Iron Control in Atmospheric Acid Laterite Leaching
Abstract
:1. Introduction
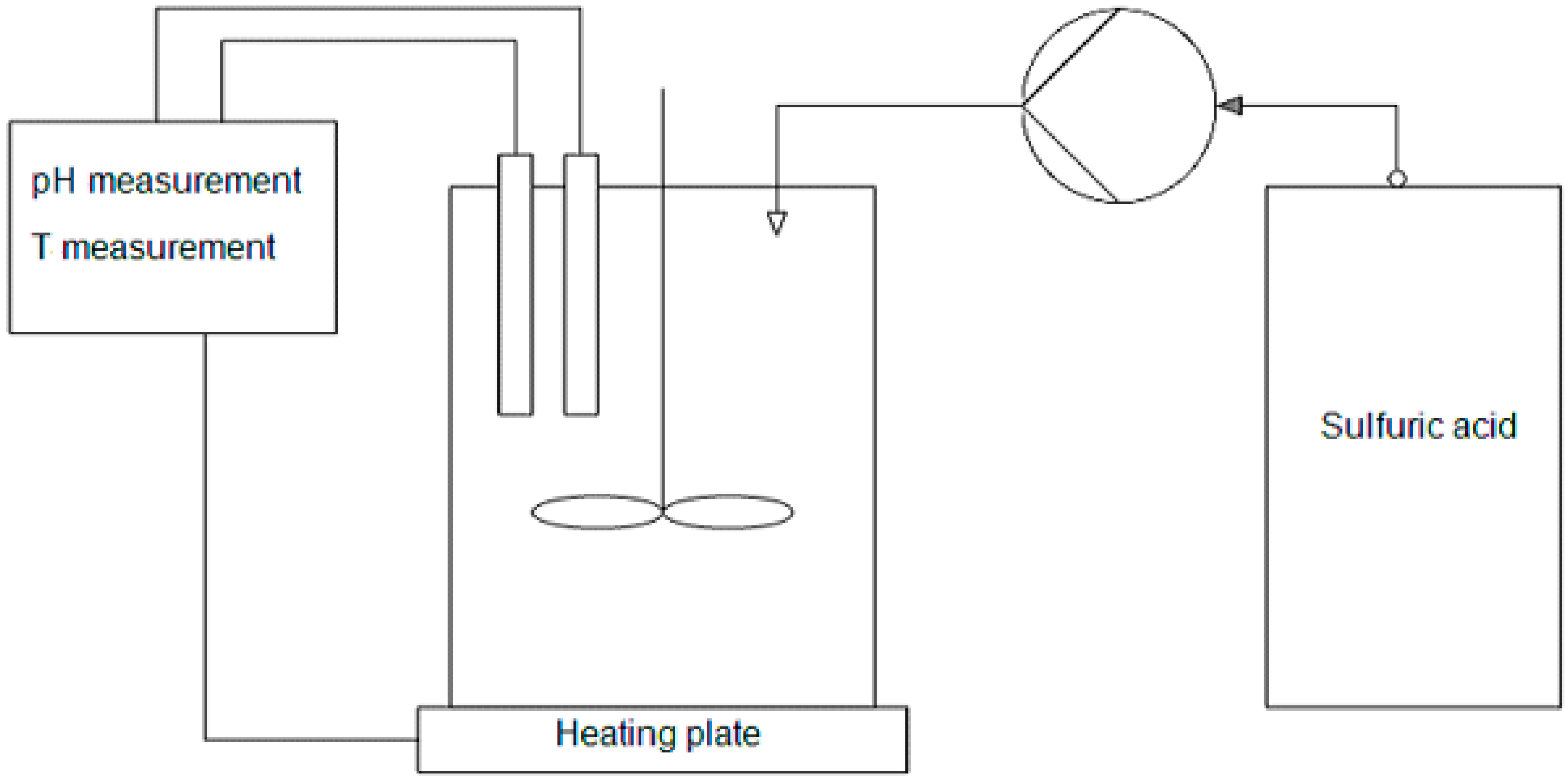
2. Materials and Methods
2.1. Materials
2.2. Leaching of Laterite Ores
2.3. Analytical Methods
3. Results and Discussion
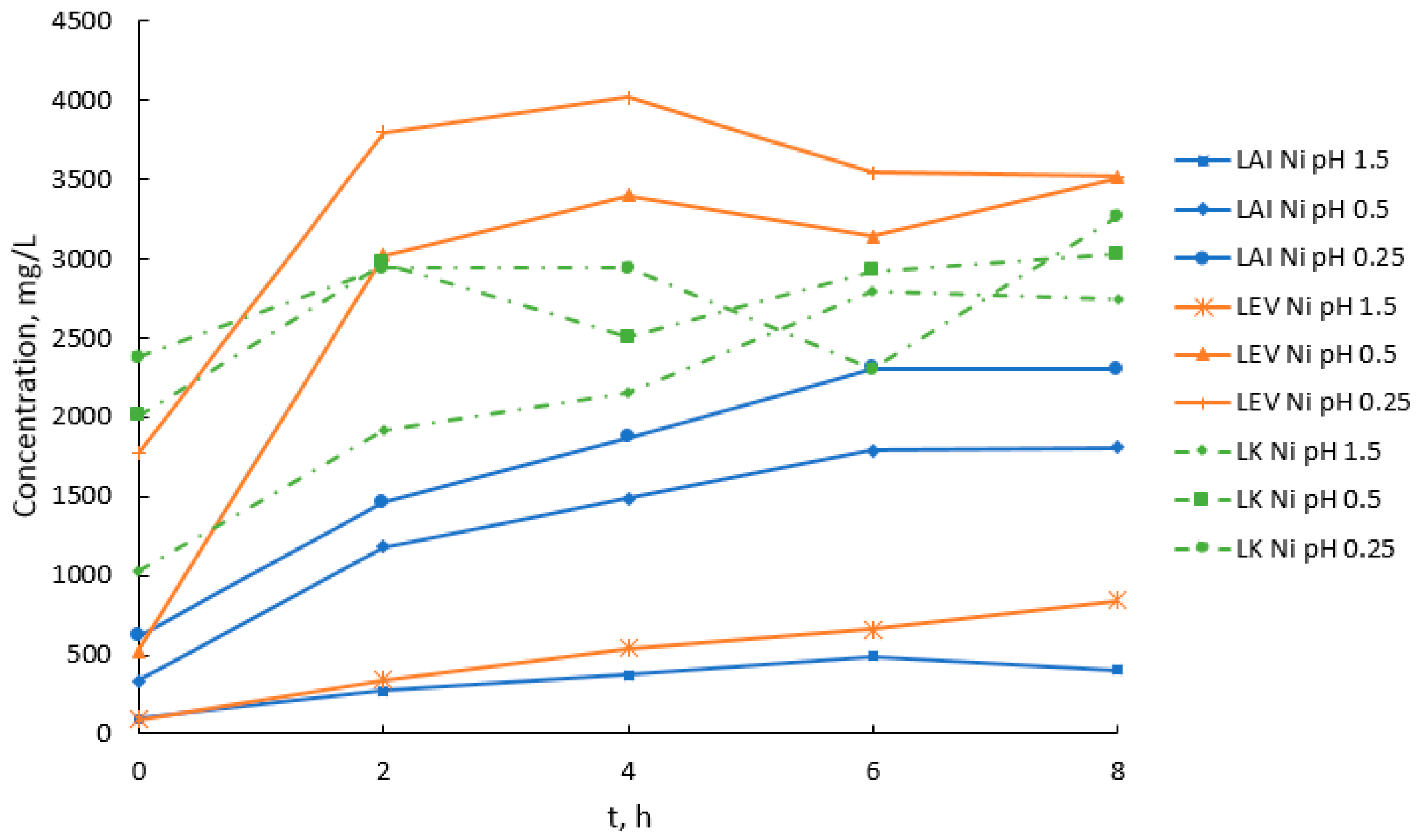
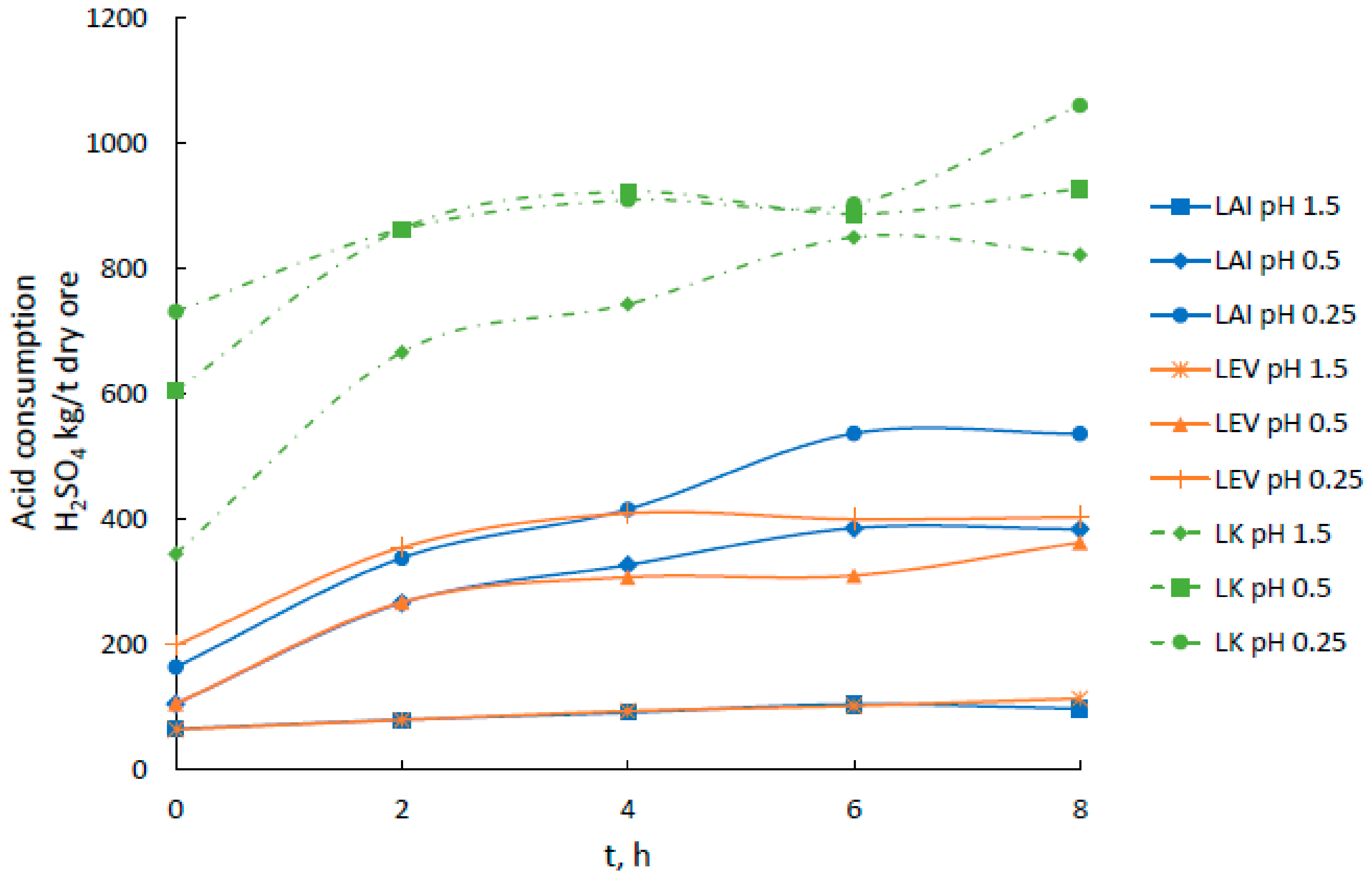
3.1. Acid Leaching of Greek Laterites
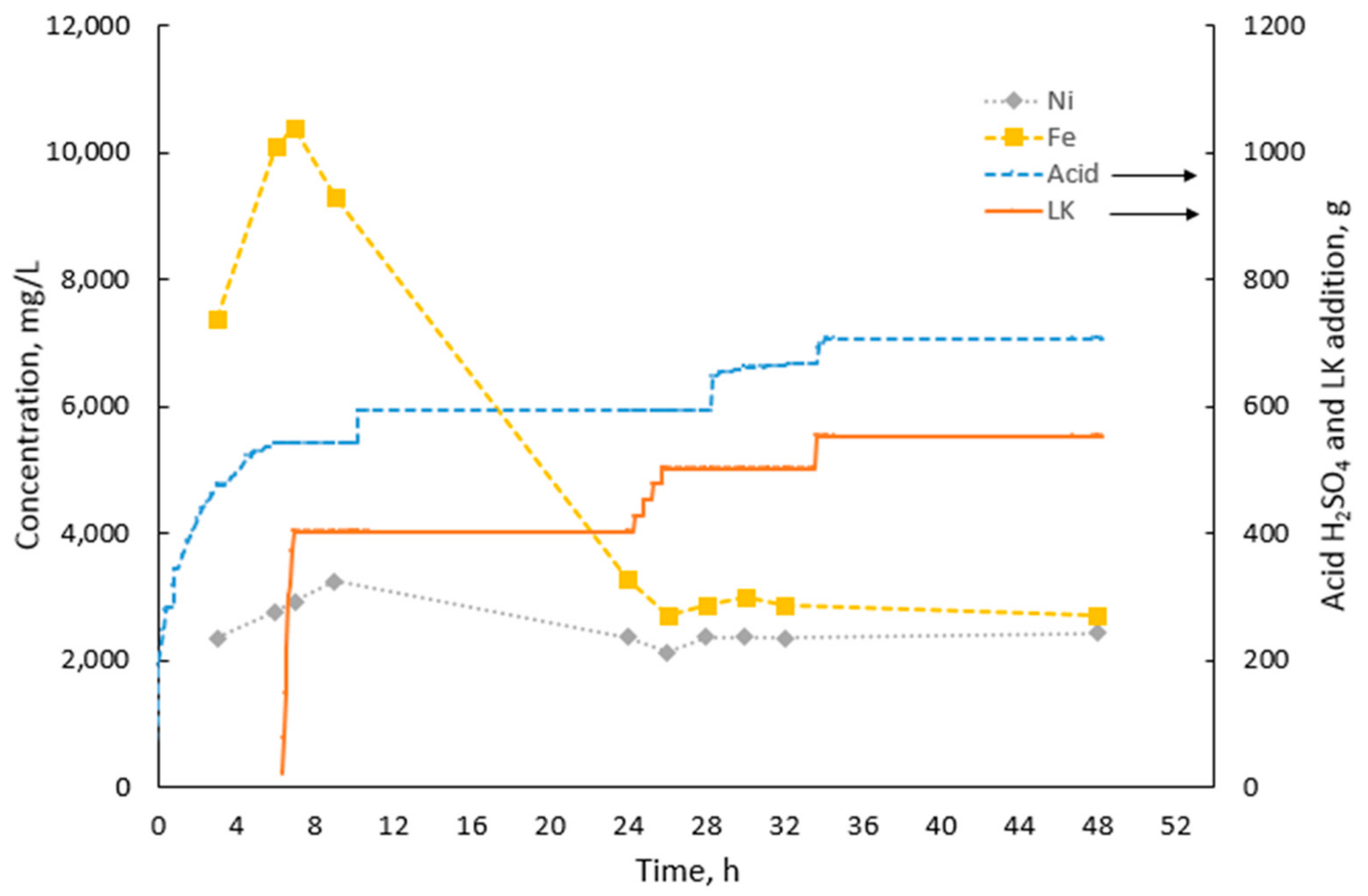
3.2. Combined Leaching and Iron Precipitation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mudd, G.M. Global trends and environmental issues in nickel mining: Sulfides versus laterites. Ore Geol. Rev. 2010, 38, 9–26. [Google Scholar] [CrossRef]
- Barnett, S. Nickel—A key material for innovation in a sustainable future. In Proceedings of the 2nd Euro Nickel Conference, London, UK, 18–19 March 2010; Informa Pty Ltd.: London, UK. [Google Scholar]
- McRae, M.E. Minerals Yearbook, Nickel (Advance Release); U.S. Geological Survey: Reston, VA, USA, June 2018.
- European Commission. Commission Staff Working Document. Report on Raw Materials for Battery Applications. Available online: https://ec.europa.eu/transport/sites/transport/files/3rd-mobility-pack/swd20180245.pdf (accessed on 24 March 2019).
- McRae, M.E. Nickel: U.S. Geological Survey, Mineral Commodity Summaries 2018. U.S. Department of Interior, U.S. Geological Survey; pp. 112–113. Available online: https://min-erals.usgs.gov/minerals/pubs/mcs/2018/mcs2018.pdf (accessed on 24 March 2019).
- McDonald, R.G.; Whittington, B.I. Atmospheric acid leaching of nickel laterites review Part, I. Sulfuric acid technologies. Hydrometallurgy 2008, 91, 35–55. [Google Scholar] [CrossRef]
- Sudol, S. The thunder from down under: everything you wanted to know about laterites but were afraid to ask. Can. Min. J. 2005, 126, 8–12. [Google Scholar]
- Oxley, A.; Smith, M.; Caceres, O. Why heap leach nickel laterites? Miner. Eng. 2016, 88, 53–60. [Google Scholar] [CrossRef]
- Li, G.; Zhou, Q.; Zhu, Z.; Luo, J.; Rao, M.; Peng, Z.; Jiang, T. Selective leaching of nickel and cobalt from limonitic laterite using phosphoric acid: An alternative for value-added processing of laterite. J. Clean. Prod. 2018, 189, 620–626. [Google Scholar] [CrossRef]
- Whittington, B.I.; Muir, D. Pressure Acid Leaching of nickel Laterites: A Review. Miner. Process. Extr. Metall. Rev. 2000, 21, 527–599. [Google Scholar] [CrossRef]
- Dalvi, A.D.; Bacon, W.G.; Osborne, R.C. The Past and Future of Nickel Laterites, PDAC 2004 Convention, March 7–10, 2004. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.732.7854&rep=rep1&type=pdf (accessed on 24 March 2019).
- Rodrigues, F.; Pickles, C.A. Factors Affecting the Upgrading of a Nickeliferous Limonitic Laterite Ore by Reduction Roasting, Thermal Growth and Magnetic Separation. Minerals 2017, 7, 176. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E. Column Leaching of Greek Low-Grade Limonitic Laterites. Minerals 2018, 8, 377. [Google Scholar] [CrossRef]
- Agatzini-Leonardou, S. Hydrometallurgical Process for the separation and recovery of nickel from sulfate heap leach liquor of nickelferrous latetrite ores. Miner. Eng. 2009, 22, 1181–1192. [Google Scholar] [CrossRef]
- Komnitsas, K.; Petrakis, E.; Bartzas, G.; Karmali, V. Column leaching of low-grade saprolitic laterites and valorization of leaching residues. Sci. Total Environ. 2019, 665, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Wang, K. Iron, Aluminium and chromium co-removal from atmospheric nickel laterite leach solutions. Miner. Eng. 2018, 116, 35–45. [Google Scholar] [CrossRef]
- Cheng, C.Y. Purification of synthetic laterite leach solution by solvent extraction using D2EHPA. Hydrometallurgy 2000, 56, 369–386. [Google Scholar] [CrossRef]
- Arroyo, J.C.; Neudorf, D.A. Atmospheric Leaching Process for the Recovery of Nickel and Cobalt from Limonite and Saprolite Laterite Ores. Patent no: 6,261,527, 2001. [Google Scholar]
- Leppinen, J.; Riihimäki, T.; Ruonala, M. Method for Separating Nickel from Material with Low Nickel Content. Patent no: CA2819224A1, 2011. [Google Scholar]
- White, D.T.; Gillaspie, J.D. Acid leaching of nickel laterites with jarosite precipitation. In Ni-Co 2013; Springer: Cham, Switzerland, 2013; pp. 75–95. [Google Scholar]
- Dutrizac, J.E. The effectiveness of jarosite species for precipitating sodium jarosite. Jom 1999, 51, 30–32. [Google Scholar] [CrossRef]
- Das, G.K.; Acharya, S. Jarosites: A Review. Miner. Process. Extr. Metall. Rev. 1996, 16, 185–210. [Google Scholar] [CrossRef]
- Mystrioti, C.; Papassiopi, N.; Xenidis, A.; Komnitsas, K. Counter-Current Leaching of Low-Grade Laterites with Hydrochloric Acid and Proposed Purification Options of Pregnant Solution. Minerals 2018, 8, 599. [Google Scholar] [CrossRef]


| Laterite | LAI | LEV | LK |
|---|---|---|---|
| Element | % | % | % |
| Ni * | 1.00 | 0.843 | 0.939 |
| Fe * | 31.6 | 26.3 | 13.2 |
| Al * | 9.58 | 2.62 | 0.388 |
| Mg * | 1.1 | 2.16 | 12.8 |
| Na * | <0.1 | <0.1 | <0.1 |
| P * | 0.025 | <0.02 | <0.02 |
| K * | 0.16 | 0.539 | <0.1 |
| Ca * | 2.2 | 2.08 | 3.37 |
| Ti * | 0.55 | 0.142 | 0.025 |
| Cr * | 1.27 | 1.72 | 0.554 |
| Mn * | 0.179 | 0.167 | 0.179 |
| Co * | 0.062 | 0.042 | 0.029 |
| Cu * | <0.005 | <0.01 | <0.01 |
| Zn * | 0.015 | 0.015 | 0.009 |
| As * | 0.02 | <0.02 | <0.02 |
| C ** | 0.69 | 0.76 | 1.1 |
| S ** | 0.02 | 0.09 | <0.02 |
| SiO2 *** | 16.3 | 40.7 | 37.1 |
| Mineralogy | LAI | Ni | LEV | Ni | LK | Ni |
|---|---|---|---|---|---|---|
| wt % | dist. % | wt % | dist. % | wt % | dist. % | |
| Hematite | 45.4 | 16 | 44.6 | 47 | ||
| Goethite | 19.5 | 24 | ||||
| Al-clays and Al-hydroxides * | 21.1 | 60 | ||||
| Smectite group minerals | 5.5 | 22 | 2.1 | 13 | ||
| Primary serpentinite silicates ** | 12.8 | 1 | 8.5 | 39 | 67.6 | 75 |
| Quartz | 6 | 34.4 | 3.2 | |||
| Carbonates | 5.8 | 3.3 | 7.6 | |||
| Chromite | 2.6 | <1 | 3.5 | <1 | 1.7 | <1 |
| Magnetite | 0.8 | <1 | 0.7 | <1 | 0.5 | <1 |
| LAI | LEV | LK | |
|---|---|---|---|
| H2SO4 g/laterite kg | H2SO4 g/laterite kg | H2SO4 g/laterite kg | |
| Ni | 16.7 | 14.0 | 15.7 |
| Fe | 832 | 693 | 348 |
| Al | 522 | 143 | 21.3 |
| Mg | 44.4 | 87.2 | 517 |
| Ca | 53.8 | 50.9 | 82.5 |
| Mn | 3.21 | 3.03 | 3.21 |
| Total | 1470 | 991 | 987 |
| LAI, pH 1.5 | LAI, pH 0.5 | LAI, pH 0.25 | LEV, pH 1.5 | LEV, pH 0.5 | LEV, pH 0.25 | LK, pH 1.5 | LK, pH 0.5 | LK, pH 0.25 | |
|---|---|---|---|---|---|---|---|---|---|
| Ni, % | 12.0 | 54.3 | 69.0 | 30.1 | >100.0 | >100.0 | 87.5 | 96.8 | >100.0 |
| Fe, % | 1.2 | 17.1 | 27.9 | 2.9 | 16.2 | 23.3 | 20.9 | 78.9 | 80.5 |
| pH | Time | LAI | LEV | LK |
|---|---|---|---|---|
| h | H2SO4 kg/t dry ore | H2SO4 kg/t dry ore | H2SO4 kg/t dry ore | |
| 1.5 | 2 | 72.9 | 89.3 | 532 |
| 4 | 92.5 | 115 | 597 | |
| 6 | 109 | 126 | 647 | |
| 8 | 123 | 142 | 647 | |
| 0.5 | 2 | 305 | 343 | 787 |
| 4 | 372 | 403 | 832 | |
| 6 | 395 | 455 | 1120 | |
| 8 | 442 | 455 | 1120 | |
| 0.25 | 2 | 463 | 4940 | 880 |
| 4 | 532 | 529 | 911 | |
| 6 | 605 | 576 | 945 | |
| 8 | 649 | 593 | 951 |
| Laterite Ore | pH | Ni (mg/L) | Co (mg/L) | Fe (mg/L) | Ca (mg/L) | Mg (mg/L) | Al (mg/L) | Ni/Fe | Ni/Mg | Ni/Al |
|---|---|---|---|---|---|---|---|---|---|---|
| LAI | 1.5 | 401 | 60 | 1220 | 447 | 591 | 1560 | 0.33 | 0.68 | 0.26 |
| LAI | 0.5 | 1810 | 156 | 18,000 | 515 | 2630 | 9020 | 0.10 | 0.69 | 0.20 |
| LAI | 0.25 | 2300 | 174 | 29,400 | 405 | 2990 | 12,400 | 0.08 | 0.77 | 0.19 |
| LEV | 1.5 | 845 | 27 | 2550 | 721 | 2350 | 1440 | 0.33 | 0.36 | 0.59 |
| LEV 1 | 0.5 | 3510 | 136 | 14,200 | 518 | 8040 | 5210 | 0.25 | 0.44 | 0.67 |
| LEV | 0.25 | 3520 | 77 | 20,400 | 417 | 7540 | 5070 | 0.17 | 0.47 | 0.69 |
| LK | 1.5 | 2740 | 63 | 9210 | 373 | 53,400 | 437 | 0.30 | 0.05 | 6.27 |
| LK | 0.5 | 3030 | 60 | 34,700 | 159 | 44,900 | 754 | 0.09 | 0.07 | 4.02 |
| LK | 0.25 | 3270 | 85 | 35,400 | 177 | 55,300 | 785 | 0.09 | 0.06 | 4.17 |
| Laterite Ore | pH | Ni (mg/L) | Co (mg/L) | Fe (mg/L) | Ca (mg/L) | Mg (mg/L) | Al (mg/L) | Ni/Fe | Ni/Mg | Ni/Al |
|---|---|---|---|---|---|---|---|---|---|---|
| LAI | 0.75 | 1100 | 82 | 10300 | 595 | 1630 | 6040 | 0.11 | 0.68 | 0.18 |
| LAI + LK | 1.8 | 2220 | 124 | 1850 | 456 | 24100 | 2000 | 1.2 | 0.09 | 1.11 |
| LEV | 0.75 | 2770 | 119 | 10100 | - | 6510 | 4560 | 0.27 | 0.43 | 0.61 |
| LEV + LK | 1.8 | 2430 | 94 | 2710 | - | 19300 | 2520 | 0.90 | 0.13 | 0.96 |
| Laterite | pH | Time, h | H2SO4 kg/t Dry Ore |
|---|---|---|---|
| LAI | 0.75 | 24 | 565.25 |
| LAI + LK | 1.8 | 48 | 369.69 |
| LEV | 0.75 | 6 | 481.71 |
| LEV + LK | 1.8 | 48 | 421.94 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miettinen, V.; Mäkinen, J.; Kolehmainen, E.; Kravtsov, T.; Rintala, L. Iron Control in Atmospheric Acid Laterite Leaching. Minerals 2019, 9, 404. https://doi.org/10.3390/min9070404
Miettinen V, Mäkinen J, Kolehmainen E, Kravtsov T, Rintala L. Iron Control in Atmospheric Acid Laterite Leaching. Minerals. 2019; 9(7):404. https://doi.org/10.3390/min9070404
Chicago/Turabian StyleMiettinen, Ville, Jarno Mäkinen, Eero Kolehmainen, Tero Kravtsov, and Lotta Rintala. 2019. "Iron Control in Atmospheric Acid Laterite Leaching" Minerals 9, no. 7: 404. https://doi.org/10.3390/min9070404





