Dissolution and Passivation of Chalcopyrite during Bioleaching by Acidithiobacillus ferrivorans at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Mineral
2.3. Bioleaching Experiments
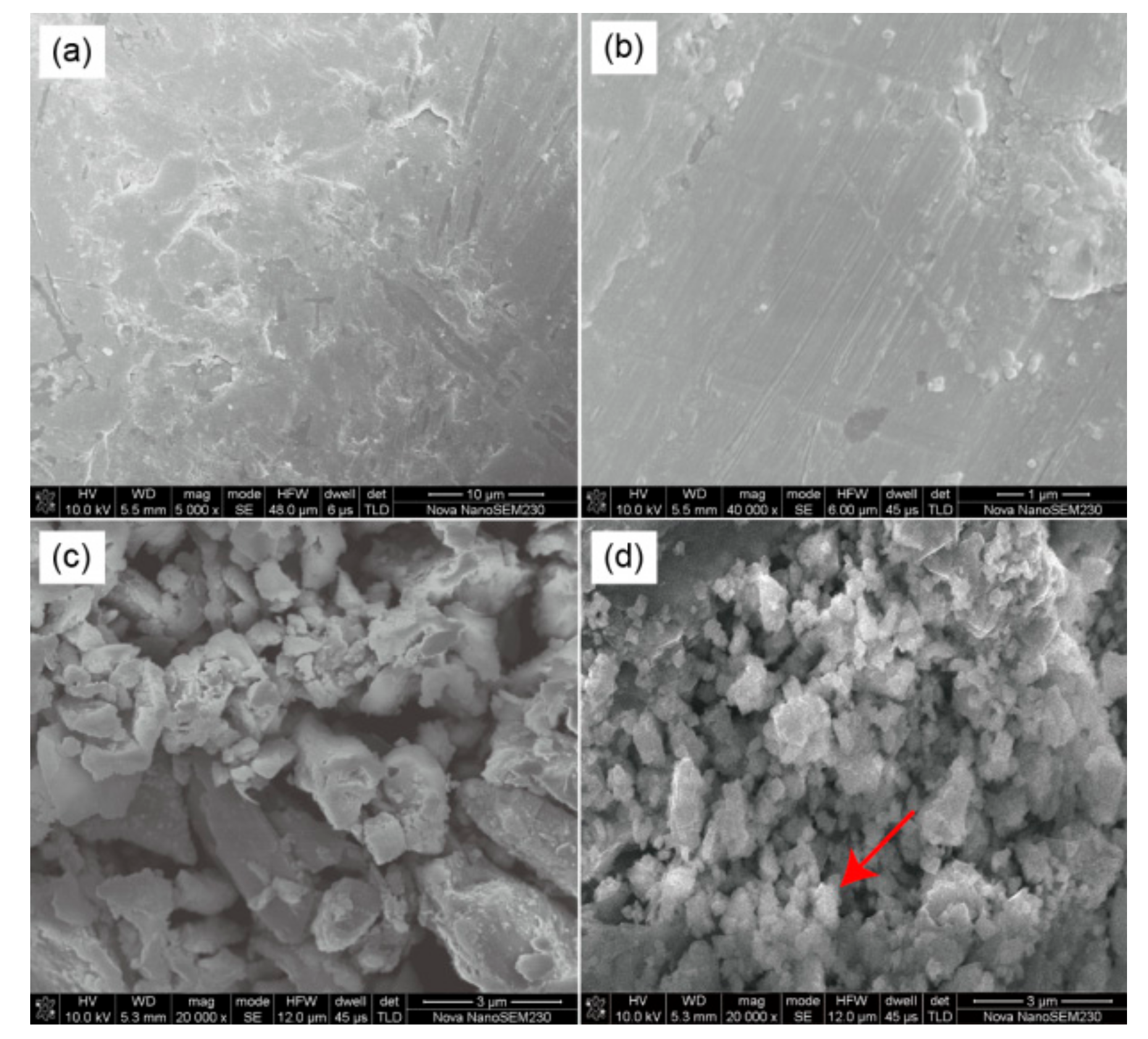
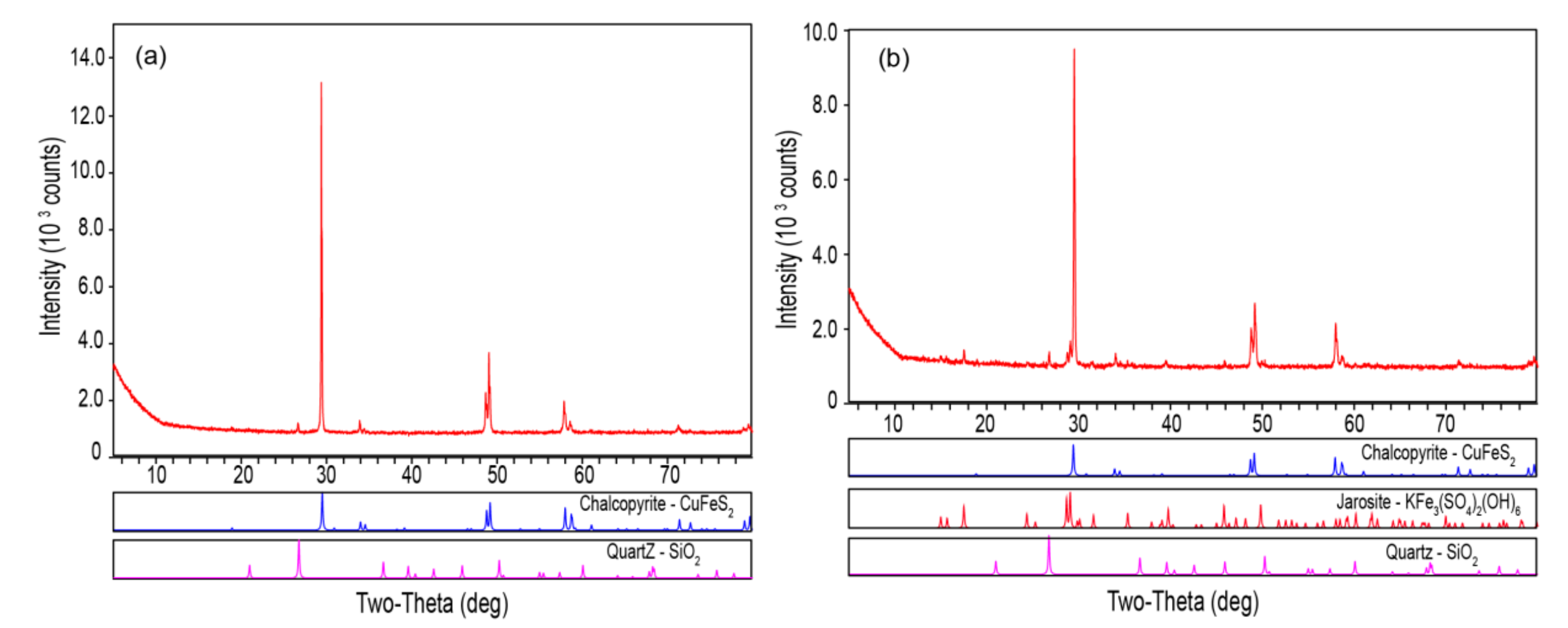
2.4. Characterization of Ore Residues
2.5. Quantification of Gene Expression during Bioleaching
3. Results and Discussion
3.1. Characterization of Strain YL15
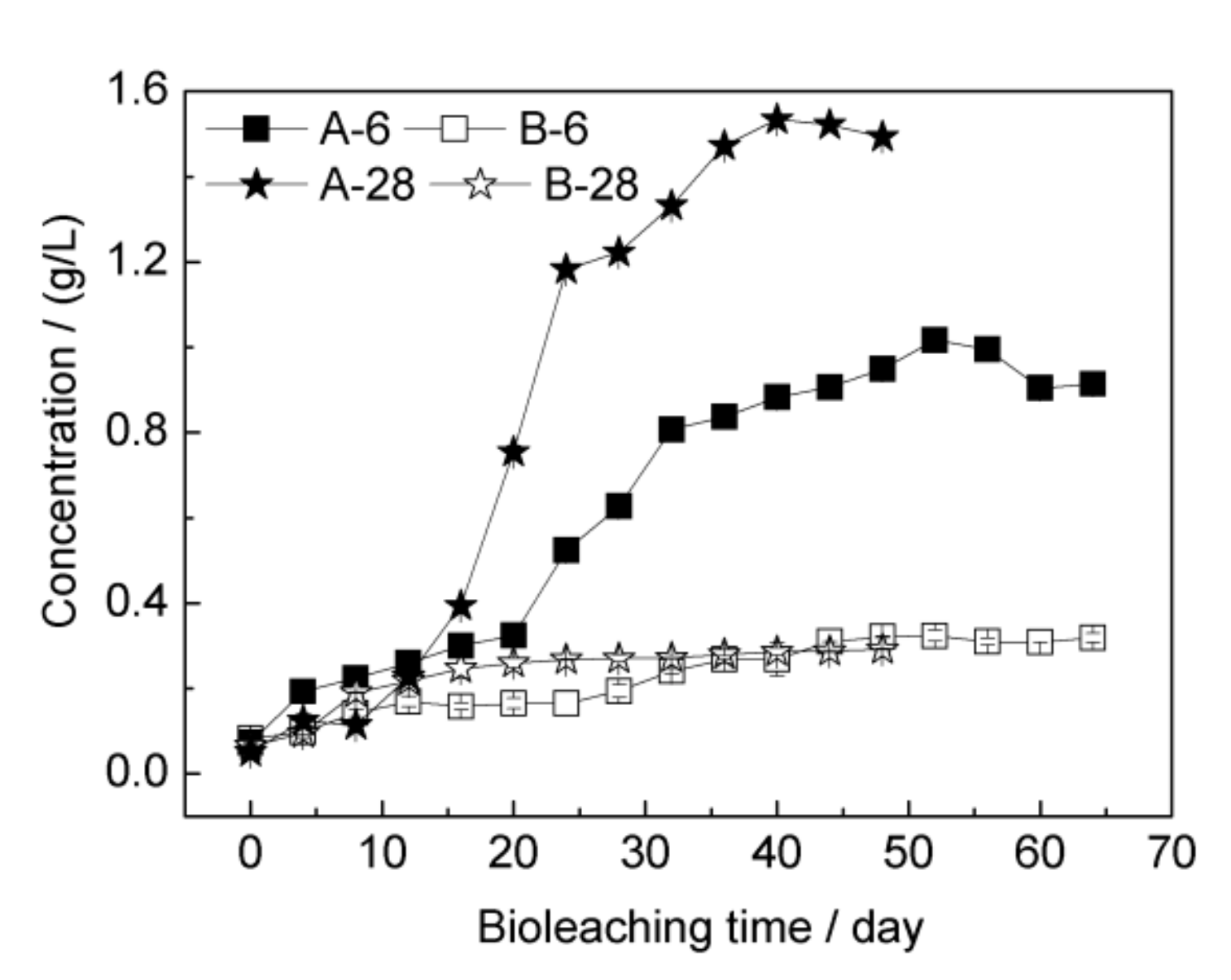
3.2. Bioleaching of Chalcopyrite by Strain YL15
3.3. Passivation of the Mineral Surface at 6 °C
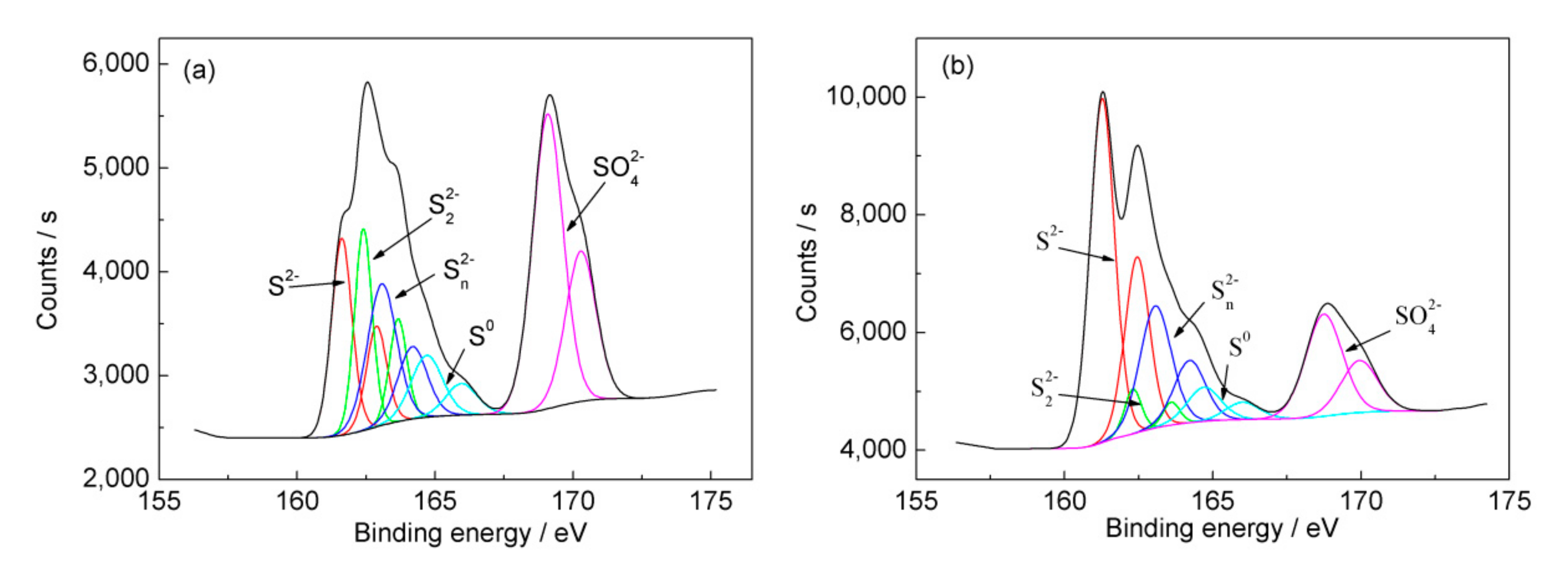
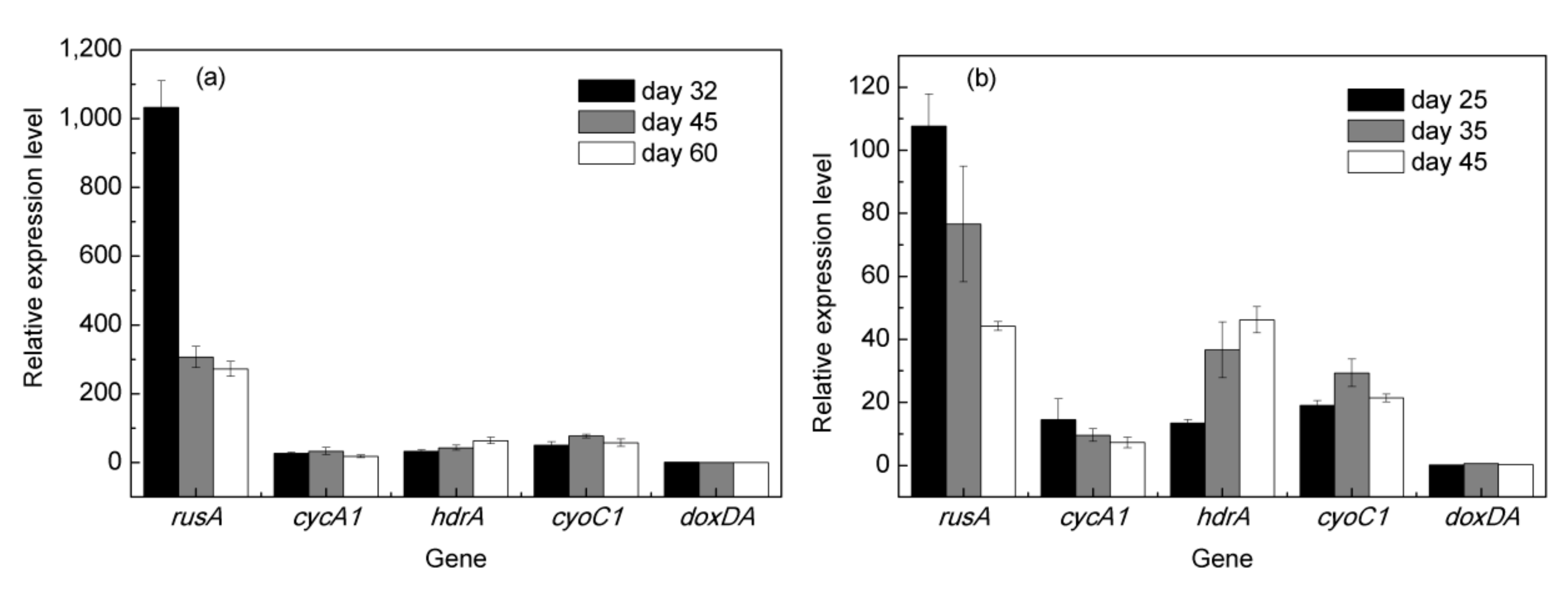
3.4. Expression of Critical Iron- and Sulfur-Oxidation Associated Genes during Bioleaching
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Johnson, D.B.; Grail, B.M.; Hallberg, K.B. A new direction for biomining: Extraction of metals by reductive dissolution of oxidized ores. Minerals 2013, 3, 49–58. [Google Scholar] [CrossRef]
- Li, Q.; Yang, B.; Zhu, J.; Jiang, H.; Li, J.; Zhang, R.; Sand, W. Comparative analysis of attachment to chalcopyrite of three mesophilic iron and/or sulfur-oxidizing acidophiles. Minerals 2018, 8, 406. [Google Scholar] [CrossRef]
- Panda, S.; Parhi, P.K.; Nayak, B.D.; Pradhan, N.; Mohapatra, U.B.; Sukla, L.B. Two step meso-acidophilic bioleaching of chalcopyrite containing ball mill spillage and removal of the surface passivation layer. Bioresour. Technol. 2013, 130, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Acosta, M.; Galleguillos, P.; Ghorbani, Y.; Tapia, P.; Contador, Y.; Velásquez, A.; Espoz, C.; Pinilla, C.; Demergasso, C. Variation in microbial community from predominantly mesophilic to thermotolerant and moderately thermophilic species in an industrial copper heap bioleaching operation. Hydrometallurgy 2014, 150, 281–289. [Google Scholar] [CrossRef]
- Behrad Vakylabad, A. A comparison of bioleaching ability of mesophilic and moderately thermophilic culture on copper bioleaching from flotation concentrate and smelter dust. Int. J. Miner. Process. 2011, 101, 94–99. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Zhou, D.; Liu, Y.; Yu, R.; Qiu, G.; Zeng, W. Effects of pH value on the expression of key iron/sulfur oxidation genes during bioleaching of chalcopyrite on thermophilic condition. Ann. Microbiol. 2019, 69, 627–635. [Google Scholar] [CrossRef]
- Ai, C.; McCarthy, S.; Liang, Y.; Rudrappa, D.; Qiu, G.; Blum, P. Evolution of copper arsenate resistance for enhanced enargite bioleaching using the extreme thermoacidophile Metallosphaera sedula. J. Ind. Microbiol. Biotechnol. 2017, 44, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Elberling, B.; Schippers, A.; Sand, W. Bacterial and chemical oxidation of pyritic mine tailings at low temperatures. J. Contam. Hydrol. 2000, 41, 225–238. [Google Scholar] [CrossRef]
- Escobar, B.; Buccicardi, S.; Morales, G.; Wiertz, J. Biooxidation of ferrous iron and sulphide at low temperatures: Implications on acid mine drainage and bioleaching of sulphide minerals. Hydrometallurgy 2010, 104, 454–458. [Google Scholar] [CrossRef]
- Halinen, A.K.; Rahunen, N.; Kaksonen, A.H.; Puhakka, J.A. Heap bioleaching of a complex sulfide ore: Part II. Effect of temperature on base metal extraction and bacterial compositions. Hydrometallurgy 2009, 98, 101–107. [Google Scholar] [CrossRef]
- Langdahl, B.R.; Ingvorsen, K. Temperature characteristics of bacterial iron solubilisation and 14C assimilation in naturally exposed sulfide ore material at Citronen Fjord, North Greenland (83oN). Fems Microbiol. Ecol. 1997, 23, 275–283. [Google Scholar] [CrossRef]
- Dopson, M.; Halinen, A.K.; Rahunen, N.; Ozkaya, B.; Sahinkaya, E.; Kaksonen, A.H.; Lindstrom, E.B.; Puhakka, J.A. Mineral and iron oxidation at low temperatures by pure and mixed cultures of acidophilic microorganisms. Biotechnol. Bioeng. 2007, 97, 1205–1215. [Google Scholar] [CrossRef]
- Hallberg, K.B.; Gonzalez-Toril, E.; Johnson, D.B. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Talla, E.; Hedrich, S.; Mangenot, S.; Ji, B.; Johnson, D.B.; Barbe, V.; Bonnefoy, V. Insights into the pathways of iron- and sulfur-oxidation, and biofilm formation from the chemolithotrophic acidophile Acidithiobacillus ferrivorans CF27. Res. Microbiol. 2014, 165, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Liljeqvist, M.; Rzhepishevska, O.I.; Dopson, M. Gene identification and substrate regulation provide insights into sulfur accumulation during bioleaching with the psychrotolerant acidophile Acidithiobacillus ferrivorans. Appl. Env. Microbol. 2013, 79, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Quatrini, R.; Appia-Ayme, C.; Denis, Y.; Jedlicki, E.; Holmes, D.S.; Bonnefoy, V. Extending the models for iron and sulfur oxidation in the extreme acidophile Acidithiobacillus ferrooxidans. BMC Genom. 2009, 10, 394–412. [Google Scholar] [CrossRef]
- Stott, M.; Watling, H.; Franzmann, P.; Sutton, D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Min. Eng. 2000, 13, 1117–1127. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Chen, M. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles. Min. Eng. 2013, 48, 31–35. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Dixon, D.G.; Asselin, E. Electrochemical and XPS analysis of chalcopyrite (CuFeS2) dissolution in sulfuric acid solution. Electrochim. Acta 2013, 87, 97–112. [Google Scholar] [CrossRef]
- Peng, T.; Ma, L.; Feng, X.; Tao, J.; Nan, M.; Liu, Y.; Li, J.; Shen, L.; Wu, X.; Yu, R. Genomic and transcriptomic analyses reveal adaptation mechanisms of an Acidithiobacillus ferrivorans strain YL15 to alpine acid mine drainage. Plos ONE 2017, 12, e0178008. [Google Scholar] [CrossRef]
- Johnson, D.B. Selective solid media for isolating and enumerating acidophilic bacteria. J. Microbiol. Methods 1995, 23, 205–218. [Google Scholar] [CrossRef]
- Falagám, C.; Johnson, D.B. Acidithiobacillus ferriphilus sp. nov., a facultatively anaerobic iron- and sulfur-metabolizing extreme acidophile. Int. J. Syst. Evol. Microbiol. 2016, 66, 206–211. [Google Scholar]
- Ma, L.; Wang, X.; Liu, X.; Wang, S.; Wang, H. Intensified bioleaching of chalcopyrite by communities with enriched ferrous or sulfur oxidizers. Bioresour. Technol. 2018, 268, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Goto, K.; Yotsuyanagi, T.; Nagayama, M. Spectrophotometric determination of iron(II) with 1,10-phenanthroline in the presence of large amounts of iron(III). Talanta 1974, 21, 314–318. [Google Scholar] [CrossRef]
- Peterson, R.E.; Bollier, M.E. Spectrophotometric determination of serum copper with biscyclohexanoneoxalyldihydrazone. Anal. Chem. 1955, 27, 1195–1197. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure, and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197–198, 1–32. [Google Scholar] [CrossRef]
- Christel, S.; Fridlund, J.; Buetti-Dinh, A.; Buck, M.; Watkin, E.L.; Dopson, M. RNA transcript sequencing reveals inorganic sulfur compound oxidation pathways in the acidophile Acidithiobacillus ferrivorans. Fems Microbiol. Lett. 2016, 363, fnw057. [Google Scholar] [CrossRef]
- Wu, X.L.; Wu, X.Y.; Shen, L.; Li, J.; Yu, R.; Liu, Y.; Qiu, G.; Zeng, W. Whole genome sequencing and comparative genomics analyses of Pandoraea sp. XY-2, a new species capable of biodegrade tetracycline. Front Microbiol. 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wei, M.; Ji, H.; Chen, X.; Qiu, G.; Zhou, H. Application of real-time PCR to monitor population dynamics of defined mixed cultures of moderate thermophiles involved in bioleaching of chalcopyrite. Appl. Microbiol. Biotechnol. 2009, 81, 1161–1168. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review part A: Progress in bioleaching: fundamentals and mechanisms of bacterial metalsulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Klauber, C.; Parker, A.; van Bronswijk, W.; Watling, H. Sulphur speciation of leached chalcopyrite surfaces as determined by X-ray photoelectron spectroscopy. Int. J. Min. Process. 2001, 62, 65–94. [Google Scholar] [CrossRef]
- Zhu, W.; Xia, J.; Yang, Y.; Nie, Z.; Zheng, L.; Ma, C.; Zhang, R.; Peng, A.; Tang, L.; Qiu, G. Sulfur oxidation activities of pure and mixed thermophiles and sulfur speciation in bioleaching of chalcopyrite. Bioresour. Technol. 2011, 102, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Kupka, D.; Rzhepishevska, O.I.; Dopson, M.; Lindstrom, E.B.; Karnachuk, O.V.; Tuovinen, O.H. Bacterial oxidation of ferrous iron at low temperatures. Biotechnol. Bioeng. 2007, 97, 1470–1478. [Google Scholar] [CrossRef]
- Liljeqvist, M.; Valdes, J.; Holmes, D.S.; Dopson, M. Draft Genome of the psychrotolerant acidophile Acidithiobacillus ferrivorans SS3. J. Bacteriol. 2011, 193, 4304–4305. [Google Scholar] [CrossRef] [PubMed]
- Barahona, S.; Dorador, C.; Zhang, R.; Aguilar, P.; Sand, W.; Vera, M.; Remonsellez, F. Isolation and characterization of a novel Acidithiobacillus ferrivorans strain from the Chilean Altiplano: Attachment and biofilm formation on pyrite at low temperature. Res. Microbiol. 2014, 165, 782–793. [Google Scholar] [CrossRef]


| Strain | Fe(II) | Fe(III) | Cu(II) | Zn(II) | Co(II) | Ni(II) | Mn(II) |
|---|---|---|---|---|---|---|---|
| YL15 | 22.3 (27.9) | 16.8 (22.3) | 25.4 (31.8) | 52.3 (58.9) | 29.5 (35.4) | 11.7 (17.6) | 22.0 (27.5) |
| ATCC 23270T | 22.3 (27.9) | 16.8 (22.3) | 19.06 (25.4) | 52.3 (58.9) | 23.6 (29.5) | 5.9 (11.7) | 16.5 (22.0) |
| Sample | S2− | S22− | Sn2− | S0 | SO42− | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| BE | FWHM | BE | FWHM | BE | FWHM | BE | FWHM | BE | FWHM | |
| Before bioleaching | 161.6 | 0.87 | 162.4 | 0.75 | 163.1 | 1.25 | 164.7 | 1.35 | 169.0 | 1.30 |
| Ore residue | 161.3 | 0.98 | 162.3 | 0.67 | 163.1 | 1.25 | 164.7 | 1.35 | 168.8 | 1.46 |
| Sample | S0 | Sn2− | SO42− | S2− | S22− |
|---|---|---|---|---|---|
| Before bioleaching | 8.6 | 10.4 | 46.7 | 18.7 | 15.6 |
| Ore residue | 6.4 | 21.4 | 20.9 | 47.1 | 4.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, T.; Chen, L.; Wang, J.; Miao, J.; Shen, L.; Yu, R.; Gu, G.; Qiu, G.; Zeng, W. Dissolution and Passivation of Chalcopyrite during Bioleaching by Acidithiobacillus ferrivorans at Low Temperature. Minerals 2019, 9, 332. https://doi.org/10.3390/min9060332
Peng T, Chen L, Wang J, Miao J, Shen L, Yu R, Gu G, Qiu G, Zeng W. Dissolution and Passivation of Chalcopyrite during Bioleaching by Acidithiobacillus ferrivorans at Low Temperature. Minerals. 2019; 9(6):332. https://doi.org/10.3390/min9060332
Chicago/Turabian StylePeng, Tangjian, Lei Chen, Jingshu Wang, Jie Miao, Li Shen, Runlan Yu, Guohua Gu, Guanzhou Qiu, and Weimin Zeng. 2019. "Dissolution and Passivation of Chalcopyrite during Bioleaching by Acidithiobacillus ferrivorans at Low Temperature" Minerals 9, no. 6: 332. https://doi.org/10.3390/min9060332





