Mineralogical Sequence of Self-Healing Products in Cracked Marine Concrete
Abstract
:1. Introduction
2. Materials
2.1. Concrete Beams
2.2. Concrete Cores
3. Methods
3.1. Phase Assemblage of Binders
3.1.1. Mass Balance Calculations
3.1.2. Scanning Electron Microscopy
3.2. Mineralogy and Chemistry of Self-Healing Products
3.2.1. Micro X-ray Fluorescence Elemental Mapping
3.2.2. Powder X-ray Diffraction
3.2.3. Optical Polarizing Microscopy
3.2.4. Scanning Electron Microscopy
3.3. Extent of Self-Healing
4. Results
4.1. Phase Assemblage of the Hydrated Binders
4.2. Extent of Self-Healing
4.3. Mineralogy and Chemistry of Self-Healing Products
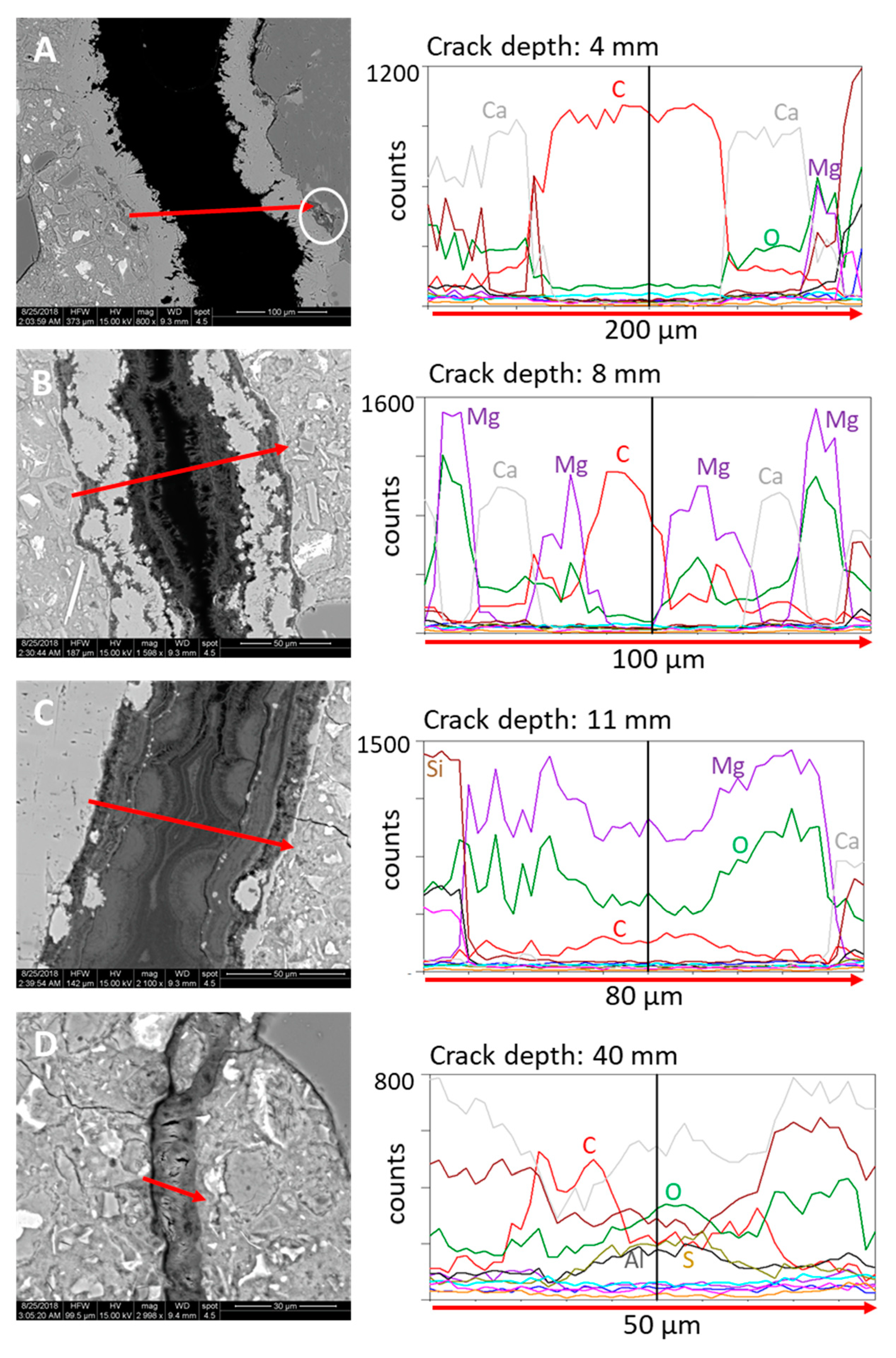
4.3.1. Micro X-ray Fluorescence
4.3.2. X-ray Diffraction
4.3.3. Optical Polarizing Microscopy
4.3.4. Scanning Electron Microscopy
5. Discussion
5.1. Phase Assemblage of Hydrated Pastes
5.2. Mechanism of Self-Healing
6. Conclusions
- There was no effect of binder combination on the mineralogy and chemistry of the self-healing products and the extent of self-healing. Crack widths smaller than 0.2 mm appeared closed.
- A sequence of changing mineralogy of self-healing products was found with increasing crack depth. In the outer faces of the crack (0–5 mm) only calcium carbonate was precipitated followed by brucite layers from 5–30 mm. The brucite was occasionally intermixed with calcite. At crack depths >30 mm only ettringite was observed.
- Two self-healing mechanisms appear to have acted: (1) Precipitation of ions from seawater partly in reaction with ions from the cement paste in the outer part of the crack. (2) Ettringite formation due to the dissolution and reprecipitation of hydrate phases at larger crack depth. It is hypothesized that the mineralogical sequence observed with increasing crack depth occurs due to an increasing pH of the solution inside the crack with increased crack depth.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Audenaert, K.; De Schutter, G.; Marsavina, L.; Boel, V. Influence of cracks and crack width on penetration depth of chlorides in concrete. Eur. J. Environ. Civ. Eng. 2009, 13, 561–572. [Google Scholar] [CrossRef]
- Marsavina, L.; Audenaert, K.; De Schutter, G.; Faur, N.; Marsavina, D. Experimental and numerical determination of the chloride penetration in cracked concrete. Constr. Build. Mater. 2009, 23, 264–274. [Google Scholar] [CrossRef]
- Concrete Society. Relevance of Cracking in Concrete to Reinforcement Corrosion—Technical Report 44; Concrete Society: Camberley, UK, 2015. [Google Scholar]
- Hornbostel, K.; Geiker, M. Influence of cracking on reinforcement corrosion. In Proceedings of Crack width Calculations Methods for Large Concrete Structures, Nordic Mini-Seminar; Nordic Concrete Federation: Oslo, Norway, 2017. [Google Scholar]
- Boschmann Käthler, A.C.; Angst, U.M.; Wagner, M.; Larsen, C.K.; Elsener, B. Effect of Cracks on Chloride Induced Corrosion of Steel in Concrete—A Review; National Public Roads Administration (NPRA): Oslo, Norway, 2017. [Google Scholar]
- De Rooij, M. (Ed.) Self Healing Phenomena in Cement-based Materials, State of the Art Report from RILEM Technical Commitee 221-SHC; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Huang, H.; Ye, G.; Qian, C.; Schlangen, E. Self-healing in cementitious materials: Materials, methods and service conditions. Mater. Des. 2016, 92, 499–511. [Google Scholar] [CrossRef]
- Schlangen, E.; Joseph, C. Self-healing process in concrete. In Self-Healing Materials: Fundamentals, Design, Strategies and Applications; Gosh, S.K., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2009. [Google Scholar]
- Van Tittelboom, K.; De Belie, N. Self-healing in cementitious materials—A review. Mater. Des. 2013, 6, 2182–2217. [Google Scholar] [CrossRef]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Reinhardt, H.W.; Jonkers, H.; Van Tittelboom, K.; Snoeck, D.; De Belie, N.; De Muynck, W.; Verstraete, W.; Wang, J.; Mechtcherine, V. Recovery Against Environmental Action. In Self-Healing Phenomena in Cement-Based Materials; De Rooij, M., Ed.; RILEM Technical Committee 221-SHC; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Savija, B.; Schlangen, E. Autogenous healing and chloride ingress in cracked concrete. Heron 2016, 61, 15–32. [Google Scholar]
- Edvardsen, C. Water Permeability and Autogenous Healing of Cracks in Concrete. Mater. J. 1999, 96, 448–454. [Google Scholar] [CrossRef]
- Jacobsen, S.; Marchand, J.; Hornain, H. Sem observations of the microstructure of frost deteriorated and self-healed concretes. Cem. Concr. Res. 1995, 25, 1781–1790. [Google Scholar] [CrossRef]
- De Weerdt, K.; Justnes, H.; Geiker, M.R. Changes in the phase assemblage of concrete exposed to sea water. Cem. Concr. Compos. 2014, 47, 53–63. [Google Scholar] [CrossRef]
- Fidjestøl, P.; Nilsen, N. Field Testof Reinforcement Corrosionin Concrete. In Performance of Concrete in Marine Environment; American Concrete Institute: Farmington Hills, MI, USA, 1980. [Google Scholar]
- Mohammed, T.U.; Otsuki, N.; Hamada, H. Corrosion of steel bars in cracked concrete under marine environment. J. Mater. Civ. Eng. 2003, 15, 460–469. [Google Scholar] [CrossRef]
- Maes, M.; Snoeck, D.; De Belie, N. Chloride penetration in cracked mortar and the influence of autogenous crack healing. Constr. Build. Mater. 2016, 115, 114–124. [Google Scholar] [CrossRef]
- Reinhardt, H.-W.; Jooss, M. Permeability and self-healing of cracked concrete as a function of temperature and crack width. Cem. Concr. Res. 2003, 33, 981–985. [Google Scholar] [CrossRef]
- Palin, D.; Jonkers, H.M.; Wiktor, V. Autogenous healing of sea-water exposed mortar: Quantification through a simple and rapid permeability test. Cem. Concr. Res. 2016, 84, 1–7. [Google Scholar] [CrossRef]
- Palin, D.; Wiktor, V.; Jonkers, H.M. Autogenous healing of marine exposed concrete: Characterization and quantification through visual crack closure. Cem. Concr. Res. 2015, 73, 17–24. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; Gruyaert, E.; Rahier, H.; De Belie, N. Influence of mix composition on the extent of autogenous crack healing by continued hydration or calcium carbonate formation. Constr. Build. Mater. 2012, 37, 349–359. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Li, Z.Q.; Xu, D.Y.; Yu, J.H. Influence of Slag and Fly Ash on the Self-Healing Ability of Concrete. Adv. Mater. Res. 2011, 306–307, 1020–1023. [Google Scholar] [CrossRef]
- Jóźwiak-Niedźwiedzka, D. Microscopic observations of self-healing products in calcareous fly ash mortars. Microsc. Res. Tech. 2015, 78, 22–29. [Google Scholar] [CrossRef]
- Holtmon, J.P.; Isaksen, H.R. Utvikling av Kloridbestandig Betong—Rapport fra Produksjon av Prøveelementer, Vegdirektoratet; National Public Roads Administration: Oslo, Norway, 1994. [Google Scholar]
- Jensen, I.S. yr.no/place/Norway/Sandnessjøen; Norwegian Meteorological Institute and Norwegian Broadcasting Corporation, Meteorological Institute of Norway: Oslo, Norway, 2018. [Google Scholar]
- Jakobsen, U.H.; De Weerdt, K.; Geiker, M.R. Elemental zonation in marine concrete. Cem. Concr. Res. 2016, 85, 12–27. [Google Scholar] [CrossRef] [Green Version]
- De Weerdt, K.; Orsáková, D.; Müller, A.C.A.; Larsen, C.K.; Pedersen, B.; Geiker, M.R. Towards the understanding of chloride profiles in marine exposed concrete, impact of leaching and moisture content. Constr. Build. Mater. 2016, 120, 418–431. [Google Scholar] [CrossRef]
- Herfort, D.; Lothenbach, B. Calculation of Ternary Diagrams by Mass Balance Calculations in MS Excel; EMPA: Dübendorf, Switzerland, 2016. [Google Scholar]
- Justnes, H.; De Weerdt, K.; Geiker, M. Chloride Binding in Concrete by Sea Water—The Role of Magnesium. In Proceedings of First International Conference on Performance-based and Life-cycle Structural Engineering; Hong Kong Polytechnic University: Hong Kong, China, 2012. [Google Scholar]
- B Buenfeld, N.R.; Newman, J.B. The development and stability of surface layers on concrete exposed to sea-water. Cem. Concr. Res. 1986, 16, 721–732. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Michel, A.; Gehlen, C.; Wong, H.; Isgor, O.B.; Elsener, B.; Hansson, C.M.; François, R.; Hornbostel, K.; et al. The steel–concrete interface. Mater. Struct. 2017, 50, 143. [Google Scholar] [CrossRef]
- Samson, E.; Marchand, J.; Zuber, B.; Skalny, J.P. Ettringite in air voids. In Proceedings of the International RILEM TC 186-ISA Workshop on Internal Sulfate Attack and Delayed Ettringite Formation, Villars, Switzerland, 4–6 September 2002. [Google Scholar]
- Jakobsen, U.H. Personal Communication; Danish Technological Institute, Concrete: Taastrup, Denmark, 2019. [Google Scholar]
- Rosenqvist, M.; Bertron, A.; Fridh, K.; Hassanzadeh, M. Concrete alteration due to 55 years of exposure to river water: Chemical and mineralogical characterisation. Cem. Concr. Res. 2017, 92, 110–120. [Google Scholar] [CrossRef]
- de Moel, P.; Van der Helm, A.; van Rijn, M.; Dijk, J.; Meer, W. Assessment of Calculation Methods for Calcium Carbonate Saturation in Drinking Water for DIN 38404-10 Compliance. Drink. Water Eng. Sci. 2013, 6, 115–124. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer, Green Chemistry and Sustainable Development Solutions: Proceedings of the World Congress Geopolymer 2005; Geopolymer Institute: Saint-Quentin, France, 2005. [Google Scholar]











| Component | Unit | PC-4SF | PC-4SF20FA | PC-12SF |
|---|---|---|---|---|
| Cement | kg/m3 | 373 | 384 | 398 |
| Cement type | - | CEM I | CEM I | CEM I |
| Silica fume | % (bwc) 2 | 4 | 4 | 12 |
| Fly ash | % (bwc) 2 | 20 | ||
| Free water | kg/m3 | 160.5 | 166.6 | 198.7 |
| Aggregate 0–8 mm | kg/m3 | 928 | 1014 | 881 |
| Aggregate 8–16 mm | kg/m3 | 904 | 820 | 841 |
| Air entrainer | kg/m3 | 0.3 | 0.6 | |
| Plasticizer | kg/m3 | 6.0 | 7.9 | 8.5 |
| Paste volume | % | 28.6 | 29.5 | 34.8 |
| Theoretical density | kg/m3 | 2.38 | 2.40 | 2.37 |
| Equivalent w/c 1 | - | 0.40 | 0.40 | 0.40 |
| Oxides | Unit | CEM I | Fly Ash [28] | Silica Fume [28] |
|---|---|---|---|---|
| CaO | wt% | 63.3 | 3.6 | 0.1 |
| SiO2 | wt% | 20.6 | 55.4 | 95.1 |
| Al2O3 | wt% | 4.8 | 27.4 | 1.0 |
| Fe2O3 | wt% | 3.5 | 3.9 | 0.1 |
| MgO | wt% | 2.2 | 1.0 | 0.4 |
| SO3 | wt% | 2.8 | N/A | 0.0 |
| K2O | wt% | 1.0 | 1.1 | 1.0 |
| Na2O | wt% | 0.35 | 0.3 | 0.1 |
| LOI | wt% | 1.0 | N/A | N/A |
| Specific surface area | m2/kg | 341 | N/A | N/A |
| Core ID | Exposure Zone | Distance from Top of Atmospheric Zone (m) | Crack Width (mm) | |
|---|---|---|---|---|
| Before Drilling | After Drilling | |||
| PC-4SF | Tidal | 1.4 | 0.15 | 0.15 |
| PC-4SF20FA | Tidal | 1.5 | 0.35 | 0.20 |
| PC-12SF | Tidal | 1.4 | 0.20 | 0.15 |
| Concrete/Core | Phase Assemblage | Mineralogy and Chemistry of Self-Healing Products | Extent of Self-Healing | ||||
|---|---|---|---|---|---|---|---|
| Mass Balance | SEM | µ-XRF | XRD 1 | Optical Polarizing Microscopy | SEM | Optical Polarizing Microscopy | |
| PC-4SF | X | X | X | X | X | X | |
| PC-4SF20FA | X | X | X | X | X | X | X |
| PC-12SF | X | X | X | X | X | X | |
| EDX (atomic%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Depth 1,2 (mm) | Na | K | Mg | S | Cl | Al | Si | Ca | Fe | |
| PC-4SF | 4–5 | 1.2 | 0.5 | 3.1 | 0.1 | 0.1 | 1.6 | 8.6 | 29.4 | 0.7 |
| 10–30 | 0.7 | 0.4 | 0.9 | 0.9 | 0. | 2.1 | 12.1 | 23.9 | 0.9 | |
| 30–35 | 1.1 | 0.4 | 0.8 | 1.7 | 0.6 | 1.6 | 12.5 | 22.5 | 0.7 | |
| Bulk | 0.6 | 0.2 | 0.7 | 1.1 | 1.1 | 0.7 | 12.7 | 23.8 | 0.6 | |
| PC-4SF20FA | 4–5 | 0.5 | 0.2 | 1.3 | 1.1 | 0.8 | 1.3 | 12.4 | 19.3 | 1.4 |
| 10–30 | 1.9 | 0.6 | 1.0 | 1.2 | 0.4 | 1.0 | 12.9 | 19.2 | 0.8 | |
| 30–35 | 0.9 | 0.6 | 0.8 | 1.6 | 0.8 | 0.8 | 12.9 | 19.4 | 0.6 | |
| Bulk | 0.8 | 0.3 | 0.8 | 1.2 | 0.7 | 0.8 | 13.4 | 19.4 | 0.7 | |
| PC-12SF | 4–5 | 0.4 | 0.1 | 0.9 | 0.9 | 1.2 | 0.9 | 13.1 | 23.6 | 0.8 |
| 10–30 | 0.7 | 0.3 | 0.7 | 1.1 | 0.9 | 0.7 | 13.0 | 23.1 | 0.7 | |
| 30–35 | 0.6 | 0.3 | 0.6 | 1.1 | 0.8 | 0.6 | 13.7 | 22.8 | 0.5 | |
| Bulk | 0.7 | 0.3 | 0.8 | 1.0 | 0.8 | 0.8 | 13.2 | 23.3 | 0.6 | |
| Hydrate | Amount (g/100 g Hydrated Binder) | ||
|---|---|---|---|
| PC-4SF | PC-4SF20FA | PC-12SF | |
| Portlandite | 15 | - | 2 |
| C-A-S-H (C1.75A0.05SH4.3) | 61 | 48 | 78 |
| C-A-S-H (C1.3A0.1SH3) | - | 22 | - |
| C-A-S-H total | 61 | 70 | 78 |
| Monoulpho-aluminate | 1 | 9 | - |
| Hemisulpho-alminate | - | 4 | - |
| Ettringite | 9 | - | 4 |
| Gypsum | - | - | 2 |
| Ferrihydrate | 2 | 2 | 2 |
| Hydrotalcite | 4 | 3 | 4 |
| Ca/Si of C-A-S-H | 1.75 | 1.6 | 1.75 |
| Ca/Al of C-A-S-H | 0.05 | 0.1 | 0.05 |
| Core | PC-4SF | PC-4SF20FA | PC-12SF | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Depth (mm) | S | w (mm) | wmax_s (mm) | S | w (mm) | wmax_s (mm) | S | w (mm) | wmax_s (mm) |
| 0–5 | ☐ | 0.1–0.25 | - | ◣ | 0.05–0.20 | 0.08 | ☐ | 0.05–0.20 | - |
| 5–10 | ◣ | 0.05–0.25 | 0.21 | ◣ | 0.20–0.30 | 0.03 1 | ◣ | 0.05–0.15 | 0.12 |
| 10–15 | ◣ | 0.05–0.15 | 0.12 | ◣ | 0.10–0.25 | 0.16 | ◣ | 0.05–0.20 | 0.18 |
| 15–20 | ◣ | 0.05–0.20 | 0.12 | ◣ | 0.10–0.20 | 0.18 | ◣ | 0.10–0.20 | 0.17 |
| 20–25 | ◣ | 0.05–0.20 | 0.12 | ◣ | 0.05–0.20 | 0.18 | ◣ | 0.10–0.15 | 0.15 |
| 25–30 | ◣ | 0.10–0.15 | 0.13 | ☐ | 0.10–0.40 | - | ☐ | 0.05–0.10 | - |
| 30–35 | ◣ | 0.05–0.15 | - | ◣ | 0.05–0.60 | - | ◣ | 0.05–0.10 | - |
| 35–40 | ◣ | 0.05–0.20 | - | ◣ | 0.05–0.15 | - | ◣ | 0.05–0.10 | 0.05 |
| Element | Crack Depth (mm) | |
|---|---|---|
| 5 | 15 | |
| Na | 2.4 ± 2.4 | 0.9 ± 0.8 |
| Mg | 1.5 ± 0.5 | 40.4 ± 3.0 |
| Al | 4.2 ± 2.7 | 8.1 ± 2.6 |
| Si | 27.9 ± 24.8 | 21.7 ± 6.2 |
| S | 0.1 ± 0.0 | 0.2 ± 0.1 |
| K | 1.0 ± 1.0 | 0.5 ± 0.1 |
| Ca | 62.4 ± 27.3 | 26.7 ± 7.2 |
| Fe | 0.4 ± 0.2 | 0.5 ± 0.2 |
| Concrete | Open Crack | Calcite | Brucite | Brucite Gel | Ettringite |
|---|---|---|---|---|---|
| PC-4SF | 0–0.5 | 0.5–30 | 8–29 | 16–29 | 38–45 |
| PC-4SF20FA | 0–2.0 | 2–25 | 9–18 | 14–32 | 32–45 |
| PC-12SF | 0–1.2 | 1.2–30 | 4–12 | 12–23 | 23–45 |
| Element | Color Code |
|---|---|
| Carbon (C) | red |
| Oxygen (O) | green |
| Magnesium (Mg) | Pink |
| Aluminium (Al) | dark grey |
| Silicon (Si) | Brown |
| Calcium (Ca) | light grey |
| Iron (Fe) | Orange |
| Sulfur (S) | Ochre |
| Chloride (Cl) | light blue |
| Sodium (Na) | dark blue |
| Potassium (K) | Violet |
| Ca | Mg | Al | Si | S | Na | K | O | |
|---|---|---|---|---|---|---|---|---|
| M-S-H | 2.6 ± 0.9 | 20.2 ± 2.3 | 3.7 ± 0.7 | 13.5 ± 1.1 | 0.2 ± 0.2 | 0.4 ± 0.4 | 0.3 ± 0.1 | 57.2 ± 0.8 |
| Calcite | 47.5 ± 0.5 | 0.4 ± 0.2 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.2 | 1.5 ± 0.4 | 0.1 ± 0.1 | 49.9 ± 0.2 |
| Brucite | 0.9 ± 0.2 | 45.6 ± 0.8 | 0.4 ± 0.2 | 1.6 ± 0.3 | 0.1 ± 0.1 | 0.0 | 0.1 ± 0.1 | 50.1 ± 0.3 |
| Ettringite | 21.8 ± 0.7 | 0.1 ± 0.1 | 3.9 ± 1.0 | 6.9 ± 2.7 | 5.9 ± 1.9 | 0.3 ± 0.1 | 0.2 ± 0.1 | 59.9 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danner, T.; Hjorth Jakobsen, U.; Geiker, M.R. Mineralogical Sequence of Self-Healing Products in Cracked Marine Concrete. Minerals 2019, 9, 284. https://doi.org/10.3390/min9050284
Danner T, Hjorth Jakobsen U, Geiker MR. Mineralogical Sequence of Self-Healing Products in Cracked Marine Concrete. Minerals. 2019; 9(5):284. https://doi.org/10.3390/min9050284
Chicago/Turabian StyleDanner, Tobias, Ulla Hjorth Jakobsen, and Mette Rica Geiker. 2019. "Mineralogical Sequence of Self-Healing Products in Cracked Marine Concrete" Minerals 9, no. 5: 284. https://doi.org/10.3390/min9050284





