Experimental Modeling of Silicate and Carbonate Sulfidation under Lithospheric Mantle P,T-Parameters
Abstract
:1. Introduction
2. Materials and Methods
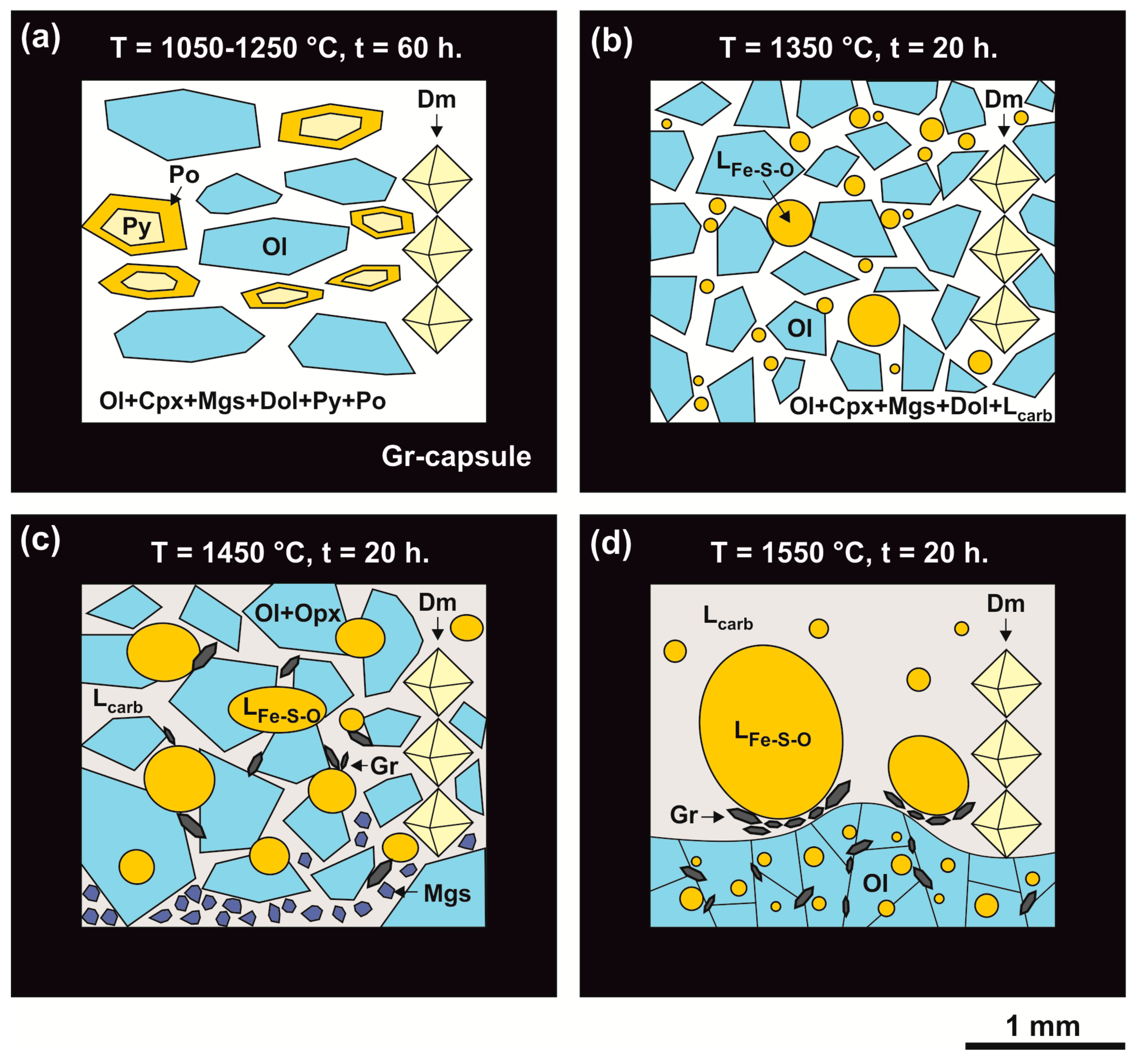
3. Results
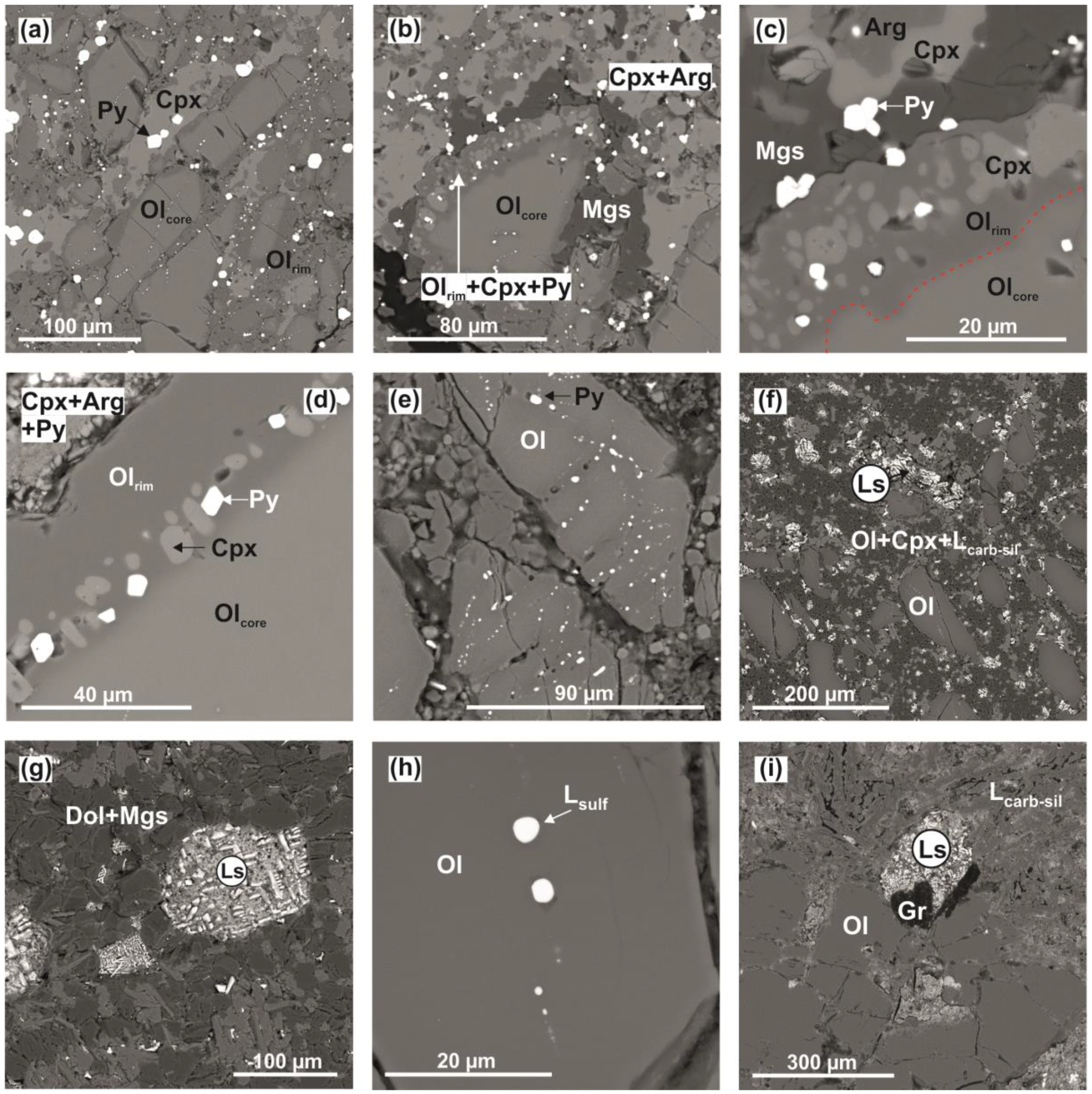
3.1. Olivine-Ankerite-Sulfur System
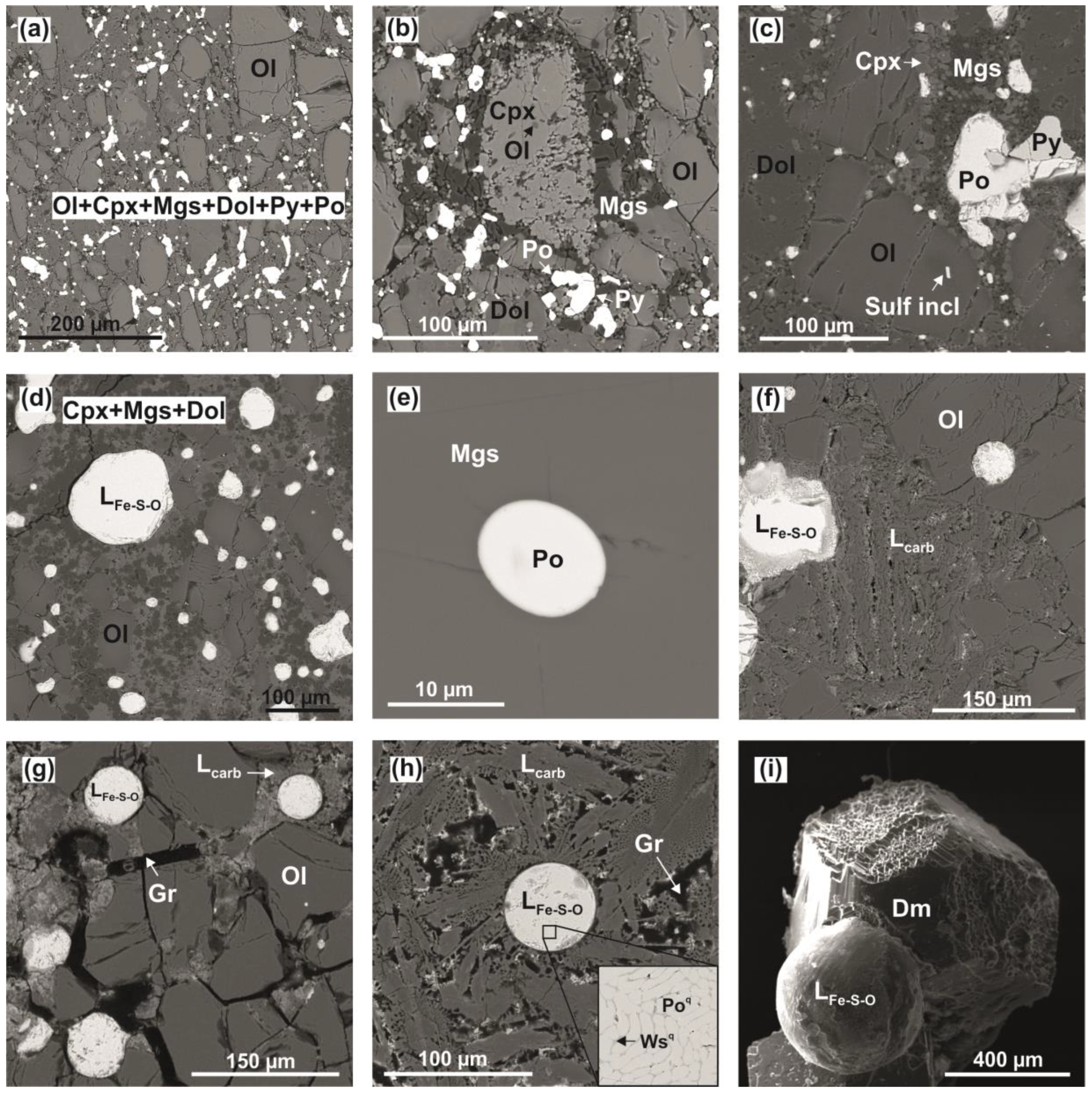
3.2. Olivine-Ankerite-Pyrite System
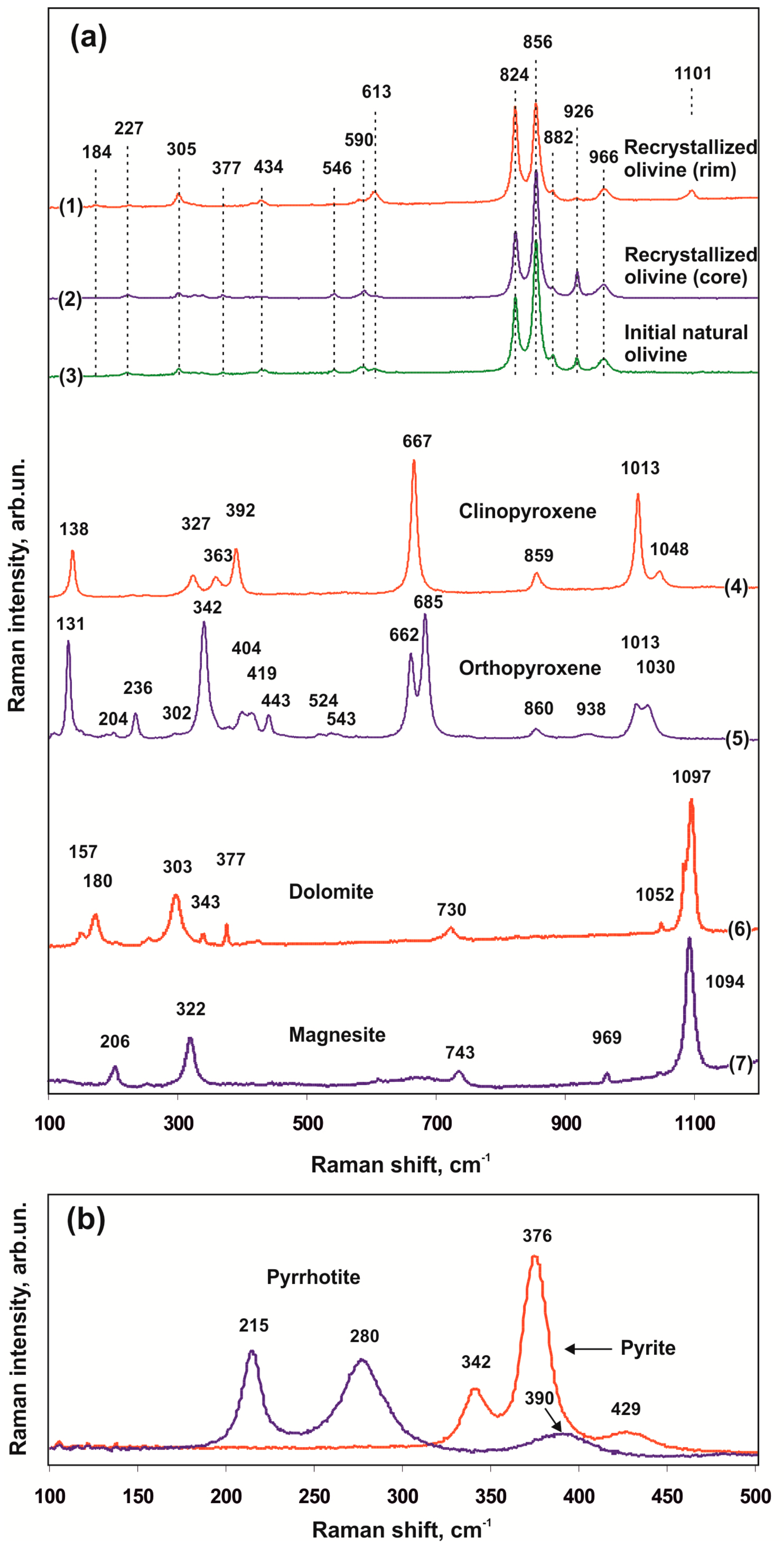
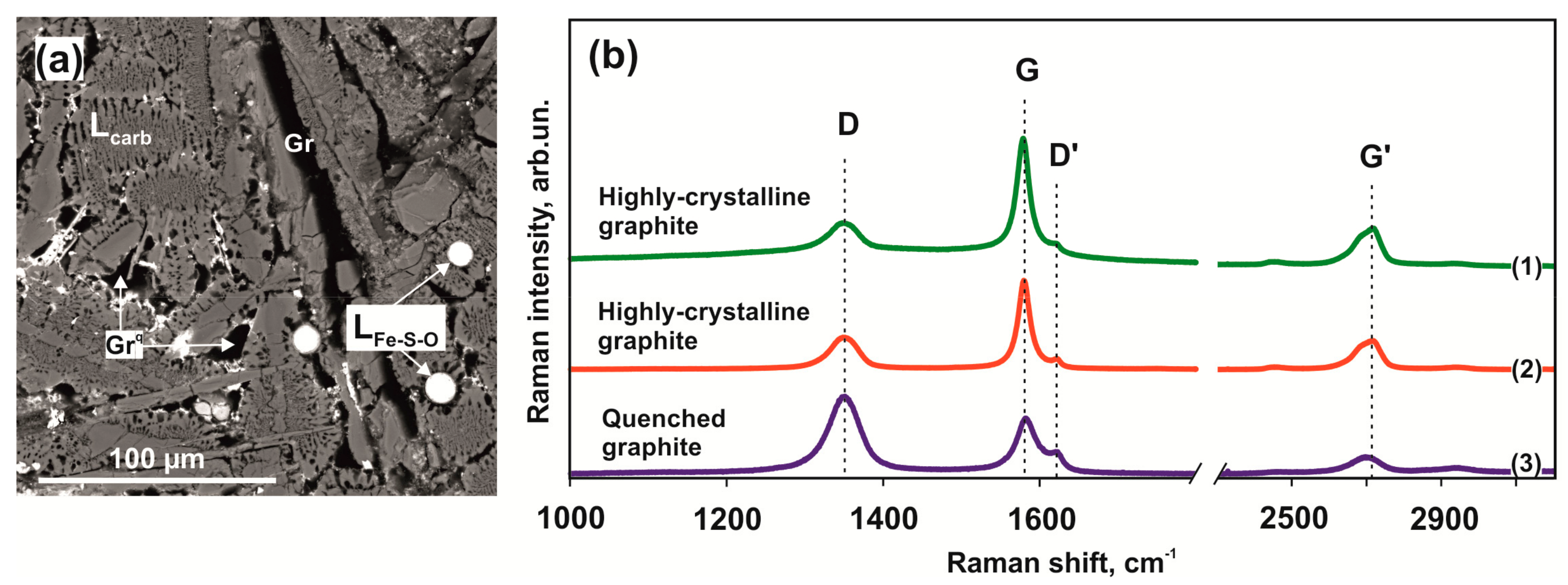
3.3. Raman Spectral Characteristics of Produced Mineral Phases
3.3.1. Olivine, Orthopyroxene, and Clinopyroxene
3.3.2. Carbonates
3.3.3. Graphite
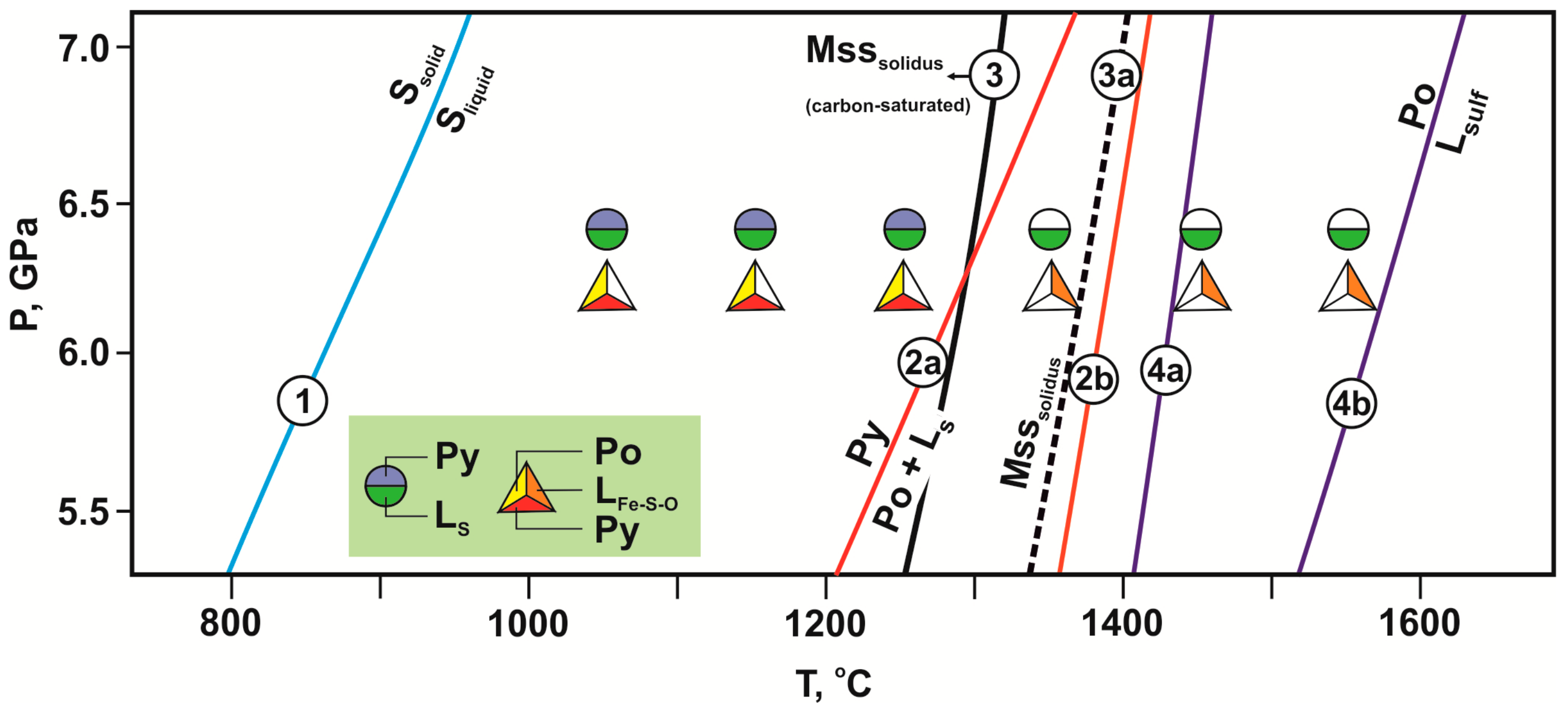
4. Discussion
4.1. Reconstruction of Interactions in the Olivine-Ankerite-Sulfur System
4.2. Reconstruction of Interactions in the Olivine-Ankerite-Pyrite System
4.3. Raman Spectral Characteristics and Indicative Properties of Mineral Phases Produced in Sulfidation Processes
4.3.1. Olivine
4.3.2. Carbonates
4.3.3. Graphite
4.4. Scenarios of Metasomatic Interactions of Reduced Sulfur-Rich Fluid with Olivine and Carbonates in the Earth’s Upper Mantle
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alt, J.; Shanks, W.C.; Jackson, M.C. Cycling of sulfur in subduction zones: The geochemistry of sulfur in the Mariana Island Arc and back-arc trough. Earth Planet. Sci. Lett. 1993, 119, 477–494. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.A. The redox budget of subduction zones. Earth Sci. Rev. 2012, 113, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Evans, K.A.; Powell, R. The effect of subduction on the sulphur, carbon and redox budget of lithospheric mantle. J. Metamorph. Geol. 2015, 33, 649–670. [Google Scholar] [CrossRef]
- Tomkins, A.G.; Evans, K.A. Separate zones of sulfate and sulfide release from subducted mafic oceanic crust. Earth Planet. Sci. Lett. 2015, 428, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Jégo, S.; Dasgupta, R. The fate of sulfur during fluid-present melting of subducting basaltic crust at variable oxygen fugacity. J. Petrol. 2014, 55, 1019–1050. [Google Scholar] [CrossRef]
- Labidi, J.; Cartigny, P.; Jackson, M.G. Multiple sulfur isotope composition of oxidized Samoan melts and the implications of a sulfur isotope ‘mantle array’ in chemical geodynamics. Earth Planet. Sci. Lett. 2015, 417, 28–39. [Google Scholar] [CrossRef]
- Wallace, P.J.; Edmonds, M. The sulfur budget in magmas: Evidence from melt inclusions, submarine glasses, and volcanic gas emissions. Rev. Mineral. Geochem. 2011, 73, 215–246. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.G.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the geology of mantle carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef]
- Oganov, A.R.; Hemley, R.J.; Hazen, R.M.; Jones, A.P. Structure, bonding, and mineralogy of carbon at extreme conditions. Rev. Mineral. Geochem. 2013, 75, 47–77. [Google Scholar] [CrossRef]
- Bulanova, G.P. The formation of diamond. J. Geochem. Explor. 1995, 53, 1–23. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; DeleDuboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.D.; Rossman, G.R.; Wasserburg, G.J. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Kaminsky, F.V.; Griffin, W.L.; Yefimova, E.S.; Win, T.T.; Ryan, C.G.; Botkunov, A.I. Mineral inclusions in diamonds from the Sputnik kimberlite pipe, Yakutia. Lithos 1997, 39, 135–157. [Google Scholar] [CrossRef]
- Lorand, J.P.; Gregoire, M. Petrogenesis of base metal sulphide assemblages of some peridotites from the Kaapvaal craton (South Africa). Contrib. Mineral. Petrol. 2006, 151, 521–538. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W. The origin of cratonic diamonds—Constraints from mineral inclusions. Ore Geol. Rev. 2008, 34, 5–32. [Google Scholar] [CrossRef]
- Leung, I.S.; Kuo, W.X. Silicon carbide cluster entrapped in a diamond from Fuxian, China. Am. Mineral. 1990, 75, 1110–1119. [Google Scholar]
- Giuliani, A.; Fitzpayne, A.; Phillips, D.; Hergt, J.; Woodhead, J.D.; James Farquhar, J.; Fiorentini, M.L.; Russell, N.; Drysdale, R.N.; Wu, N. Mantle oddities: A sulphate fluid preserved in a MARID xenolith from the Bultfontein kimberlite (Kimberley, South Africa). Earth Planet. Sci. Lett. 2013, 376, 74–86. [Google Scholar] [CrossRef]
- Andersen, T.; Neumann, E.R. Fluid inclusions in mantle xenoliths. Lithos 2001, 55, 301–320. [Google Scholar] [CrossRef]
- Newton, R.C.; Manning, C.E. Solubility of anhydrite, CaSO4, in NaCl-H2O solutions at high pressures and temperatures: Applications to fluid-rock interaction. J. Petrol. 2005, 46, 701–716. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle. Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Carroll, M.R.; Rutherford, M.J. Sulfide and sulfate saturation in hydrous silicate melts. J. Geophys. Res. 1985, 90, 601–612. [Google Scholar] [CrossRef]
- Lorand, J.P.; Alard, O.; Godard, M. Highly siderophile elements and the multi-stage metasomatic history of Kerguelen lithospheric mantle (south Indian Ocean). Chem. Geol. 2004, 208, 195–215. [Google Scholar] [CrossRef]
- Zajacz, Z.; Candela, P.A.; Piccoli, P.M.; Sanchez-Valle, C.; Waelle, M. Solubility and partitioning behavior of Au, Cu, Ag and reduced S in magmas. Geochim. Cosmochim. Acta 2013, 112, 288–304. [Google Scholar] [CrossRef]
- Alard, O.; Lorand, J.-P.; Reisberg, L.; Bodinier, J.-L.; Dautria, J.-M.; O’Reilly, S. Volatile-rich metasomatism in Montferrier xenoliths (Southern France): Implications for the abundances of chalcophile and highly siderophile elements in the subcontinental mantle. J. Petrol. 2011, 52, 2009–2045. [Google Scholar] [CrossRef]
- Delpech, G.; Lorand, J.P.; Grégoire, M.; Cottin, J.-Y.; O’Reilly, S.Y. In-situ geochemistry of sulfides in highly metasomatized mantle xenoliths from Kerguelen, southern Indian Ocean. Lithos 2012, 154, 296–314. [Google Scholar] [CrossRef]
- Eggler, D.H.; Lorand, J.P. Mantle sulfide geobarometry. Geochim. Cosmochim. Acta 1993, 57, 2213–2222. [Google Scholar] [CrossRef]
- Fleet, M.E.; MacRae, N.D. Sulfidation of Mg-rich olivine and the stability of niningerite in enstatite chondrites. Geochim. Cosmochim. Acta 1987, 51, 1511–1521. [Google Scholar] [CrossRef]
- Papike, J.J.; Spilde, M.N.; Fowler, G.W.; Layne, G.D.; Shearer, C.K. The Lodran primitive achondrite: Petrogenetic insights from electron and ion microprobe analysis of olivine and orthopyroxene. Geochim. Cosmochim. Acta 1995, 59, 3061–3070. [Google Scholar] [CrossRef]
- Lehner, W.; Petaev, M.I.; Buseck, P.R. Formation of niningerite by silicate sulfidation in EH3 enstatite chondrites. Geochim. Cosmochim. Acta 2013, 101, 34–56. [Google Scholar] [CrossRef]
- Ballhaus, C.G.; Stumpfl, E.F. Sulfide and platinum mineralization in the Merensky Reef: Evidence from hydrous silicates and fluid inclusions. Contrib. Mineral. Petrol. 1986, 94, 193–204. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Kullerud, G.; Yoder, H.S., Jr. Sulfide-Silicate Relations: Carnegie Institution of Washington Year Book; Carnegie Institution of Washington: Washington, DC, USA, 1963; Volume 62, pp. 215–218. [Google Scholar]
- Los, C.; Bach, W. Sulfidation of major rock types of the oceanic lithosphere; An experimental study at 250 °C and 400 bars. Lithos 2018, 323, 208–217. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Sobolev, N.V. Sulfidation of silicate mantle by reduced S-bearing metasomatic fluids and melts. Geology 2016, 44, 271–274. [Google Scholar] [CrossRef]
- Zhang, Z.; von der Handt, A.; Hirschmann, M. An experimental study of Fe–Ni exchange between sulfide melt and olivine at upper mantle conditions: Implications for mantle sulfide compositions and phase equilibria. Contrib. Mineral. Petrol. 2018, 173, 19. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M. Sulfide formation as a result of sulfate subduction into silicate mantle (experimental modeling under high P,T-parameters). Minerals 2018, 8, 373. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Zdrokov, E.V.; Sobolev, N.V. Experimental modeling of the interaction of subducted carbonates and sulfur with mantle silicates. Dokl. Earth Sci. 2016, 470, 953–956. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F. Fluid-bearing alkaline carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: An experimental study. Lithos 2002, 60, 145–159. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Brazhkin, V.V.; Popova, S.V.; Voloshin, R.N. Pressure-temperature phase diagram of molten elements: Selenium, sulfur and iodine. Phys. B Condens. Matter 1999, 265, 64–71. [Google Scholar] [CrossRef]
- Sharp, W.E. Melting curves of sphalerite, galena, and pyrrhotite and the decomposition curve of pyrite between 30 and 65 kilobars. J. Geophys. Res. 1969, 74, 1645–1652. [Google Scholar] [CrossRef]
- Boehler, R. Melting of the Fe-FeO and the Fe-FeS systems at high pressure—Constraints on core temperatures. Earth Planet. Sci. Lett. 1992, 111, 217–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Lentsch, N.; Hirschmann, M.M. Carbon-saturated monosulfide melting in the shallow mantle: Solubility and effect on solidus. Contrib. Mineral. Petrol. 2015, 170, 47. [Google Scholar] [CrossRef]
- Zhang, Z.; Hirschmann, M.M. Experimental constraints on mantle sulfide melting up to 8 GPa. Am. Mineral. 2016, 101, 181–192. [Google Scholar] [CrossRef]
- Iishi, K. Lattice dynamics of forsterite. Am. Mineral. 1978, 63, 1198–1208. [Google Scholar]
- Guyot, F.; Boyer, H.; Madon, M.; Velde, B.; Poirier, J.P. Comparison of the Raman microprobe spectra of (Mg,Fe)zSiO4 and MgzSiO4 with olivine and spinel structure. Phys. Chem. Miner. 1986, 13, 91–95. [Google Scholar] [CrossRef]
- Price, G.D.; Parker, S.C.; Leslie, M. Lattice dynamics of forsterite. Mineral. Mag. 1986, 51, 157–170. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Geiger, C.A. A Raman spectroscopic study of Fe-Mg olivines. Phys. Chem. Miner. 2004, 31, 142–154. [Google Scholar] [CrossRef]
- Huang, E.; Chen, C.H.; Huang, T.; Lin, E.H.; Xu, J.-A. Raman spectroscopic characteristics of Mg-Fe-Ca pyroxenes. Am. Miner. 2000, 85, 473–479. [Google Scholar] [CrossRef]
- Gillet, P.; Biellmann, C.; Reynard, B.; McMillan, P. Raman Spectroscopic Studies of Carbonates Part I: High-Pressure and High-Temperature Behaviour of Calcite, Magnesite, Dolomite and Aragonite. Phys. Chem. Miner. 1993, 20, 1–18. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman study on certain carbonates. Analele Ştiinţifice Ale Universităţii “AL. I. CUZA” IAŞI 2009, 55, 97–112. [Google Scholar]
- Gunn, S.C.; Luth, R.W. Carbonate reduction by Fe-S-O melts at high pressure and high temperature. Am. Mineral. 2006, 91, 1110–1116. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Sokol, A.G. Synthesis of diamonds with mineral, fluid and melt inclusions. Lithos 2016, 265, 292–303. [Google Scholar] [CrossRef]
- Fleet, M.E.; MacRae, N.D. Partition of Ni between olivine and sulfide and its application to Ni–Cu sulfide deposits. Contrib. Mineral. Petrol. 1983, 83, 75–81. [Google Scholar] [CrossRef]
- Heymann, D. Raman study of olivines in 37 heavily and moderately shocked ordinary chondrites. Geochim. Cosmochim. Acta 1990, 54, 2507–2510. [Google Scholar] [CrossRef]
- Michel, R.; Ammar, M.R.; Poiriern, J.; Simon, P. Phase transformation characterization of olivine subjected to high temperature in air. Ceram. Int. 2013, 39, 5287–5294. [Google Scholar] [CrossRef]
- Weber, I.; Böttger, U.; Pavlov, S.G.; Jessberger, E.K.; Hübers, H.-W. Mineralogical and Raman spectroscopy studies of natural olivines exposed to different planetary environments. Planet. Space Sci. 2014, 104, 163–172. [Google Scholar] [CrossRef] [Green Version]
- Wickham-Eade, J.E.; Burchell, M.J.; Price, M.C.; Hicks, L.J.; MacArthur, J.L.; Bridges, J.C. Raman identification of olivine grains in fine grained mineral assemblages fired into aerogel. Proc. Inst. Mech. Eng. 2017, 204, 413–420. [Google Scholar] [CrossRef]
- Crepisson, C.; Blanchard, M.; Lazzeri, M.; Balan, E.; Sanloup, C. New constraints on Xe incorporation mechanisms in olivine from first-principles calculations. Geochim. Cosmochim. Acta 2018, 222, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Park, S.Y.; Park, C. Spatially-resolved mineral identification and depth profiling on chondrules from the primitive chondrite Elephant Moraine 14017 with confocal Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 207, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Solovova, I.P.; Kogarko, L.N.; Averin, A.A. Conditions of sulfide formation in the metasomatized mantle beneath East Antarctica. Petrology 2015, 23, 519–542. [Google Scholar] [CrossRef]
- Mungall, J.E.; Su, S. Interfacial tension between magmatic sulfide and silicate liquids: Constraints on kinetics of sulfide liquation and sulfide migration through silicate rocks. Earth Planet. Sci. Lett. 2005, 234, 135–140. [Google Scholar] [CrossRef]
- Griffin, W.L.; Graham, S.; O’Reilly, S.Y.; Pearson, N.J. Lithosphere evolution beneath the Kaapvaal Craton: Re-Os systematics of sulfides in mantle-derived peridotites. Chem. Geol. 2004, 208, 89–118. [Google Scholar] [CrossRef]



| Mineral | Si | Ca | Fe | Mg | Mn | Ni | S | C | O | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Olivine | 19.17 | - | 7.22 | 29.43 | - | 0.35 | - | - | 43.96 | 99.86 |
| Ankerite | - | 19.27 | 13.7 | 5.87 | 0.55 | - | - | 11.98 | 47.93 | 100 |
| Pyrite | - | - | 46.82 | - | - | 0.03 | 53.04 | - | - | 99.89 |
| Run N | T,°C | t, h | Final Phases | |||
| Silicates | Carbonates | S-Bearing Phases | Phases of Elemental Carbon | |||
| Olivine-Ankerite-Sulfur System | ||||||
| 932-3 | 1050 | 60 | Ol, Cpx | Dol, Mgs, Arg | Py, Ls | - |
| 1476-3 | 1150 | 60 | Ol, Cpx | Dol, Mgs, Arg | Py, Ls | - |
| 1997-3 | 1250 | 60 | Ol, Cpx | Dol, Mgs | Py, Ls | - |
| 2000-3 | 1350 | 20 | Ol, Cpx | Dol, Mgs | Ls | Gr |
| 2001-3 | 1450 | 20 | Ol, Opx | Mgs, Dol, Lcarb-sil | Ls | Gr |
| 1181-3 | 1550 | 20 | Ol | Lcarb-sil | Ls | Gr, Dm |
| Olivine-Ankerite-Pyrite System | ||||||
| 932-6 | 1050 | 60 | Ol, Cpx | Dol, Mgs | Py, Po | - |
| 1476-6 | 1150 | 60 | Ol, Cpx | Dol, Mgs | Py, Po | - |
| 1997-6 | 1250 | 60 | Ol, Cpx | Dol, Mgs | Py, Po | - |
| 2000-6 | 1350 | 20 | Ol, Cpx | Dol, Mgs, Lcarb | LFe-S-O | - |
| 2001-6 | 1450 | 20 | Ol, Opx | Mgs, Lcarb | LFe-S-O | Gr |
| 1181-6 | 1550 | 20 | Ol | Lcarb | LFe-S-O | Gr, Dm |
| Run N | T,°C | Phase | Mass Concentrations. wt. % | n(O) | Cations Per Formula Unit | ||||||||||
| SiO2 | FeO | MgO | CaO | NiO | Total | Si | Fe | Mg | Ca | Ni | Sum | ||||
| Olivine-Ankerite-Sulfur System | |||||||||||||||
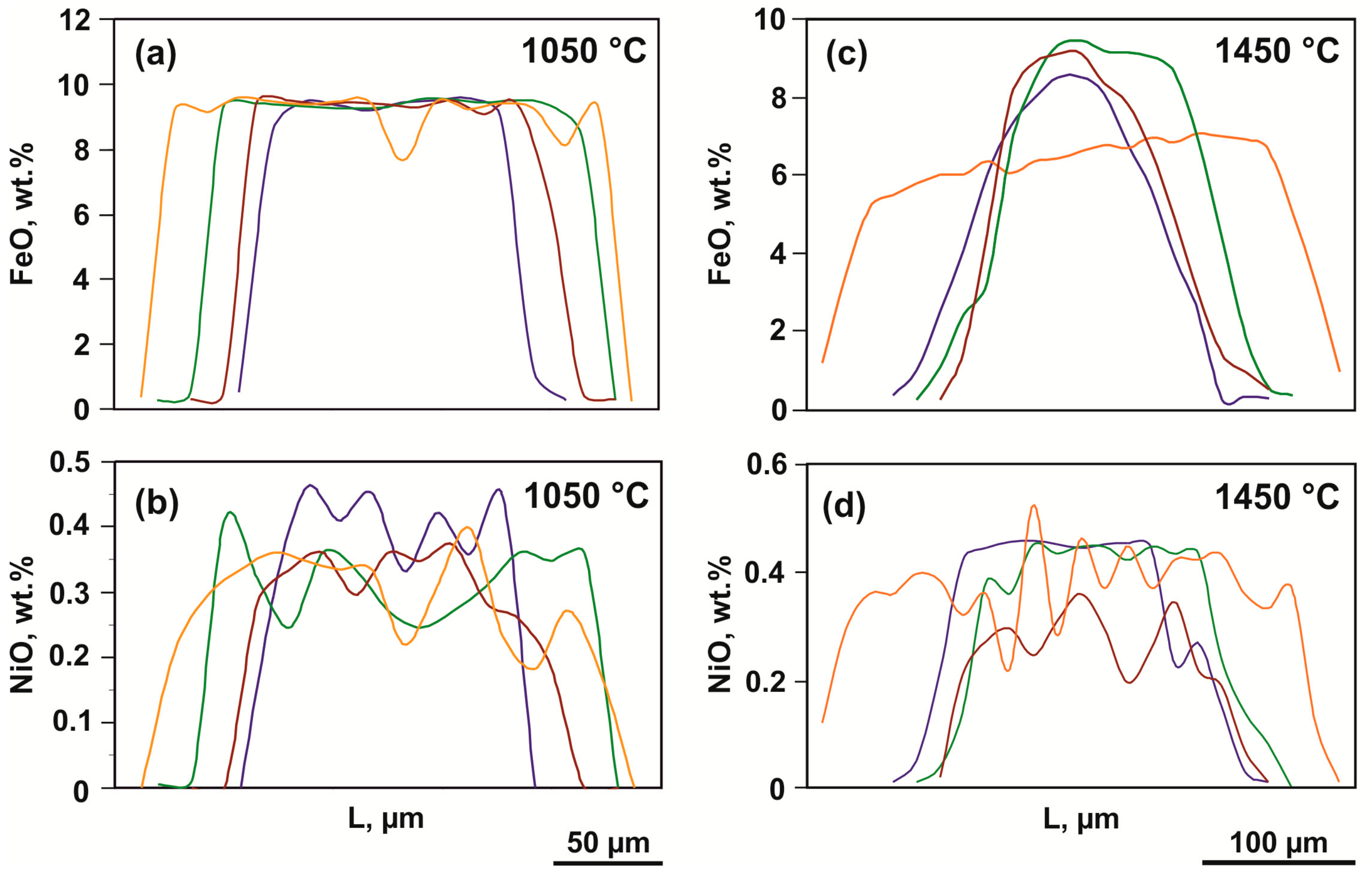
| 932-3 | 1050 | Olcore | 40.5(3) | 9.1(3) | 49.2(2) | 0.07(7) | 0.45(2) | 99.47 | 4 | 1.00(0) | 0.19(1) | 1.81(1) | - | 0.01(0) | 3.01 |
| Olrim | 42.3(2) | 0.1(1) | 56.8(3) | 0.3(2) | - | 99.62 | 4 | 0.99(0) | - | 2.00(1) | 0.01(1) | - | 3.01 | ||
| Cpx | 54.6(9) | 0.5(5) | 22(3) | 22(3) | - | 99.57 | 6 | 1.96(4) | 0.01(1) | 1.2(2) | 0.9(1) | - | 4.04 | ||
| 1476-3 | 1150 | Olcore | 40.6(3) | 9.0(7) | 49.3(6) | 0.02(2) | 0.45(2) | 99.46 | 4 | 1.00(0) | 0.19(2) | 1.81(1) | - | 0.01(0) | 3.01 |
| Olrim | 42.3(3) | 0.6(6) | 56.6(7) | 0.06(6) | - | 99.62 | 4 | 1.00(1) | 0.01(1) | 2.00(2) | - | - | 3.00 | ||
| Cpx | 54.3(6) | 0.5(1) | 22(3) | 22(3) | - | 99.76 | 6 | 1.95(3) | 0.01(0) | 1.2(1) | 0.9(1) | - | 4.03 | ||
| 1997-3 | 1250 | Olcore | 40.8(4) | 8.7(9) | 49.5(9) | 0.06(6) | 0.45(3) | 99.52 | 4 | 1.00(0) | 0.18(2) | 1.82(2) | - | 0.01(0) | 3.01 |
| Olrim | 41.3(5) | 2.9(1) | 54.4(3) | 0.1(1) | 0.3(0) | 99.16 | 4 | 0.99(1) | 0.06(0) | 1.95(2) | - | 0.01(0) | 3.01 | ||
| Cpx | 54.9(6) | 0.4(2) | 20.5(9) | 23(1) | - | 99.55 | 6 | 1.98(1) | 0.01(1) | 1.11(5) | 0.92(5) | - | 4.02 | ||
| 2000-3 | 1350 | Olcore | 40.8(4) | 8.5(9) | 49.7(8) | 0.02(2) | 0.45(2) | 99.49 | 4 | 1.00(1) | 0.17(2) | 1.83(2) | - | 0.01(0) | 3.01 |
| Olrim | 41.9(1) | 1.7(5) | 55.6(4) | 0.2(1) | 0.1(1) | 99.66 | 4 | 0.99(0) | 0.03(1) | 1.97(1) | 0.01(0) | - | 3.01 | ||
| Cpx | 55(1) | 0.2(2) | 21.8(7) | 22.6(8) | - | 99.75 | 6 | 1.98(3) | 0.01(0) | 1.18(4) | 0.87(4) | - | 4.02 | ||
| 2001-3 | 1450 | Olcore | 40.8(4) | 6(1) | 52(1) | 0.02(2) | 0.4(1) | 99.67 | 4 | 0.99(0) | 0.13(3) | 1.88(4) | - | 0.01(0) | 3.01 |
| Olrim | 41.9(5) | 1.5(9) | 56.1(9) | 0.1(1) | 0.1(1) | 99.64 | 4 | 0.99(1) | 0.03(2) | 1.99(2) | - | - | 3.01 | ||
| Opx | 58.8(5) | 0.3(1) | 38.1(6) | 2.4(4) | - | 99.62 | 6 | 1.99(1) | 0.01(0) | 1.93(3) | 0.09(1) | - | 4.01 | ||
| 1811-3 | 1550 | Ol | 42.5(3) | 0.3(2) | 56.5(2) | 0.1(1) | - | 99.37 | 4 | 1.00(0) | 0.01(0) | 1.99(1) | - | - | 3.00 |
| Olivine-Ankerite-Pyrite System | |||||||||||||||
| 932-6 | 1050 | Olcore | 40.6(2) | 9.2(2) | 49.1(1) | - | 0.44(8) | 99.5 | 4 | 1.00(0) | 0.19(0) | 1.81(1) | - | 0.01(0) | 3.00 |
| Olrim | 43(1) | 1.4(5) | 54(2) | - | - | 99.8 | 4 | 1.01(2) | 0.03(1) | 1.91(9) | - | - | 2.99 | ||
| Cpx | 55.4(6) | 0.6(4) | 19.2(5) | 24.4(3) | - | 99.8 | 6 | 2.00(2) | 0.02(1) | 1.04(3) | 0.94(1) | - | 4.00 | ||
| 1476-6 | 1150 | Olcore | 40.5(4) | 9.3(6) | 49.3(4) | - | 0.49(6) | 99.6 | 4 | 0.99(1) | 0.19(1) | 1.81(1) | - | 0.01(0) | 3.01 |
| Olrim | 41.4(6) | 3(1) | 53(1) | - | - | 99.3 | 4 | 0.99(1) | 0.07(2) | 1.91(5) | - | - | 3.01 | ||
| Cpx | 55.0(2) | 0.8(3) | 18.8(3) | 24.7(3) | - | 100,0 | 6 | 2.00(1) | 0.02(1) | 1.02(2) | 0.96(1) | - | 4.00 | ||
| 1997-6 | 1250 | Olcore | 41.0(3) | 9(1) | 50(1) | - | 0.42(8) | 99.5 | 4 | 1.00(0) | 0.18(3) | 1.81(3) | - | 0.01(0) | 3.00 |
| Olrim | 42.2(5) | 3(1) | 54.3(9) | - | - | 99.7 | 4 | 1.00(1) | 0.06(2) | 1.94(2) | - | - | 3.00 | ||
| Cpx | 55(1) | 1.0(6) | 21(1) | 23(1) | - | 99.5 | 6 | 1.98(4) | 0,03(2) | 1.12(7) | 0.89(6) | - | 4,02 | ||
| 2000-6 | 1350 | Olcore | 41.0(3) | 8(1) | 50(1) | - | 0.47(9) | 99.7 | 4 | 1.00(0) | 0.16(2) | 1.83(2) | - | 0.01(0) | 2.99 |
| Olrim | 42.0(5) | 3(1) | 54(1) | - | - | 99,7 | 4 | 1.00(1) | 0.06(3) | 1.93(3) | - | - | 3.00 | ||
| Cpx | 55.4(6) | 0.9(5) | 21.3(7) | 22.2(9) | - | 99.8 | 6 | 1.99(1) | 0.03(1) | 1.14(4) | 0.85(4) | - | 4.01 | ||
| 2001-6 | 1450 | Olcore | 40.9(5) | 8(2) | 51(1) | - | 0,5(1) | 99.9 | 4 | 1.00(1) | 0.16(3) | 1.85(3) | - | 0.01(0) | 3.00 |
| Olrim | 41.8(4) | 3.8(6) | 53.9(6) | - | - | 99.8 | 4 | 1.00(1) | 0.08(1) | 1.92(2) | - | - | 3.00 | ||
| Opx | 58.3(7) | 2.1(2) | 36.7(9) | 2.9(9) | - | 100.1 | 6 | 1.99(1) | 0.06(1) | 1.86(4) | 0.10(3) | - | 4.01 | ||
| 1811-6 | 1550 | Olcore | 41.3(3) | 6(1) | 52(1) | - | - | 99.6 | 4 | 1.00(1) | 0.12(2) | 1.88(3) | - | - | 3.00 |
| Olrim | 42.4(2) | 0.41(8) | 56.5(5) | - | - | 99,6 | 4 | 1.00(0) | 0.01(0) | 1.99(2) | - | - | 3.00 | ||
| Run N | T, °C | Phase | Mass Concentrations. wt. % | n(O) | Cations Per Formula Unit | ||||||||||
| SiO2 | FeO | MgO | CaO | SO3 | CO2 * | Total | Fe | Mg | Ca | C | Sum | ||||
| Olivine-Ankerite-Sulfur System | |||||||||||||||
| 932-3 | 1050 | Arg | - | 0.1(1) | 0.1(1) | 54.2(9) | - | 45.7(9) | 100 | 3 | - | - | 0.91(1) | 1.04(1) | 1.96 |
| Mgs | - | 0.1(1) | 43(1) | 3.6(1) | - | 52(1) | 100 | 3 | - | 0.92(3) | 0.05(0) | 1.01(1) | 1.99 | ||
| Dol | - | - | 19(2) | 32(3) | - | 47(1) | 100 | 6 | - | 0.91(7) | 1.1(1) | 2.00(3) | 4.00 | ||
| 1476-3 | 1150 | Arg | - | 0.3(2) | - | 55.2(3) | - | 44.5(2) | 100 | 3 | - | - | 0.94(1) | 1.03(0) | 1.97 |
| Dol | - | - | 19(2) | 31.9(5) | - | 48(1) | 100 | 6 | - | 0.93(5) | 0.99(1) | 2.05(3) | 3.97 | ||
| Mgs | - | 0.2(1) | 43.5(5) | 3.5(9) | - | 52(1) | 100 | 3 | - | 0.92(2) | 0.05(1) | 1.01(1) | 1.99 | ||
| 1997-3 | 1250 | Mgs | - | 0.23(1) | 41.7(7) | 6.8(6) | - | 51(1) | 100 | 3 | - | 0.89(2) | 0.10(1) | 1.00(2) | 2.00 |
| Dol | - | - | 23.1(4) | 28(1) | - | 48.9(8) | 100 | 6 | - | 1.06(1) | 0.87(3) | 2.04(2) | 3.97 | ||
| 2000-3 | 1350 | Mgs | - | 0.3(2) | 38.9(9) | 8.5(9) | - | 52.3(6) | 100 | 3 | - | 0.83(3) | 0.12(1) | 1.02(1) | 1.98 |
| Dol | - | 0.21(5) | 25.8(9) | 25.1(1) | - | 48.9(9) | 100 | 6 | - | 1.18(5) | 0.77(0) | 2.03(3) | 3.97 | ||
| 2001-3 | 1450 | Mgs | - | 0.09(9) | 40.3(3) | 4.9(2) | - | 54.6(3) | 100 | 3 | - | 0.85(1) | 0.07(0) | 1.04(0) | 1.97 |
| Dol | - | - | 20(2) | 30(1) | - | 48.9(3) | 100 | 3 | - | 0.95(6) | 0.99(4) | 2.03(1) | 3.97 | ||
| Lcarb-sil | 15(3) | 0.4(3) | 24(2) | 21(4) | 1.0(7) | 38(4) | 100 | - | - | - | - | - | - | ||
| 1811-3 | 1550 | Lcarb-sil | 11(1) | 0.5(3) | 21(2) | 17.3(1) | 1.7(5) | 48(2) | 100 | - | - | - | - | - | - |
| Olivine-Ankerite-Pyrite System | |||||||||||||||
| 932-6 | 1050 | Dolcore | - | 8(2) | 17(2) | 27.9(3) | - | 47.3(5) | 100 | 6 | 0.22(7) | 0.78(7) | 0.94(0) | 2.01(1) | 3.98 |
| Dolmantle | - | 3(1) | 20.4(4) | 27.6(3) | - | 49.0(9) | 100 | 6 | 0.08(3) | 0.94(1) | 0.9(2) | 2.02(1) | 3.98 | ||
| Dolrim | - | 0.6(1) | 20.9(4) | 30.1(4) | - | 48.5(4) | 100 | 6 | 0.01(0) | 0.96(2) | 0.98(1) | 2.01(0) | 3.99 | ||
| Mgs | - | 0.5(2) | 42.3(5) | 4(1) | - | 53(2) | 100 | 3 | 0.01(0) | 0.90(2) | 0.06(2) | 1.00(0) | 1.98 | ||
| 1476-6 | 1150 | Dol | - | 0.4(1) | 22.0(5) | 28.2(2) | - | 49.5(7) | 100 | 6 | 0.01(1) | 1.00(3) | 0.91(1) | 2.01(1) | 3.97 |
| Mgs | - | 0.5(2) | 41.2(1) | 4.8(4) | - | 53.5(5) | 100 | 3 | 0.01(0) | 0.87(0) | 0.07(0) | 1.01(0) | 1.97 | ||
| 1997-6 | 1250 | Dol | - | 0.4(2) | 23.0(5) | 27.1(7) | - | 49.5(5) | 100 | 6 | 0.01(0) | 1.04(2) | 0.88(2) | 2.02(1) | 3.98 |
| Mgs | - | 1.4(0) | 37.0(1) | 7.49(9) | - | 53.4(9) | 100 | 3 | 0.01(0) | 0.79(0) | 0.11(0) | 1.01(1) | 1.97 | ||
| 2000-6 | 1350 | Dol | - | 0.4(1) | 26.0(3) | 23.5(6) | - | 50.2(2) | 100 | 6 | 0.01(0) | 1.16(1) | 0.75(2) | 2.01(1) | 3.97 |
| Mgs | - | - | 38(2) | 9(1) | - | 52(1) | 100 | 3 | - | 0.82(4) | 0.13(2) | 1.01(1) | 1.99 | ||
| Lcarb | 2.7(1) | 1.1(2) | 16.6(7) | 34.2(4) | - | 45.4(7) | 100 | - | - | - | - | - | - | ||
| 2001-6 | 1450 | Lcarb | 0.4(2) | 3(1) | 17(7) | 25(3) | - | 48(2) | 100 | - | - | - | - | - | - |
| Mgs | - | 1.8(2) | 42(1) | 3.7(4) | - | 52.4(9) | 100 | 3 | 0.02(0) | 0.89(3) | 0.06(1) | 1.00(1) | 1.98 | ||
| 1811-6 | 1550 | Lcarb | 2(1) | 5.0(5) | 15(2) | 16(1) | - | 55(3) | 100 | - | - | - | - | - | - |
| Run N | T, °C | Phase | Mass Concentrations, wt. % | Cations Per Formula Unit | ||||||||||
| Fe | Mg | Mn | Ca | Ni | S | O | C | Total | Fe | Ni | S | |||
| Olivine-Ankerite-Sulfur System | ||||||||||||||
| 932-3 | 1050 | Py | 45.0(5) | - | - | - | 1.1(5) | 53.6(5) | - | - | 99.8 | 0.96(2) | 0.02(1) | 2 |
| Ls | 1.5(6) | - | - | - | 0.4(2) | 90.5(7) | 7.5(9) | - | 100 | - | - | - | ||
| 1476-3 | 1150 | Py | 45.0(6) | - | - | - | 1.1(6) | 54.6(7) | - | - | 99.8 | 0.94(1) | 0.02(1) | 2 |
| Ls | 1.9(8) | - | - | - | 0.3(9) | 89.5(4) | 8.3(7) | - | 100 | - | - | - | ||
| 1997-3 | 1250 | Py | 45(1) | - | - | - | 1.2(9) | 54(1) | - | - | 99.7 | 0.96(1) | 0.02(2) | 2 |
| Ls | 2.2(5) | - | - | - | 0.4(1) | 88.8(5) | 8(1) | - | 100 | - | - | - | ||
| 2000-3 | 1350 | Ls | 20.7(4) | 0.2(2) | 0.4(1) | 0.4(1) | 0.8(2) | 67.1(5) | 8(1) | 2.4(5) | 100 | - | - | - |
| 2001-3 | 1450 | Ls | 22.6(8) | 0.4(1) | 0.3(1) | 0.5(2) | 0.7(2) | 65(1) | 7.6(8) | 3.0(9) | 100 | - | - | - |
| 1811-3 | 1550 | Ls | 22.5(1) | 0.7(1) | - | 0.4(1) | 0.5(1) | 53(2) | 16(1) | 6.3(5) | 100 | - | - | - |
| Olivine-Ankerite-Pyrite System | ||||||||||||||
| 932-6 | 1050 | Po | 59.4(9) | - | - | - | 0.51(7) | 39.7(8) | - | - | 99.6 | 0.85(3) | 0.01(0) | 1 |
| Py | 46.4(3) | - | - | - | - | 53.2(3) | - | - | 99.6 | 1.00(1) | - | 2 | ||
| 1476-6 | 1150 | Po | 59.0(5) | - | - | - | 0.4(1) | 40.0(4) | - | - | 99.5 | 0.84(1) | 0.01(0) | 1 |
| Py | 46.1(1) | - | - | - | - | 53.6(6) | - | - | 99.7 | 0.98(0) | - | 2 | ||
| 1997-6 | 1250 | Po | 59.0(5) | - | - | - | 0.5(2) | 40.1(4) | - | - | 99.6 | 0.84(1) | 0.01(0) | 1 |
| Py | 45.7(4) | - | - | - | - | 53.8(2) | - | - | 99.5 | 0.97(1) | - | 2 | ||
| 2000-6 | 1350 | LFe-S-O | 58.0(3) | - | - | - | 0.7(2) | 39.9(1) | 1.1(1) | - | 99.7 | - | - | - |
| 2001-6 | 1450 | LFe-S-O | 58.9(2) | - | - | - | 0.9(1) | 38.9(2) | 0.9(1) | - | 99.5 | - | - | - |
| 1811-6 | 1550 | LFe-S-O | 61.3(3) | - | 0.2(2) | - | 0.9(1) | 33.6(3) | 3.4(3) | - | 99.5 | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zdrokov, E.; Novoselov, I.; Bataleva, Y.; Borzdov, Y.; Palyanov, Y. Experimental Modeling of Silicate and Carbonate Sulfidation under Lithospheric Mantle P,T-Parameters. Minerals 2019, 9, 425. https://doi.org/10.3390/min9070425
Zdrokov E, Novoselov I, Bataleva Y, Borzdov Y, Palyanov Y. Experimental Modeling of Silicate and Carbonate Sulfidation under Lithospheric Mantle P,T-Parameters. Minerals. 2019; 9(7):425. https://doi.org/10.3390/min9070425
Chicago/Turabian StyleZdrokov, Evgeniy, Ivan Novoselov, Yuliya Bataleva, Yuri Borzdov, and Yuri Palyanov. 2019. "Experimental Modeling of Silicate and Carbonate Sulfidation under Lithospheric Mantle P,T-Parameters" Minerals 9, no. 7: 425. https://doi.org/10.3390/min9070425





