A Comparative Study of Different Columns Sizes for Ultrafine Apatite Flotation
Abstract
:1. Introduction
2. Experimental
2.1. Ore Sample
2.2. Desliming
3. Flotation Studies
3.1. Reagents
3.2. Pilot Plant Flotation Tests
4. Results and Discussion
4.1. Desliming
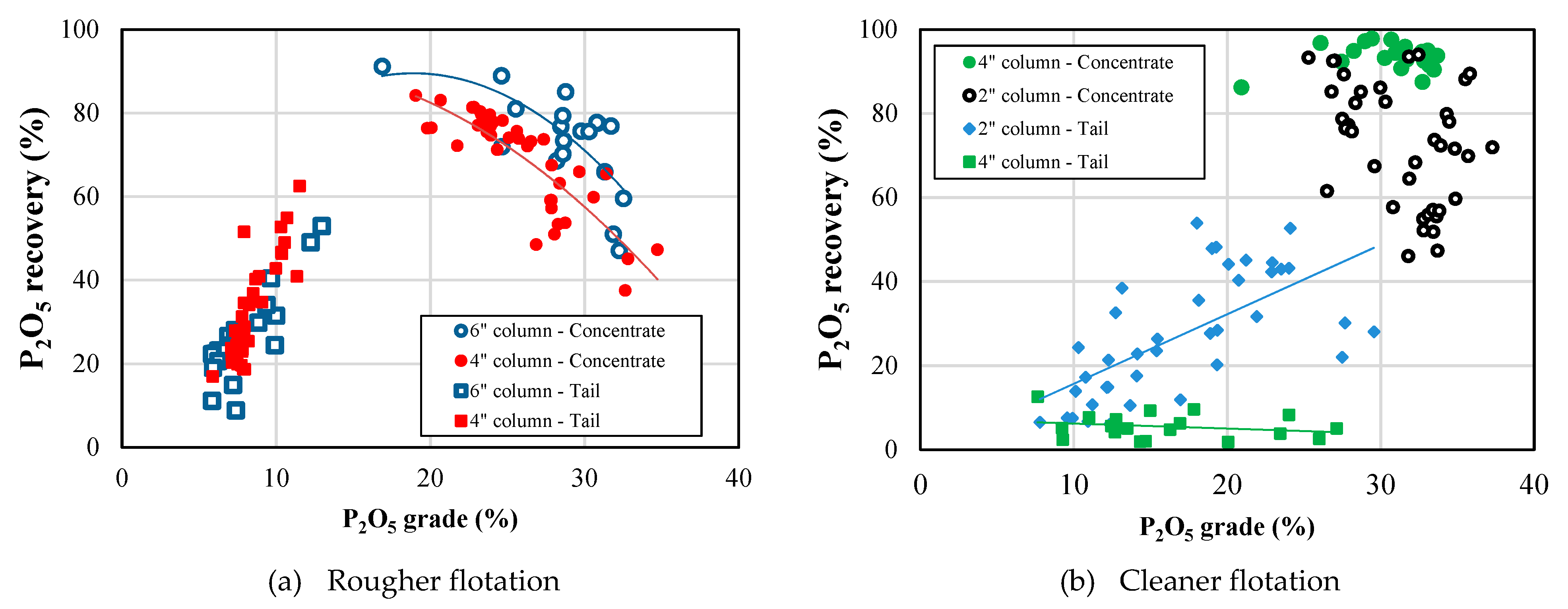
4.2. Flotation Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Houot, R. Beneficiation of phosphatic ores through flotation: Review of industrial applications and potential developments. Int. J. Miner. Process. 1982, 9, 353–384. [Google Scholar] [CrossRef]
- Dong, X.; Liu, S.; Yao, Y.; Liu, H.; Pei, Y. A review of new technological progress for benefication of refractory phosphate ore in China. IOP Conf. Ser.: Earth Environ. Sci. 2017, 63, 1–6. [Google Scholar]
- Pourkarimi, Z.; Rezai, B.; Noaparast, M. Nanobubbles effect on the mechanical flotation of phosphate ore fine particles. Physicochem. Probl. Miner. Process. 2018, 54, 278–292. [Google Scholar]
- Huynh, L.; Feiler, A.; Michelmore, A.; Ralston, J.; Jenkins, P. Control of slime coatings by the use of anionic phosphates: A fundamental study. Min. Eng. 2000, 13, 1050–1069. [Google Scholar] [CrossRef]
- Ahmed, H.A.M. Optimization of desliming prior to phosphate ore upgrading flotation. Phys. Probl. Min. Proc. 2007, 41, 79–88. [Google Scholar]
- Teague, A.J.; Lollback, M.C. The beneficiation of ultrafine phosphate. Min. Eng. 2012, 27–28, 52–59. [Google Scholar] [CrossRef]
- Al-Thyabat, S. Evaluation of mechanical flotation of non-slimed Jordanian siliceous phosphate. Arab. J. Sci. Eng. 2012, 31, 877–887. [Google Scholar] [CrossRef]
- Zhang, P.; Bogan, M. Recovery of phosphate from Florida beneficiation slimes I. Re-identifying the problem. Min. Eng. 1995, 8, 523–534. [Google Scholar] [CrossRef]
- Pradip, R.S.; Sankar, T.A.P. Selective flotation of Maton (India) phosphate ore slimes with particular reference to the effects of particle size. Internet J. Min. Proc. 1992, 36, 283–293. [Google Scholar]
- Al-Thyabat, S.; Yoon, R.H.; Shin, D. Floatability of fine phosphate in a batch column flotation cell. Min. Metal. Proc. 2011, 28, 1110–1116. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Liu, T.; Cai, Z.; Sun, K. Characterization and separation studies of fine sedimentary phosphate ore slime. Minerals 2017, 7, 94. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Peres, A.E.C. Industrial practice of phosphate ore flotation at Serrana-Araxá, Brazil. In Proceedings of the XXI International Mineral Processing Congress, Rome, Italy, 23–27 July 2000. B9-17. [Google Scholar]
- Guimarães, R.C.; Peres, A.E.C. Experiência brasileira de produção de concentrado fosfático a partir de lamas. In Proceedings of the XIX Encontro Nacional de Tratamento de Minérios e Metalurgia Extrativa, Recife, Brasil, 26–29 November 2002; Volume I, pp. 247–253. (In Portuguese). [Google Scholar]
- Guimarães, R.C.; Peres, A.E.C. Production of phosphate concentrates from slimes: Brazilian experience. In Proceedings of the XXII International Mineral Processing Congress, Cape-Town, South Africa, 29 September–3 October 2003; pp. 606–612. [Google Scholar]
- Matiolo, E.; Couto, H.J.B.; Teixeira, M.F.L.; Freitas, A.S.; Almeida, R.N. Recovery of apatite from slimes of a Brazilian phosphate ore. J. Wuhan Inst. Technol. 2017, 39, 39–48. [Google Scholar]
- Ipek, H.; Ozdag, H. An investigation into the enrichment of phosphate slime by column flotation. Dev. Miner. Process. 2000, 13, C8a-1–C8a-5. [Google Scholar]
- Tao, Y.; Liu, J.; Yu, S.; Tao, D. Picobubble enhanced fine coal. Sep. Sci. Technol. 2006, 41, 3597–3607. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Fich, J.A.; Hu, H.; Rao, S.R. Role of hydrodynamic cavitation in fine particle flotation. Int. J. Miner. Process. 1997, 51, 139–149. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Finch, J.A.; Masliyah, J.H.; Chow, R.S. On the role of cavitation in particle collection in flotation—A critical review. II. Min. Eng. 2009, 22, 419–433. [Google Scholar] [CrossRef]
- Abdel-Khalek, N.A.; Hassan, F.; Arafa, M.A. Separation of valuable fine phosphate particles from their slimes by column flotation. Sep. Sci. Tech. 2000, 35, 1077–1086. [Google Scholar] [CrossRef]




| Sample | Al2O3 | BaO | CaO | Fe2O3 | MgO | P2O5 | SiO2 | Nb2O5 | CaO/P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.9 | 1.2 | 14.0 | 27.7 | 2.4 | 13.3 | 19.7 | 0.62 | 1.05 |
| 2 | 2.8 | 1.6 | 16.5 | 30.0 | 1.0 | 14.0 | 12.5 | 0.89 | 1.18 |
| Stage | Stream | Mass Rec (%) | Grade (%) | Distribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | Fe2O3 | MgO | P2O5 | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | P2O5 | SiO2 | |||
| 1 | Over | 29.9 | 4.5 | 9.9 | 33.9 | 2.3 | 10.1 | 16.5 | 35.4 | 21.4 | 36.9 | 29.0 | 23.1 | 25.3 |
| Under | 70.1 | 3.5 | 15.5 | 24.7 | 2.4 | 14.3 | 20.7 | 64.5 | 78.5 | 63.0 | 70.9 | 76.8 | 74.6 | |
| 2 | Over | 29.8 | 4.4 | 11.5 | 31.1 | 2.5 | 11.3 | 17.3 | 37.5 | 21.3 | 40.0 | 31.5 | 22.5 | 24.0 |
| Under | 70.2 | 3.1 | 17.9 | 19.7 | 2.3 | 16.4 | 23.2 | 62.4 | 78.6 | 59.9 | 68.4 | 77.4 | 76.0 | |
| Feed | 100 | 3.8 | 13.8 | 27.4 | 2.4 | 13.0 | 19.4 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Stage | Stream | Mass Rec (%) | Grade (%) | Distribution (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | CaO | Fe2O3 | MgO | P2O5 | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | P2O5 | SiO2 | |||
| 1 | Over | 43.2 | 3.7 | 12.9 | 35.3 | 1.2 | 11.5 | 12.3 | 58.4 | 33.9 | 51.4 | 43.1 | 34.8 | 42.6 |
| Under | 56.8 | 2.0 | 19.2 | 25.5 | 1.2 | 16.4 | 12.6 | 41.6 | 66.1 | 48.6 | 56.9 | 65.2 | 57.4 | |
| 2 | Over | 15.0 | 2.8 | 10.9 | 28.8 | 1.1 | 12.7 | 10.5 | 20.3 | 8.9 | 17.9 | 13.5 | 11.7 | 12.0 |
| Under | 85.0 | 1.9 | 19.8 | 23.4 | 1.2 | 16.9 | 13.3 | 79.7 | 91.1 | 82.1 | 86.5 | 88.3 | 88.0 | |
| Feed | 100 | 2.7 | 16.5 | 29.7 | 1.2 | 14.3 | 12.5 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Test | Recovery (%) | Grade (%) | Consumption (g/t) | ||||
|---|---|---|---|---|---|---|---|
| Mass | P2O5 | P2O5 | Fe2O3 | SiO2 | Collector | Depressant | |
| 1 | 24.2 | 52.7 | 34.3 | 2.9 | 6.2 | 123 | 2353 |
| 2 | 25.3 | 57.7 | 35.5 | 3.0 | 5.1 | 116 | 2737 |
| 3 | 22.7 | 53.5 | 35.8 | 3.0 | 4.2 | 113 | 3259 |
| Average | 24.1 | 54.6 | 35.2 | 3.0 | 5.2 | 117 | 2783 |
| Test | Recovery (%) | Grade (%) | Consumption (g/t) | |||||
|---|---|---|---|---|---|---|---|---|
| Mass | P2O5 | P2O5 | Fe2O3 | SiO2 | pH | Collector | Depressant | |
| 1 | 35.0 | 72.1 | 33.7 | 5.2 | 5.2 | 10.8 | 132 | 3004 |
| 2 | 33.5 | 70.2 | 33.5 | 5.6 | 5.2 | 127 | 2905 | |
| 3 | 35.3 | 73.8 | 33.1 | 5.9 | 5.5 | 129 | 2947 | |
| Average | 34.6 | 72.0 | 33.4 | 5.6 | 5.3 | 129 | 2952 | |
| 1 | 33.5 | 68.2 | 31.7 | 5.2 | 9.1 | 9.7 | 123 | 2815 |
| 2 | 37.9 | 72.6 | 31.6 | 4.9 | 9.7 | 125 | 2844 | |
| 3 | 36.2 | 69.7 | 31.3 | 5.1 | 9.8 | 133 | 3029 | |
| 4 | 39.9 | 74.9 | 31.0 | 5.2 | 10.2 | 130 | 2959 | |
| Average | 36.9 | 71.3 | 31.4 | 5.1 | 9.7 | 127 | 2911 | |
| Test | Recovery (%) | Grade (%) | Consumption (g/t) | ||||
|---|---|---|---|---|---|---|---|
| Mass | P2O5 | P2O5 | Fe2O3 | SiO2 | Collector | Depressant | |
| 1 | 25.6 | 47.2 | 32.8 | 4.8 | 0.7 | 72 | 3000 |
| 2 | 24.6 | 44.6 | 32.7 | 3.8 | 0.6 | 69 | 2880 |
| 3 | 27.4 | 54.5 | 33.1 | 4.2 | 1.2 | 94 | 2380 |
| 4 | 30.7 | 57.7 | 32.7 | 4.4 | 1.5 | 87 | 2215 |
| Average | 27.1 | 51.0 | 32.8 | 4.3 | 1.0 | 80 | 2618 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matiolo, E.; Couto, H.J.B.; de Lira Teixeira, M.F.; de Almeida, R.N.; de Freitas, A.S. A Comparative Study of Different Columns Sizes for Ultrafine Apatite Flotation. Minerals 2019, 9, 391. https://doi.org/10.3390/min9070391
Matiolo E, Couto HJB, de Lira Teixeira MF, de Almeida RN, de Freitas AS. A Comparative Study of Different Columns Sizes for Ultrafine Apatite Flotation. Minerals. 2019; 9(7):391. https://doi.org/10.3390/min9070391
Chicago/Turabian StyleMatiolo, Elves, Hudson Jean Bianquini Couto, Michelle Fernanda de Lira Teixeira, Renata Nigri de Almeida, and Amanda Soares de Freitas. 2019. "A Comparative Study of Different Columns Sizes for Ultrafine Apatite Flotation" Minerals 9, no. 7: 391. https://doi.org/10.3390/min9070391
APA StyleMatiolo, E., Couto, H. J. B., de Lira Teixeira, M. F., de Almeida, R. N., & de Freitas, A. S. (2019). A Comparative Study of Different Columns Sizes for Ultrafine Apatite Flotation. Minerals, 9(7), 391. https://doi.org/10.3390/min9070391




