The System K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for Carbonatite Melt Compositions in the Shallow Continental Lithosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. High-Pressure Technique
2.2. Starting Materials
2.3. Analytical Technique
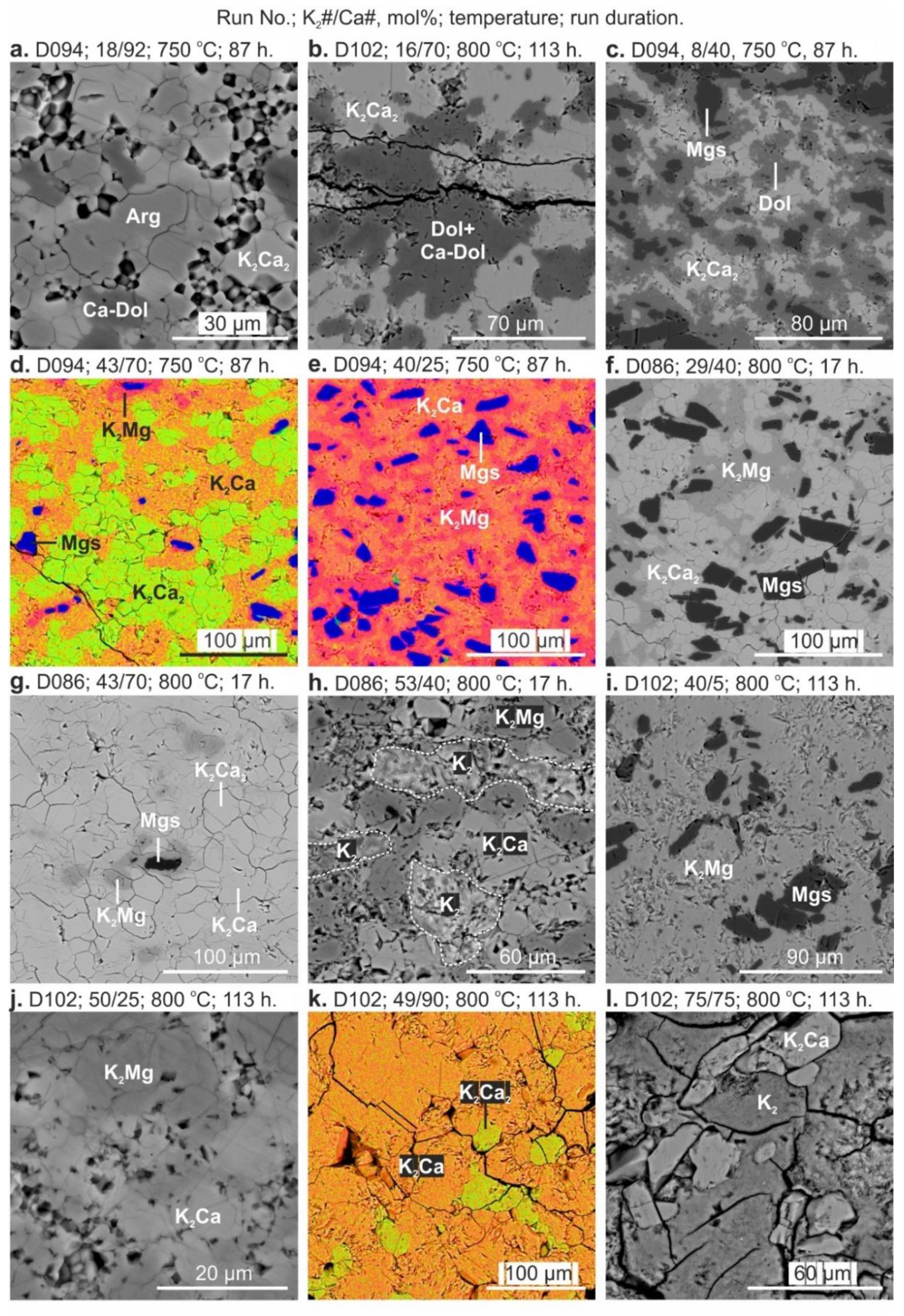
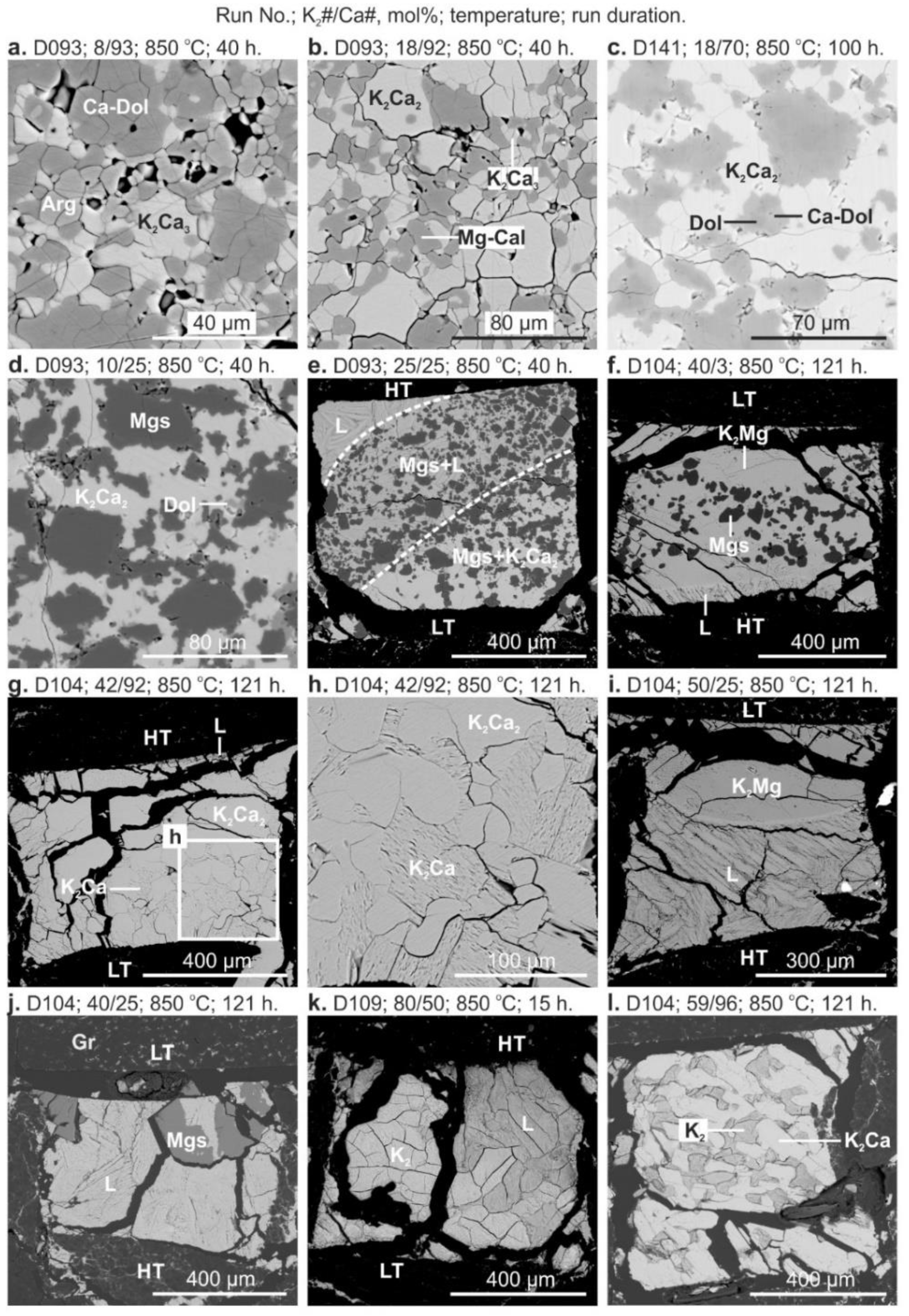
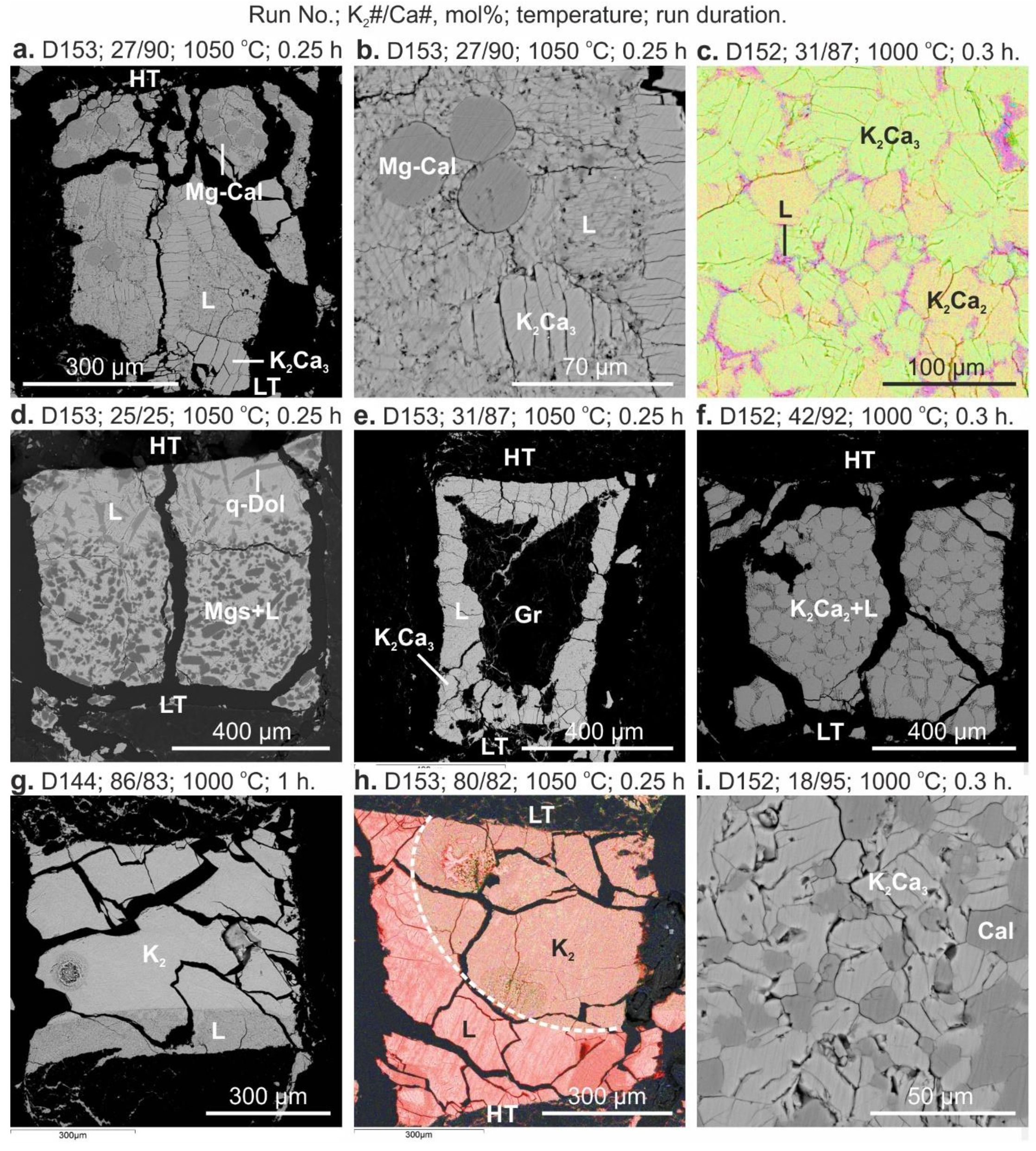
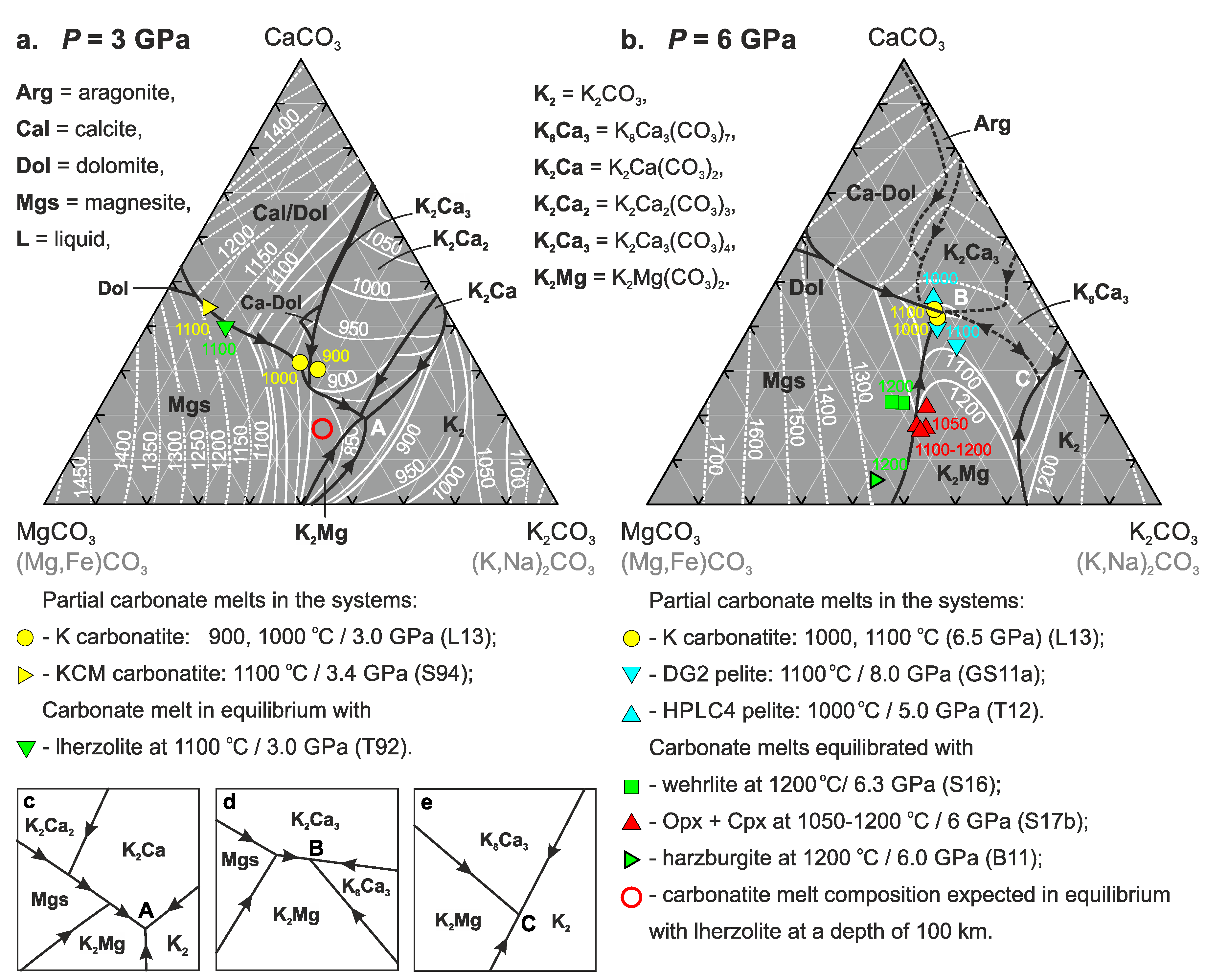
3. Results
4. Discussion
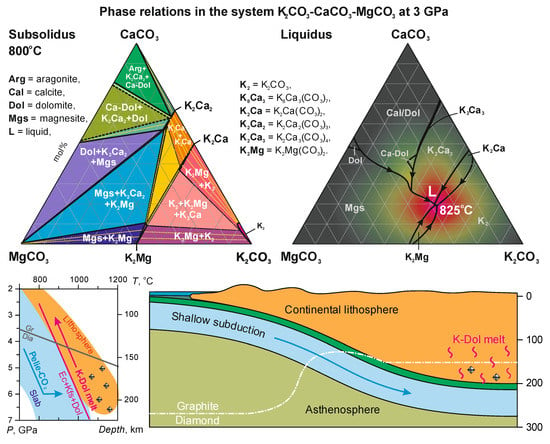
4.1. Phase Relations in the Join K2CO3–CaCO3–MgCO3 at 3 and 6 GPa
4.2. Temperature Stability Limit of Carbonate Melt vs. Alkalinity
4.3. Comparison with Carbonate–Silicate Systems
4.4. Origin of K-Rich Carbonatite Melt
4.5. The Compositional Trend of Carbonate Melts during Upward Percolation via Lithosphere
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomson, A.R.; Walter, M.J.; Kohn, S.C.; Brooker, R.A. Slab melting as a barrier to deep carbon subduction. Nature 2016, 529, 76–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grassi, D.; Schmidt, M.W. The melting of carbonated pelites from 70 to 700 km depth. J. Petrol. 2011, 52, 765–789. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R.; Matsyuk, S.; Schreiber, A.; Thomas, R. Nyerereite and nahcolite inclusions in diamond: Evidence for lower-mantle carbonatitic magmas. Mineral. Mag. 2009, 73, 797–816. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: New minerals in the carbonate-halide association. Can. Mineral. 2013, 51, 669–688. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Ryabchikov, I.D.; Wirth, R. A primary natrocarbonatitic association in the Deep Earth. Mineral. Petrol. 2016, 110, 387–398. [Google Scholar] [CrossRef]
- Sharygin, I.S.; Golovin, A.V.; Korsakov, A.V.; Pokhilenko, N.P. Eitelite in sheared peridotite xenoliths from Udachnaya-East kimberlite pipe (Russia)—A new locality and host rock type. Eur. J. Mineral. 2013, 25, 825–834. [Google Scholar] [CrossRef]
- Golovin, A.; Sharygin, I.; Kamenetsky, V.; Korsakov, A.; Yaxley, G. Alkali-carbonate melts from the base of cratonic lithospheric mantle: Links to kimberlites. Chem. Geol. 2018, 483, 261–274. [Google Scholar] [CrossRef]
- Kamenetsky, V.S.; Kamenetsky, M.B.; Weiss, Y.; Navon, O.; Nielsen, T.F.D.; Mernagh, T.P. How unique is the Udachnaya-East kimberlite? Comparison with kimberlites from the Slave Craton (Canada) and SW Greenland. Lithos 2009, 112, 334–346. [Google Scholar] [CrossRef]
- Golovin, A.V.; Sharygin, I.S.; Korsakov, A.V. Origin of alkaline carbonates in kimberlites of the Siberian craton: Evidence from melt inclusions in mantle olivine of the Udachnaya-East pipe. Chem. Geol. 2017, 455, 357–375. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Ohtani, E. Composition of primary kimberlite melt in a garnet lherzolite mantle source: Constraints from melting phase relations in anhydrous Udachnaya-East kimberlite with variable CO2 content at 6.5 GPa. Gondwana Res. 2017, 45, 208–227. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Egonin, I.A.; Mironov, A.M.; Palyanov, Y.N.; Ohtani, E. The system Na2CO3-CaCO3-MgCO3 at 6 GPa and 900–1250 °C and its relation to the partial melting of carbonated mantle. High Press. Res. 2016, 36, 23–41. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Litasov, K.D. Phase relations in the system Na2CO3-CaCO3-MgCO3 at 3 GPa with implications for carbonatite genesis and evolution. Lithos 2019, 330–331, 74–89. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.; Rossman, G.; Wasserburg, G. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Rege, S.; Griffin, W.L.; Kagi, H.; Shatsky, V.S. Composition of trapped fluids in cuboid fibrous diamonds from the Udachnaya kimberlite: LAM-ICPMS analysis. Chem. Geol. 2007, 240, 151–162. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Logvinova, A.M.; Schrauder, M.; Spetius, Z.V.; Weiss, Y.; Hauri, E.H.; Kaminsky, F.V.; Sobolev, N.V.; Navon, O. High-Mg carbonatitic microinclusions in some Yakutian diamonds—A new type of diamond-forming fluid. Lithos 2009, 112, 648–659. [Google Scholar] [CrossRef]
- Weiss, Y.; Kessel, R.; Griffin, W.L.; Kiflawi, I.; Klein-BenDavid, O.; Bell, D.R.; Harris, J.W.; Navon, O. A new model for the evolution of diamond-forming fluids: Evidence from microinclusion-bearing diamonds from Kankan, Guinea. Lithos 2009, 112, 660–674. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L. Fibrous diamonds from the placers of the northeastern Siberian Platform: Carbonate and silicate crystallization media. Russ. Geol. Geophys. 2011, 52, 1298–1309. [Google Scholar] [CrossRef]
- Skuzovatov, S.; Zedgenizov, D.; Howell, D.; Griffin, W.L. Various growth environments of cloudy diamonds from the Malobotuobia kimberlite field (Siberian craton). Lithos 2016, 265, 96–107. [Google Scholar] [CrossRef]
- Jablon, B.M.; Navon, O. Most diamonds were created equal. Earth Planet. Sci. Lett. 2016, 443, 41–47. [Google Scholar] [CrossRef]
- Kruk, A.N.; Sokol, A.G.; Palyanov, Y.N. Phase relations in the harzburgite–hydrous carbonate melt at 5.5–7.5 GPa and 1200–1350 °С. Petrology 2018, 26, 575–587. [Google Scholar] [CrossRef]
- Sokol, A.G.; Kruk, A.N.; Chebotarev, D.A.; Palyanov, Y.N. Carbonatite melt–peridotite interaction at 5.5–7.0 GPa: Implications for metasomatism in lithospheric mantle. Lithos 2016, 248, 66–79. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V. Melting of K-rich carbonated peridotite at 6–10 GPa and the stability of K-phases in the upper mantle. Chem. Geol. 2011, 281, 333–342. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation from mantle carbonate fluids. Nature 1999, 400, 417–418. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [Green Version]
- Shatskiy, A.; Sharygin, I.S.; Gavryushkin, P.N.; Litasov, K.D.; Borzdov, Y.M.; Shcherbakova, A.V.; Higo, Y.; Funakoshi, K.; Palyanov, Y.N.; Ohtani, E. The system K2CO3-MgCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2013, 98, 1593–1603. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Sharygin, I.S.; Palyanov, Y.N.; Ohtani, E. Phase relationships in the system K2CO3-CaCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2015, 100, 223–232. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Ohtani, E.; Borzdov, Y.M.; Khmelnicov, A.I.; Palyanov, Y.N. Phase relations in the K2CO3-FeCO3 and MgCO3-FeCO3 systems at 6 GPa and 900–1700 °C. Eur. J. Mineral. 2015, 27, 487–499. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Palyanov, Y.N.; Ohtani, E. Phase relations on the K2CO3-CaCO3-MgCO3 join at 6 GPa and 900–1400 °C: Implications for incipient melting in carbonated mantle domains. Am. Mineral. 2016, 101, 437–447. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Crawford, A.J.; Green, D.H. Evidence for carbonatite metasomatism in spinel peridotite xenoliths from western Victoria, Australia. Earth Planet. Sci. Lett. 1991, 107, 305–317. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Green, D.H.; Kamenetsky, V. Carbonatite metasomatism in the southeastern Australian lithosphere. J. Petrol. 1998, 39, 1917–1930. [Google Scholar] [CrossRef]
- Dautria, J.; Dupuy, C.; Takherist, D.; Dostal, J. Carbonate metasomatism in the lithospheric mantle: Peridotitic xenoliths from a melilititic district of the Sahara basin. Contrib. Mineral. Petrol. 1992, 111, 37–52. [Google Scholar] [CrossRef]
- Thibault, Y.; Edgar, A.D.; Lloyd, F.E. Experimental investigation of melts from a carbonated phlogopite lherzolite: Implications for metasomatism in the continental lithosphere. Am. Mineral. 1992, 77, 784–794. [Google Scholar]
- Rudnick, R.L.; McDonough, W.F.; Chappell, B.W. Carbonatite metasomatism in the northern Tanzanian mantle: Petrographic and geochemical characteristics. Earth Planet. Sci. Lett. 1993, 114, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, T.; Gruber, B.; Reinier, C. Effects of pressure and water on electrical conductivity of carbonate melt with implications for conductivity anomaly in continental mantle lithosphere. Phys. Earth Planet. Inter. 2018, 281, 8–16. [Google Scholar] [CrossRef]
- Jones, A.G.; Ferguson, I.J.; Chave, A.D.; Evans, R.L.; McNeice, G.W. Electric lithosphere of the Slave craton. Geology 2001, 29, 423–426. [Google Scholar] [CrossRef]
- Jones, A.G.; Lezaeta, P.; Ferguson, I.J.; Chave, A.D.; Evans, R.L.; Garcia, X.; Spratt, J. The electrical structure of the Slave craton. Lithos 2003, 71, 505–527. [Google Scholar] [CrossRef]
- Pinto, L.G.R.; de Pádua, M.B.; Ussami, N.; Vitorello, Í.; Padilha, A.L.; Braitenberg, C. Magnetotelluric deep soundings, gravity and geoid in the South São Francisco craton: Geophysical indicators of cratonic lithosphere rejuvenation and crustal underplating. Earth Planet. Sci. Lett. 2010, 297, 423–434. [Google Scholar] [CrossRef]
- Giuliani, A.; Kamenetsky, V.S.; Phillips, D.; Kendrick, M.A.; Wyatt, B.A.; Goemann, K. Nature of alkali-carbonate fluids in the sub-continental lithospheric mantle. Geology 2012, 40, 967–970. [Google Scholar] [CrossRef]
- Osugi, J.; Shimizu, K.; Inoue, K.; Yasunami, K. A compact cubic anvil high pressure apparatus. Rev. Phys. Chem. Jpn. 1964, 34, 1–6. [Google Scholar]
- Shatskiy, A.; Litasov, K.D.; Terasaki, H.; Katsura, T.; Ohtani, E. Performance of semi-sintered ceramics as pressure-transmitting media up to 30 GPa. High Press. Res. 2010, 30, 443–450. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Minin, D.A.; Chanyshev, A.D.; Litasov, K.D. Revision of the CaCO3–MgCO3 phase diagram at 3 and 6 GPa. Am. Mineral. 2018, 103, 441–452. [Google Scholar] [CrossRef]
- Hernlund, J.; Leinenweber, K.; Locke, D.; Tyburczy, J.A. A numerical model for steady-state temperature distributions in solid-medium high-pressure cell assemblies. Am. Mineral. 2006, 91, 295–305. [Google Scholar] [CrossRef]
- Brey, G.P.; Kohler, T. Geothermobarometry in four-phase lherzolites II. New thermobarometers, and practical assessment of existing thermobarometers. J. Petrol. 1990, 31, 1353–1378. [Google Scholar] [CrossRef]
- Lavrent’ev, Y.G.; Karmanov, N.; Usova, L. Electron probe microanalysis of minerals: Microanalyzer or scanning electron microscope? Russ. Geol. Geophys. 2015, 56, 1154–1161. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Litasov, K.D. Melting and subsolidus phase relations in the system K2CO3-MgCO3 at 3 GPa. High Press. Res. 2018, 38, 422–439. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Rashchenko, S.V.; Chanyshev, A.D.; Litasov, K.D. The system K2CO3-CaCO3 at 3 GPa: Link between phase relations and variety of K-Ca double carbonates at ≤ 0.1 and 6 GPa. Phys. Chem. Miner. 2019, 46, 229–244. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E.; Yaxley, G.M. The solidus of alkaline carbonatite in the deep mantle. Geology 2013, 41, 79–82. [Google Scholar] [CrossRef]
- Sweeney, R.J. Carbonatite melt compositions in the Earth’s mantle. Earth Planet. Sci. Lett. 1994, 128, 259–270. [Google Scholar] [CrossRef]
- Grassi, D.; Schmidt, M.W. Melting of carbonated pelites at 8–13 GPa: Generating K-rich carbonatites for mantle metasomatism. Contrib. Mineral. Petrol. 2011, 162, 169–191. [Google Scholar] [CrossRef]
- Tsuno, K.; Dasgupta, R.; Danielson, L.; Righter, K. Flux of carbonate melt from deeply subducted pelitic sediments: Geophysical and geochemical implications for the source of Central American volcanic arc. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Litasov, K.D.; Chanyshev, A.D.; Sharygin, I.S.; Karmanov, N.S.; Ohtani, E. Effect of alkalis on the reaction of clinopyroxene with Mg-carbonate at 6 GPa: Implications for partial melting of carbonated lherzolite. Am. Mineral. 2017, 102, 1934–1946. [Google Scholar] [CrossRef]
- Byrnes, A.P.; Wyllie, P.J. Subsolidus and melting relations for the join CaCO3-MgCO3 at 10 kbar. Geochim. Cosmochim. Acta 1981, 45, 321–328. [Google Scholar] [CrossRef]
- Gudfinnsson, G.H.; Presnall, D.C. Melting relations of model lherzolite in the system CaO–MgO–Al2O3–SiO2 at 2.4–3.4 GPa and the generation of komatiites. J. Geophys. Res. Solid Earth 1996, 101, 27701–27709. [Google Scholar] [CrossRef]
- Gudfinnsson, G.H.; Presnall, D.C. Continuous gradations among primary carbonatitic, kimberlitic, melilititic, basaltic, picritic, and komatiitic melts in equilibrium with garnet lherzolite at 3–8 GPa. J. Petrol. 2005, 46, 1645–1659. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef]
- Syracuse, E.M.; van Keken, P.E.; Abers, G.A. The global range of subduction zone thermal models. Phys. Earth Planet. Inter. 2010, 183, 73–90. [Google Scholar] [CrossRef]
- Tychkov, N.; Agashev, A.; Malygina, E.; Nikolenko, E.; Pokhilenko, N. Thermal perturbations in the lithospheric mantle as evidenced from PT equilibrium conditions of xenoliths from the Udachnaya kimberlite pipe. Doklady Earth Sci. 2014, 454, 84–88. [Google Scholar] [CrossRef]
- Bell, D.R.; Schmitz, M.D.; Janney, P.E. Mesozoic thermal evolution of the southern African mantle lithosphere. Lithos 2003, 71, 273–287. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L.; Kagi, H. Mg and Fe-rich carbonate-silicate high-density fluids in cuboid diamonds from the Internationalnaya kimberlite pipe (Yakutia). Lithos 2009, 112, 638–647. [Google Scholar] [CrossRef]
- Sobolev, N.; Shatsky, V.S.; Vavilov, M.A. Inclusions of microdiamonds and coexisting minerals in garnets and zircons from metamorphic rocks of Kokchetav Massif, USSR. Terra Abstr. 1991, 3, 83. [Google Scholar]
- Shatsky, V.S.; Ragozin, A.L.; Sobolev, N.V. Some aspects of metamorphic evolution of ultrahigh-pressure calc-silicate rocks of the Kokchetav Massif. Russ. Geol. Geophys. 2006, 47, 105–119. [Google Scholar]
- Nasdala, L.; Massonne, H.-J. Microdiamonds from the Saxonian Erzgebirge, Germany: In situ micro-Raman characterisation. Eur. J. Mineral. 2000, 12, 495–498. [Google Scholar] [CrossRef]
- De Corte, K.; Cartigny, P.; Shatsky, V.; Sobolev, N.; Javoy, M. Evidence of fluid inclusions in metamorphic microdiamonds from the Kokchetav massif, northern Kazakhstan. Geochim. Cosmochim. Acta 1998, 62, 3765–3773. [Google Scholar] [CrossRef]
- Stöckhert, B.; Duyster, J.; Trepmann, C.; Massonne, H.-J. Microdiamond daughter crystals precipitated from supercritical COH+ silicate fluids included in garnet, Erzgebirge, Germany. Geology 2001, 29, 391–394. [Google Scholar] [CrossRef]
- Hwang, S.-L.; Shen, P.; Chu, H.-T.; Yui, T.-F.; Liou, J.G.; Sobolev, N.V.; Shatsky, V.S. Crust-derived potassic fluid in metamorphic microdiamond. Earth Planet. Sci. Lett. 2005, 231, 295–306. [Google Scholar] [CrossRef]
- Dobson, D.P.; Jones, A.P.; Rabe, R.; Sekine, T.; Kurita, K.; Taniguchi, T.; Kondo, T.; Kato, T.; Shimomura, O.; Urakawa, S. In-situ measurement of viscosity and density of carbonate melts at high pressure. Earth Planet. Sci. Lett. 1996, 143, 207–215. [Google Scholar] [CrossRef]
- Kono, Y.; Kenney-Benson, C.; Hummer, D.; Ohfuji, H.; Park, C.; Shen, G.; Wang, Y.; Kavner, A.; Manning, C.E. Ultralow viscosity of carbonate melts at high pressures. Nat. Commun. 2014, 5, 5091. [Google Scholar] [CrossRef] [Green Version]
- Stagno, V.; Stopponi, V.; Kono, Y.; Manning, C.E.; Irifune, T. Experimental determination of the viscosity of Na2CO3 melt between 1.7 and 4.6 GPa at 1200–1700 °C: Implications for the rheology of carbonatite magmas in the Earth’s upper mantle. Chem. Geol. 2018, 501, 19–25. [Google Scholar] [CrossRef]
- Minarik, W.G.; Watson, E.B. Interconnectivity of carbonate melt at low melt fraction. Earth Planet. Sci. Lett. 1995, 133, 423–437. [Google Scholar] [CrossRef]
- Hammouda, T.; Laporte, D. Ultrafast mantle impregnation by carbonatite melts. Geology 2000, 28, 283–285. [Google Scholar] [CrossRef]
- McKenzie, D. The extraction of magma from the crust and mantle. Earth Planet. Sci. Lett. 1985, 74, 81–91. [Google Scholar] [CrossRef]
- Hunter, R.H.; McKenzie, D. The equilibrium geometry of carbonate melts in rocks of mantle composition. Earth Planet. Sci. Lett. 1989, 92, 347–356. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Bekhtenova, A.; Litasov, K.D. The System K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for Carbonatite Melt Compositions in the Shallow Continental Lithosphere. Minerals 2019, 9, 296. https://doi.org/10.3390/min9050296
Arefiev AV, Shatskiy A, Podborodnikov IV, Bekhtenova A, Litasov KD. The System K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for Carbonatite Melt Compositions in the Shallow Continental Lithosphere. Minerals. 2019; 9(5):296. https://doi.org/10.3390/min9050296
Chicago/Turabian StyleArefiev, Anton V., Anton Shatskiy, Ivan V. Podborodnikov, Altyna Bekhtenova, and Konstantin D. Litasov. 2019. "The System K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for Carbonatite Melt Compositions in the Shallow Continental Lithosphere" Minerals 9, no. 5: 296. https://doi.org/10.3390/min9050296