reduction and Magnetic Separation
2.3. Thermodynamic Mechanism
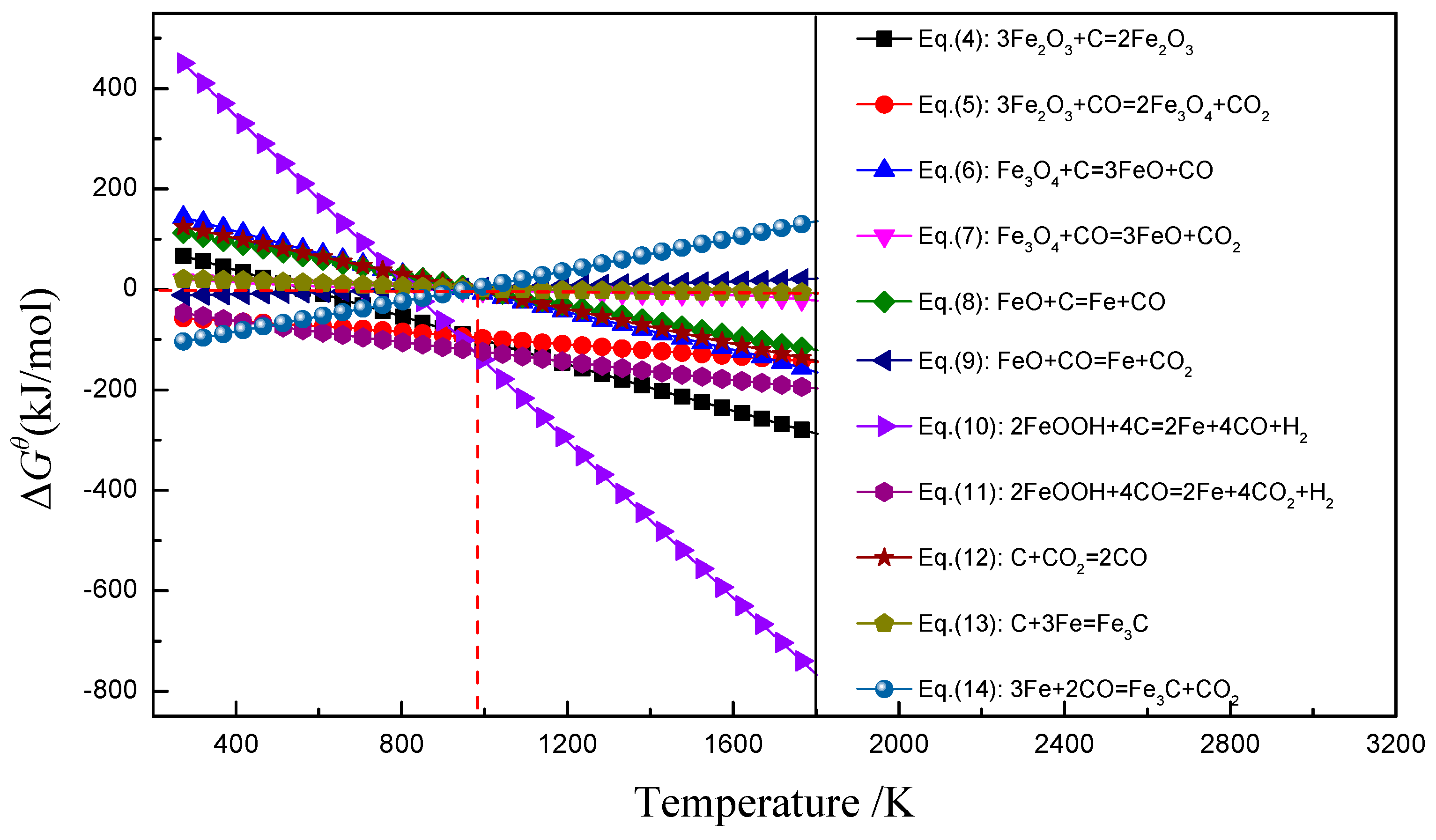
2.3.1. Thermodynamic Mechanism of Reduction Reaction
2.3.2. Thermodynamic Mechanism of Calcium Aluminate Slag
3. Results and Discussion
3.1. Recovery of Iron and Alumina Slag
3.1.1. Effect of Basicity
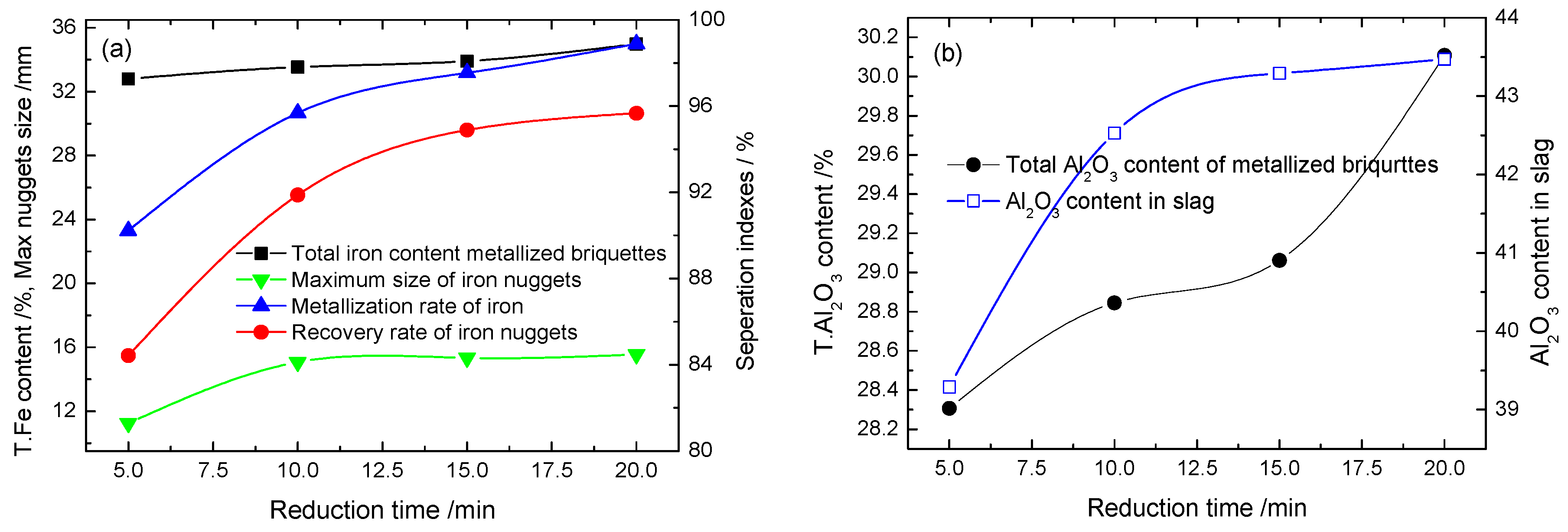
3.1.2. Effect of Reducing Time
3.1.3. Effect of Reduction Temperature
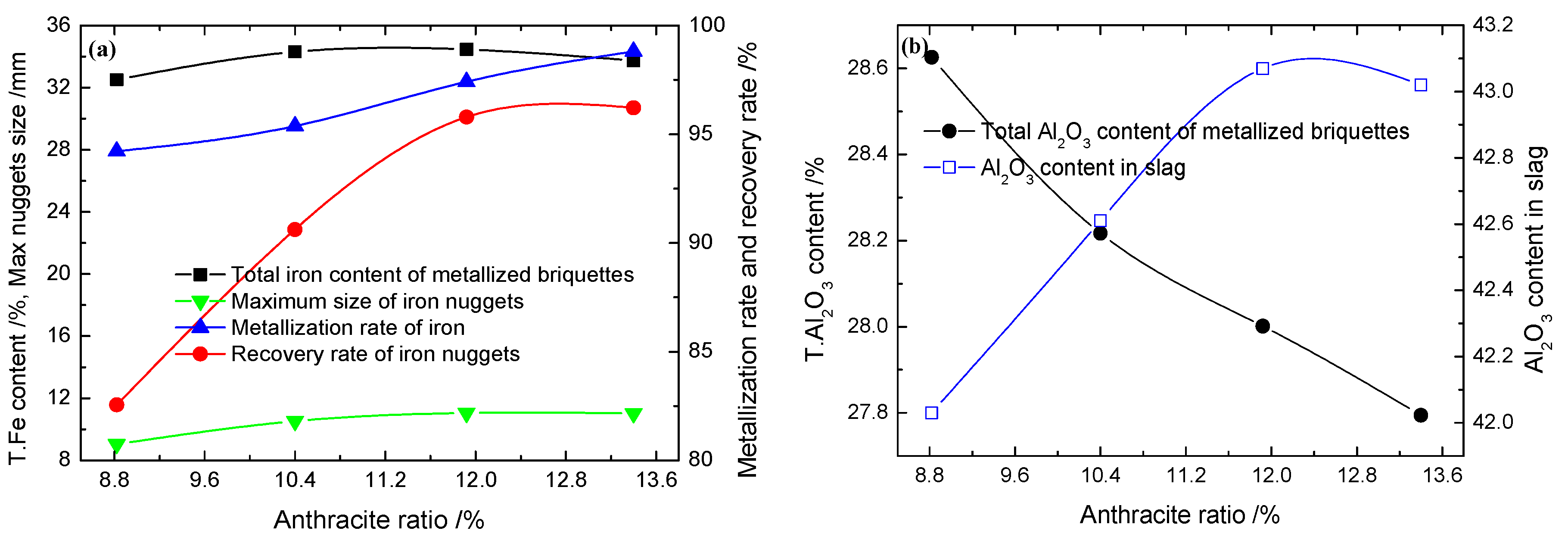
3.1.4. Effect of the Anthracite Ratio
3.1.5. Effect of the CaF2 Ratio
3.2. Properties Characterization of Reduction Products
3.2.1. X-Ray Diffraction Analysis
3.2.2. SEM–EDS Analysis
3.2.3. Chemical Composition Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.Y.; Hu, P.; Zhang, Z.Y.; Qi, Y.H.; Zou, Z.S. Mineralogical and geochemical characteristics of the Guigang Salento-type bauxite deposits, western Guangxi, China. Acta Geodyn. Geomater. 2014, 105, 1–7. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Q.F.; Zhang, Q.Z.; Feng, Y.W.; Cai, S.H. Mineralogical characteristics of the superlarge Quaternary bauxite deposits in Jingxi and Debao counties, western Guangxi, China. J. Asian Earth Sci. 2012, 52, 53–62. [Google Scholar] [CrossRef]
- Liu, X.F.; Wang, Q.F.; Zhang, Q.Z.; Yang, S.J.; Liang, Y.Y.; Zhang, Y.; Li, Y.; Guan, T. Genesis of the Permian karstic Pingguo bauxite deposit, western Guangxi, China. Miner. Deposita 2017, 52, 1031–1048. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Mikhailova, A.B.; Dorofievich, I.V.; Zheleznyi, M.V.; Gol’dberg, M.A.; Kutsev, S.V. Reaction of bauxite with hydrochloric acid under autoclave conditions. Metallurgist 2016, 60, 204–211. [Google Scholar] [CrossRef]
- Zhao, A.C.; Liu, Y.; Zhang, T.A.; Lv, G.Z.; Dou, Z.H. Thermodynamics study on leaching process of gibbsitic bauxite by hydrochloric acid. Tran. Nonferr. Metal. Soc. China 2013, 23, 266–270. [Google Scholar] [CrossRef]
- Guan, C.P.; Chen, L.Z.; Zheng, Y.M.; Sun, W.; Zheng, Y.X. Hydrochloric acid leaching for upgrading flotation concentrate from a low-grade bauxite ore. Physicochem. Probl. Miner. Process. 2017, 53, 1038–1046. [Google Scholar] [CrossRef]
- Mendelovici, E. Acid and thermal treatments of lateritic bauxites. J. Therm. Anal. Calorim. 2004, 75, 957–964. [Google Scholar] [CrossRef]
- Valeev, D.V.; Lainer, Y.A.; Pak, V.I. Autoclave leaching of boehmite-kaolinite bauxites by hydrochloric acid. Inorg. Mater. Appl. Res. 2016, 7, 272–277. [Google Scholar] [CrossRef]
- Reddy, B.R.; Mishra, S.K.; Banerjee, G.N. Kinetics of leaching of a gibbsitic bauxite with hydrochloric acid. Hydrometallurgy 1999, 51, 131–138. [Google Scholar] [CrossRef]
- Liu, Y.; Naidu, R. Hidden values in bauxite residue (red mud): recovery of metals. Waste Manage. 2014, 34, 2662–2673. [Google Scholar] [CrossRef]
- Parhi, B.R.; Sahoo, S.K.; Bhoi, B.; Satapathy, B.K.; Paramguru, R.K. Application of Hydrogen for the Reduction of Bauxite Mineral. Mate. Sci. Eng. 2016, 115, 1–7. [Google Scholar] [CrossRef]
- Samouhos, M.; Taxiarchou, M.; Pilatos, G.; Tsakiridis, P.E.; Devlin, E.; Pissas, M. Controlled reduction of red mud by H2 followed by magnetic separation. Miner. Eng. 2017, 105, 36–43. [Google Scholar] [CrossRef]
- Kurunov, I.F. The direct production of iron and alternatives to the blast furnace in iron metallurgy for the 21st century. Metallurgist 2010, 54, 335–342. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qi, Y.H.; Zou, Z.S.; Li, Y.G. Development Prospect of Rotary Hearth Furnace Process in China. Adv. Mater. Res. 2013, 746, 533–538. [Google Scholar] [CrossRef]
- Fortini, O.M.; Fruehan, R.J. Rate of reduction of ore-carbon composites: Part, I. Determination of intrinsic rate constants. Metall. Mater. Trans. B 2005, 36, 709–712. [Google Scholar] [CrossRef]
- He, A.P.; Zeng, J.M. Effect of bayer red mud on laterite nickel ore reduction. J. Iron Steel Res. Int. 2017, 29, 26–31. [Google Scholar] [CrossRef]
- Ding, Y.G.; Wang, J.S.; Wang, G.; Ma, S.; Xue, Q.G. Comprehensive Utilization of Paigeite Ore Using Iron Nugget Making Process. J. Iron Steel Res. Int. 2012, 19, 9–13. [Google Scholar] [CrossRef]
- Sawa, Y.; Yamamoto, T.; Takeda, K.; Itaya, H. New coal-based process to produce high quality DRI for the EAF. ISIJ Int. 2001, 41, 17–21. [Google Scholar] [CrossRef]
- Kapure, G.U.; Rao, C.B.; Tathavadkar, V.D.; Sen, R. Direct reduction of low grade chromite overburden for recovery of metals. Ironmak. Steelmak. 2011, 38, 590–596. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zhao, J.; Qi, Y.H.; Cheng, X.L.; Zou, Z.S. Reduction and melting behavior of carbon composite lateritic bauxite pellets. Int. J. Min. Metall. Mater. 2015, 22, 381–388. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Z.G.; Chu, M.S.; Wang, H.T.; Zhao, W.; Gao, L.H. Modeling assessment of recovering iron from red mud by direct reduction: Magnetic separation based on response surface methodology. J. Iron Steel Res. Int. 2018, 25, 497–505. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wei, L.; Qi, Y.H.; Zou, Z.S. Recovery of iron and calcium aluminate slag from high-ferrous bauxite by high-temperature reduction and smelting process. Int. J. Min. Met. Mater. 2016, 23, 881–890. [Google Scholar] [CrossRef]
- Azof, F.I.; Kolbeinsen, L.; Safarian, J. Characteristics of calcium-aluminate slags and pig iron produced from smelting-reduction of low-grade bauxites. Metall. Mater. Trans. B 2018, 49, 2400–2420. [Google Scholar] [CrossRef]
- Stamboulis, A.; Hill, R.G.; Law, R.V. Characterization of the structure of calcium alumino-silicate and calcium fluoro-alumino-silicate glasses by magic angle spinning nuclear magnetic resonance (MAS-NMR). J. Non-Cryst. Solids 2004, 333, 101–107. [Google Scholar] [CrossRef]
- Gao, J.X.; Wen, G.F.; Huang, T.; Bai, B.W.; Tang, P.; Liu, Q. Effect of Al speciation on the structure of high-Al steels mold fluxes containing fluoride. J. Am. Ceram. Soc. 2016, 99, 1–7. [Google Scholar] [CrossRef]
- Borra, C.R.; Blanpain, B.; Pontikes, Y.; Binnemans, K.; Gerven, T.V. Smelting of bauxite residue (red mud) in view of iron and selective rare earths recovery. J. Sustain. Metall. 2016, 2, 28–37. [Google Scholar] [CrossRef]
- Gao, J.X.; Wen, G.F.; Huang, T.; Bai, B.W.; Tang, P.; Liu, Q. Effect of slag-steel reaction on the structure and viscosity of CaO-SiO2-based mold flux during high-Al steel casting. J. Non-Cryst. Solids 2016, 452, 119–124. [Google Scholar] [CrossRef]
- Zhe, W.; Tang, P.; Wen, G.H.; Liu, Q. Effect of F− replacing O2− on crystallization behavior of CaO-SiO2-Al2O3 Continuous Casting Mold Flux. ISIJ Int. 2019, 59, 367–374. [Google Scholar] [CrossRef]
- Anameric, B.; Kawatra, K.S. Manipulation of slag separation properties from pig iron nuggets with flux additions to dried greenball mixture. Miner. Process. Extr. Metall. Rev. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Biswas, C.; Chaudhuri, M.G.; Dey, R. Reduction behaviour of agglomerated iron ore nuggets by devolatisation of high-ash, high-volatile lignite coal for pig iron production. Ironmak. Steelmak. 2016, 44, 1–11. [Google Scholar] [CrossRef]
- Birol, B.; Saridede, M.N. The effect of reduction parameters on iron nugget production from composite pellets. Miner. Process. Extr. Metall. Rev. 2013, 34, 195–201. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Qi, Y.H.; Zou, Z.S. Recycling of High Ferrous Bauxite Reducing Slag for Synthesis of CaAl2Si2O8-Al2O3-CaAl12O19 Composite. J. Iron Steel Res. Int. 2016, 23, 1255–1261. [Google Scholar] [CrossRef]













| Raw Materials | Fetot | Fe2O3 | FeO | SiO2 | Al2O3 | TiO2 | MnO | MgO | CaO | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Bauxite | 28.29 | 40.42 | 0.20 | 11.77 | 26.53 | 1.57 | 1.21 | 0.48 | 1.38 | 16.42 |
| Anthracite Composition | Moisture | Ash | Volatiles | Fixed Carbon | Sulfur | Phosphorus |
|---|---|---|---|---|---|---|
| Content (wt %) | 2.56 | 10.16 | 8.55 | 78.73 | 0.28 | 0.023 |
| Fe | C | Si | Mn | S | P |
|---|---|---|---|---|---|
| 94.48 | 3.94 | 0.10 | 1.42 | 0.002 | 0.058 |
| Test Positions | Analysis Result/at % | Mineral Names/Chemical Structural Formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | C | Si | Al | Ca | O | Mn | Ti | Co | Ni | ||
| Figure 16a-spot 1 | - | - | - | 49.62 | - | 50.38 | - | - | - | - | Alumina/α-Al2O3 |
| Figure 16a-area 2 | 12.60 | - | - | 35.26 | 0.82 | 45.74 | 1.73 | - | - | - | Hercynite/FeAl4O7 |
| Figure 16a-area 3 | 2.91 | - | 9.72 | 18.01 | 15.25 | 51.61 | 0.64 | 1.22 | - | - | Gehlenite/Ca2Al2SiO7 |
| Figure 16b-spot 1 | 90.41 | - | - | - | 0.63 | - | - | - | 1.18 | 2.51 | Metallic iron/Fe |
| Figure 16b-area 2 | 15.13 | - | - | 34.34 | 0.36 | 46.05 | 1.42 | - | - | - | Hercynite/FeAl4O7 |
| Figure 16b-area 3 | 2.93 | - | 9.57 | 17.78 | 15.01 | 52.43 | 0.44 | 1.20 | - | - | Gehlenite/Ca2Al2SiO7 |
| Figure 16b-area 4 | 3.70 | - | 0.72 | 38.76 | 4.97 | 51.85 | - | - | - | - | Calcium hexaluminate/CaAl12O19 |
| Fe | C | Si | S | P | Mn |
|---|---|---|---|---|---|
| 94.31 | 3.86 | 0.13 | 0.005 | 0.065 | 1.63 |
| FeO | Al2O3 | SiO2 | CaO | MnO | TiO2 | V2O5 |
|---|---|---|---|---|---|---|
| 2.26 | 43.36 | 21.75 | 27.11 | 2.56 | 2.65 | 0.31 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Gao, Q.; Zhao, J.; Li, M.; Qi, Y. Semi-Smelting Reduction and Magnetic Separation for the Recovery of Iron and Alumina Slag from Iron Rich Bauxite. Minerals 2019, 9, 223. https://doi.org/10.3390/min9040223
Zhang Y, Gao Q, Zhao J, Li M, Qi Y. Semi-Smelting Reduction and Magnetic Separation for the Recovery of Iron and Alumina Slag from Iron Rich Bauxite. Minerals. 2019; 9(4):223. https://doi.org/10.3390/min9040223
Chicago/Turabian StyleZhang, Yingyi, Qiangjian Gao, Jie Zhao, Mingyang Li, and Yuanhong Qi. 2019. "Semi-Smelting Reduction and Magnetic Separation for the Recovery of Iron and Alumina Slag from Iron Rich Bauxite" Minerals 9, no. 4: 223. https://doi.org/10.3390/min9040223




