Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Location
2.2. Laboratory Analyses
2.2.1. Petrography and Mineralogical Studies
2.2.2. Bulk Geochemical Analyses
2.2.3. In Situ Trace Element Analyses by Femtosecond-LA-ICP-MS
2.2.4. Hydrometallurgical Method
3. Results
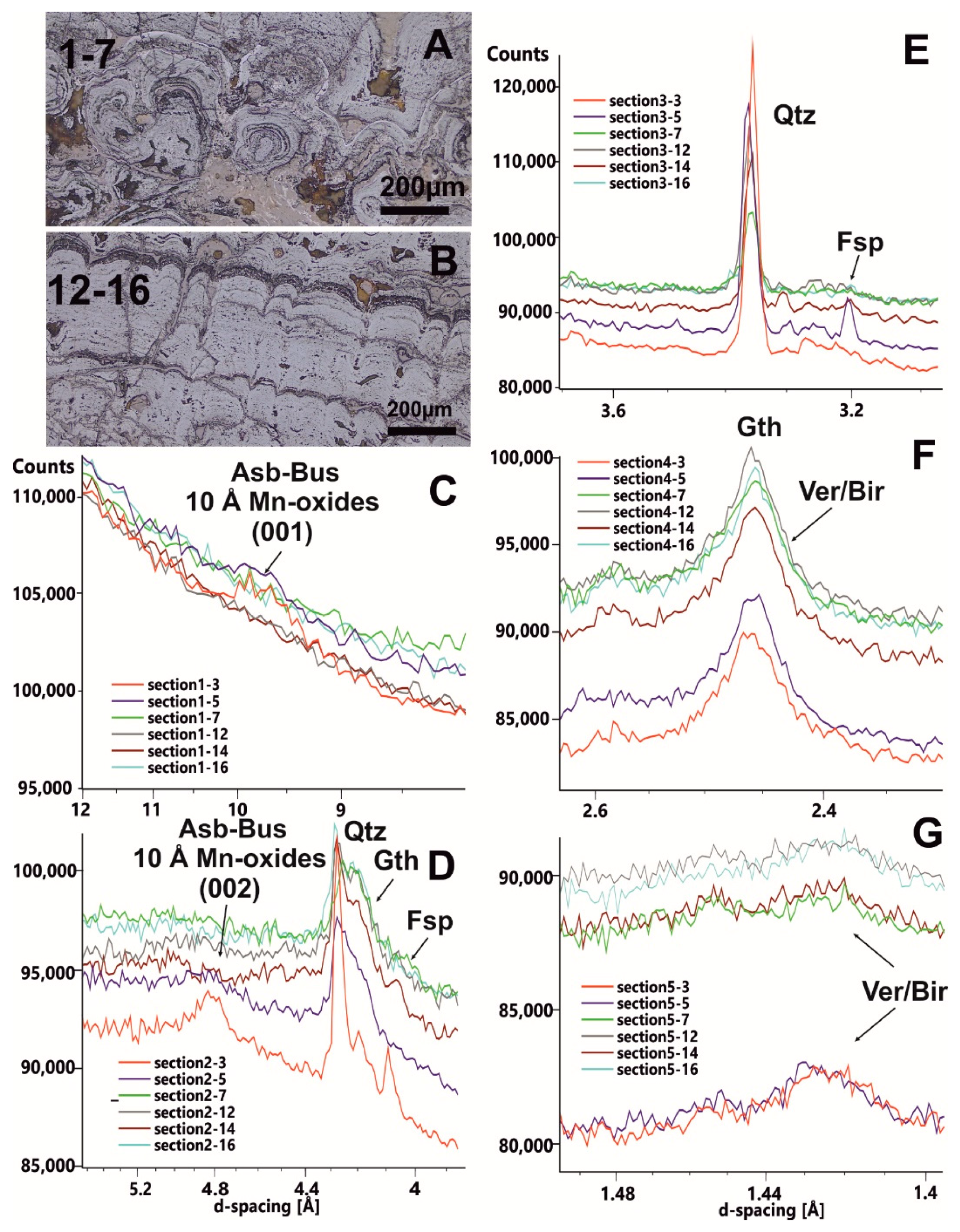
3.1. Fe-Mn Crusts Structure
3.2. Mineralogy
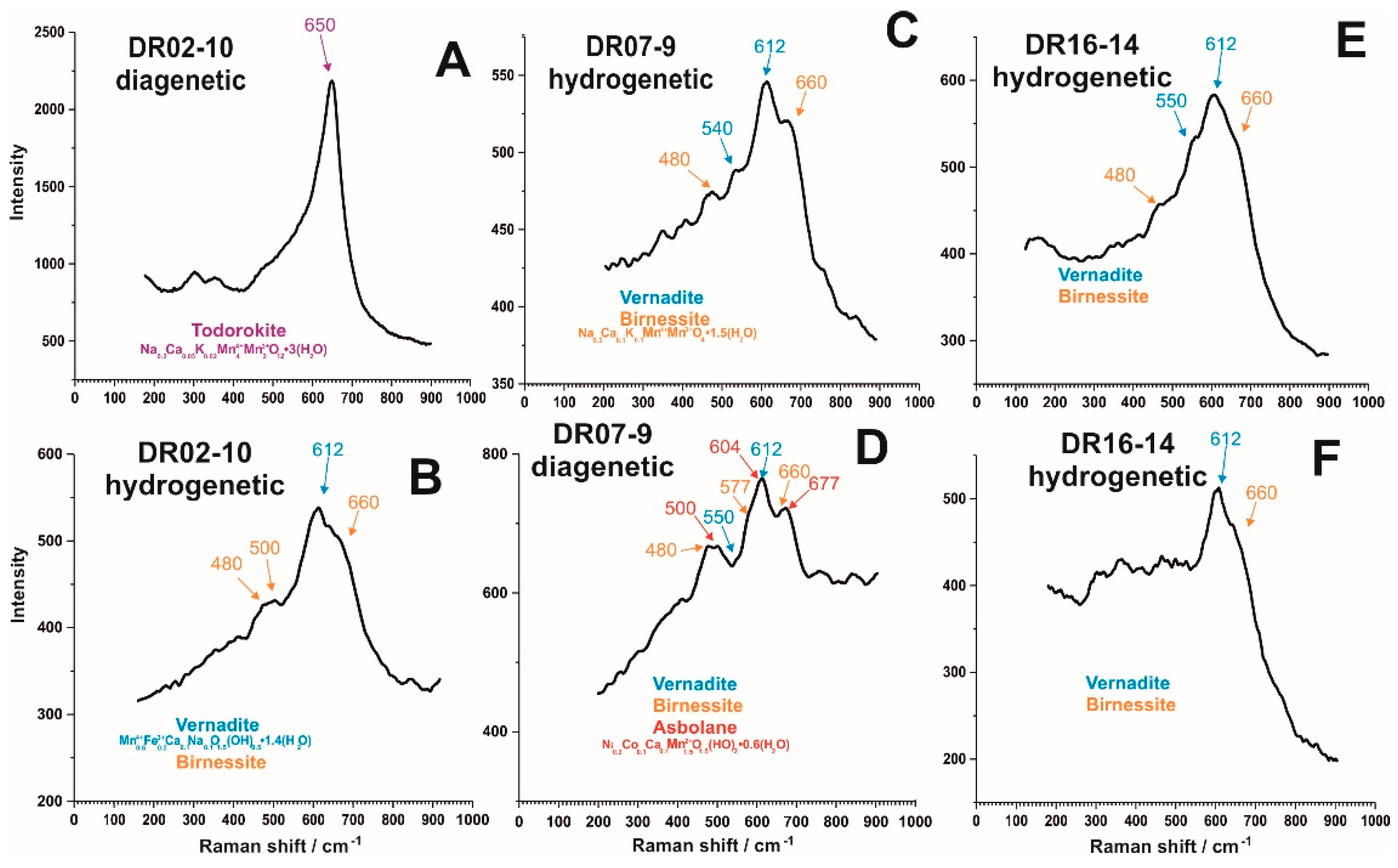
3.2.1. Petrography, XRD and Micro-XRD
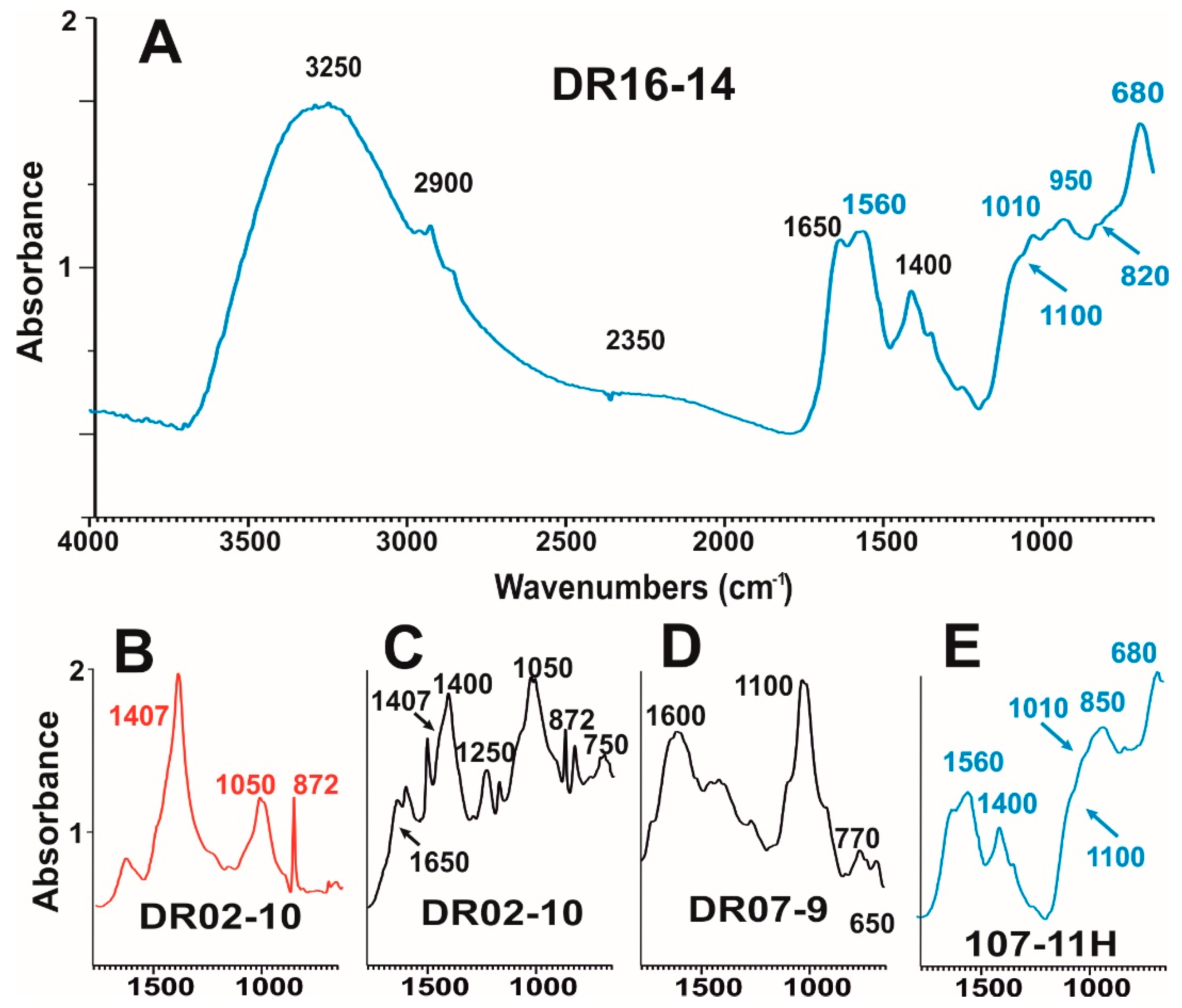
3.2.2. Raman and FT-IR Spot Analysis
3.3. Geochemistry
3.3.1. Bulk Geochemistry
3.3.2. Electron Probe Micro Analysis
3.3.3. LA-ICP-MS Punctual Analysis
3.3.4. Hydrometallurgical Treatment
4. Discussion
4.1. Bulk Analysis vs. High-Resolution Analysis
4.2. Environmental Influence in Fe-Mn Crusts
4.3. Hydrothermal Influence in Fe-Mn Crusts
4.4. CISP Crusts Composition and Metallurgic Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.-K.; Roberts, L. Cobalt-rich ferromanganese crusts in the Pacific. In Handbook of Marine Mineral Deposits; Cronan, D.S., Ed.; CRC Press: London, UK, 2000; pp. 239–279. [Google Scholar]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Halbach, P.; Hebisch, U.; Scherhag, C. Geochemical variations of ferromanganese nodules and crusts from different provinces of the Pacific Ocean and their genetic control. Chem. Geol. 1981, 34, 3–17. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Bogdanova, O.Y.; Dubinin, A.V.; Gorand, A.; Gorshkov, A.I.; Gurvich, E.G.; Isaeva, A.B.; Ivanov, G.V.; Jansa, L.F.; Monaco, A. Composition of Ferromanganese Crusts and Nodules at Northwestern Pacific Guyots and Geologic and Paleoceanographic Considerations. In Proceedings of the Ocean Drilling Program: Scientific Results; Haggerty, J.A., Premoli Silva, I., Rack, F., McNutt, M.K., Eds.; Ocean Drilling Program: College Station, TX, USA, 1995; Volume 144, p. 1059. [Google Scholar]
- Kuhn, T.; Wegorzewski, A.V.; Rühlemann, C.; Vink, A. Composition, Formation, and Occurrence of Polymetallic Nodules. In Deep-Sea Mining; Sharma, R., Ed.; Springer: Cham, Switzerland, 2017; pp. 23–63. [Google Scholar]
- Commission, E. Critical Raw Materials. 2019. Available online: http://ec.europa.eu/growth/sectors/ raw-materials/specific-interest/critical_en (accessed on 8 January 2019).
- Hein, J.R.; Koschinsky, A. Deep-Ocean Ferromanganese Crusts and Nodules. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. [Google Scholar]
- International Seabed Authority (ISA). 2019. Available online: https://www.isa.org.jm (accessed on 10 January 2019).
- Glasby, G. Manganese deposits in the southwest Pacific. In Phase I Report-Inter-University Program of Research on Ferromanganese Deposits of the Ocean Floor; Seabed Assessment Program; National Science Foundation: Washington, DC, USA, 1973; pp. 137–169. [Google Scholar]
- Burns, R.G.; Burns, V.M. Mineralogy. In Marine Manganese Deposits; Elsevier: Amsterdam, The Netherlands, 1977; Volume 15, pp. 185–248. [Google Scholar]
- Usui, A. Minerals, Metal Contents, and Mechanism of Formation of Manganese Nodules from the Central Pacific Basin (GH76-1 and GH77-1 Areas). In Marine Geology and Oceanography of the Pacific Manganese Nodule Province; Springer: Boston, MA, USA, 1979; pp. 651–679. [Google Scholar]
- Koschinsky, A.; van Gerven, M.; Halbach, P. First investigations of massive ferromanganese crusts in the NE Atlantic in comparison with hydrogenetic pacific occurrences. Mar. Georesour. Geotechnol. 1995, 13, 375–391. [Google Scholar] [CrossRef]
- Hein, J.R.; Schwab, W.C.; Davis, A. Cobalt- and platinum-rich ferromanganese crusts and associated substrate rocks from the Marshall Islands. Mar. Geol. 1988, 78, 255–283. [Google Scholar] [CrossRef]
- Hein, J.R.; Bohrson, W.A.; Schulz, M.S.; Noble, M.; Clague, D.A. Variations in the Fine-Scale Composition of a Central Pacific Ferromanganese Crust: Paleoceanographic Implications. Paleoceanogrphy 1992, 7, 63–67. [Google Scholar] [CrossRef]
- Cronan, D.S.; Varnavas, S.P.; Hodkinson, R. Hydrothermal Mineralizing Processes and Associated Sedimentation in the Santorini Hydrothermal Embayments. Mar. Georesour. Geotechnol. 2000, 18, 77–118. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Lusty, P.A.J.; Murton, B.J. Deep-Ocean Mineral Deposits: Metal Resources and Windows into Earth Processes. Elememts 2018, 14, 301–306. [Google Scholar] [CrossRef] [Green Version]
- Varentsov, I.M.; Drits, V.A.; Gorshkov, A.I.; Sivtsov, A.V.; Sakharov, B.A. Mn-Fe oxyhydroxide crusts from Krylov Seamount (Eastern Atlantic): Mineralogy, geochemistry and genesis. Mar. Geol. 1991, 96, 53–70. [Google Scholar] [CrossRef]
- Baturin, G.N.; Dubinchuk, V.T.; Rashidov, V.A. Ferromanganese crusts from the Sea of Okhotsk. Oceanology 2012, 52, 88–100. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-Resolution Analysis of Critical Minerals and Elements in Fe–Mn Crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3545. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.-K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Lond. Spec. Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- González, F.J.; Somoza, L.; Hein, J.R.; Medialdea, T.; León, R.; Urgorri, V.; Reyes, J.; Martín-Rubí, A.J. Phosphorites, Co-rich Mn nodules, and Fe-Mn crusts from Galicia Bank, NE Atlantic: Reflections of Cenozoic tectonics and paleoceanography, Geochemistry. Geophys. Geosyst. 2016, 17, 346–374. [Google Scholar] [CrossRef]
- Pelleter, E.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Labanieh, S.; Josso, P.; Bollinger, C.; Langlade, J. Ni-Cu-Co-rich hydrothermal manganese mineralization in the Wallis and Futuna back-arc environment (SW Pacific). Ore Geol. Rev. 2017, 87, 126–146. [Google Scholar] [CrossRef] [Green Version]
- Hein, J.R.; Koschinsky, A.; McIntyre, B.R. Mercury- and silver-rich ferromanganese oxides, southern California Borderland: Deposit model and environmental implications. Econ. Geol. 2005, 100, 1151–1168. [Google Scholar] [CrossRef]
- Bonatti, E.; Kraemer, T.; Rydell, H. Classification and genesis of submarine iron-manganese deposits. In Ferromanganese Deposits on the Ocean Floor; Horn, D.R., Ed.; Arden House: New York, NY, USA, 1972; pp. 149–165. [Google Scholar]
- Dymond, J.; Lyle, M.; Finney, B.; Piper, D.Z.; Murphy, K.; Conard, R.; Pisias, N. Ferromanganese nodules from MANOP Sites H, S, and R—Control of mineralogical and chemical composition by multiple accretionary processes. Geochim. Cosmochim. Acta 1984, 48, 931–949. [Google Scholar] [CrossRef]
- Lyle, M. Formation and growth of ferromanganese oxides on the Nazca plate, In GSA MEMOIRS Nazca Plate: Crustal Formation and Andean Convergence; Kulm, L.D., Dymond, J., Dasch, E.J., Hussong, D.M., Roderick, R., Eds.; Geological Society of America: Boulder, CO, USA, 1981; pp. 269–294. [Google Scholar]
- Wegorzewski, A.V.; Kuhn, T.; Dohrmann, R.; Wirth, R.; Grangeon, S. Mineralogical characterization of individual growth structures of Mn-nodules with different Ni+Cu content from the central Pacific Ocean. Am. Miner. 2015, 100, 2497–2508. [Google Scholar] [CrossRef]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Menendez, A.; James, R.; Shulga, N.; Connelly, D.; Roberts, S.; Menendez, A.; James, R.; Shulga, N.; Connelly, D.; Roberts, S. Linkages between the Genesis and Resource Potential of Ferromanganese Deposits in the Atlantic, Pacific, and Arctic Oceans. Minerals 2018, 8, 197. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Kuhn, T. The influence of suboxic diagenesis on the formation of manganese nodules in the Clarion Clipperton nodule belt of the Pacific Ocean. Mar. Geol. 2014, 357, 123–138. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]
- Josso, P.; Parkinson, I.; Horstwood, M.; Lusty, P.; Chenery, S.; Murton, B.J. Improving confidence in ferromanganese crust age models: A composite geochemical approach. Chem. Geol. 2019, 513, 108–119. [Google Scholar] [CrossRef]
- Zubkov, M.V.; Plucinski, P.K.; Dartiguelongue, A.C.Y.; Lusty, P.A.J. Metal Extraction from Deep-Ocean Mineral Deposits. Elememts 2018, 14, 319–324. [Google Scholar] [CrossRef]
- Vasil’chikov, N.V.; Shirer, G.B.; Matsepon, Y.A.; Krasnykh, I.F.; Grishankova, E.A. Iron-Manganese Nodules from the Ocean Floor—Raw Materials for the Production of Cobalt, Nickel, Manganese, and Copper. Sov. J. Non-Ferrous Met. (Engl. Transl.) 1968, 9, 46–49. [Google Scholar]
- Friedmann, D.; Pophanken, A.K.; Friedrich, B. Pyrometallurgical extraction of valuable metals from polymetallic deep-sea nodules. In Proceedings of the EMC 2015, Aachen, Germany, 14–17 June 2015. [Google Scholar]
- Abramovski, T.; Stefanova, V.; Causse, R.; Romanchuk, A. Technologies for the processing of polymetallic nodules from Clarion Clipperton zone in the Pacific Ocean. J. Chem. Technol. Metall. 2017, 52, 258–269. [Google Scholar]
- Pophanken, A.; Friedrich, B. Challenges in the Metallurgical Processing of Marine Mineral Resources. In Proceedings of the EMC 2013, Weimar, Germany, 23–26 June 2013. [Google Scholar]
- Friedmann, D.; Pophanken, A.K.; Friedrich, B. Pyrometallurgical Treatment of High Manganese Containing Deep Sea Nodules. J. Sustain. Metall. 2017, 3, 219–229. [Google Scholar] [CrossRef]
- Wegorzewski, A.V.; Köpcke, M.; Kuhn, T.; Sitnikova, M.; Wotruba, H. Thermal Pre-Treatment of Polymetallic Nodules to Create Metal (Ni, Cu, Co)-Rich Individual Particles for Further Processing. Minerals 2018, 8, 523. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Sherman, D.M.; Peacock, C.L. Surface complexation of Cu on birnessite (δ-MnO2): Controls on Cu in the deep ocean. Geochim. Cosmochim. Acta 2010, 74, 6721–6730. [Google Scholar] [CrossRef]
- Peacock, C.L. Physiochemical controls on the crystal-chemistry of Ni in birnessite: Genetic implications for ferromanganese precipitates. Geochim. Cosmochim. Acta 2009, 73, 3568–3578. [Google Scholar] [CrossRef]
- Peacock, C.L.; Sherman, D.M. Sorption of Ni by birnessite: Equilibrium controls on Ni in seawater. Chem. Geol. 2007, 238, 94–106. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, M.; Takahashi, Y. Mineralogy and crystal chemistry of Mn, Fe, Co, Ni, and Cu in a deep-sea Pacific polymetallic nodule. Am. Miner. 2014, 99, 2068–2083. [Google Scholar] [CrossRef]
- Bodeï, S.; Manceau, A.; Geoffroy, N.; Buatier, M. Formation of todorokite from vernadite in Ni-rich hemipelagic sediments. Geochim. Cosmochim. Acta 2007, 71, 5698–5716. [Google Scholar] [CrossRef]
- Liu, L.; Min, M.; Liu, F.; Yin, H.; Zhang, Y.; Qiu, G. Influence of vanadium doping on the supercapacitance performance of hexagonal birnessite. J. Power Sources 2015, 277, 26–35. [Google Scholar] [CrossRef]
- GeoERA-MINDeSEA. 2019. Available online: http://geoera.eu/projects/mindesea/ (accessed on 15 March 2019).
- Redfern, A.S.T. A.S. Marfunin (Ed.) Advanced Mineralogy Volume 1. Composition, Structure, and Properties of Mineral Matter; Volume 2. Methods and Instrumentations. Berlin, Heidelberg and New York (Springer-Verlag). Vol. 1: 1994, xxvii + 551 pp. Price DM198.00, ISBN 3-540-57254-6. Vol. 2: 1995, xxi + 441 pp. Price DM198.00. ISBN 0-387-57255-4. Mineral. Mag. 62, 877. [CrossRef]
- Roqué-Rosell, J.; Mosselmans, J.F.W.; Proenza, J.A.; Labrador, M.; Galí, S.; Atkinson, K.D.; Quinn, P.D. Sorption of Ni by “lithiophorite-asbolane” intermediates in Moa Bay lateritic deposits, eastern Cuba. Chem. Geol. 2010, 275, 9–18. [Google Scholar] [CrossRef]
- Little, S.H.; Sherman, D.M.; Vance, D.; Hein, J.R. Molecular controls on Cu and Zn isotopic fractionation in Fe-Mn crusts. Earth Planet. Sci. Lett. 2014, 396, 213–222. [Google Scholar] [CrossRef]
- Ostrooumov, M. Raman and Infrared Reflection Spectroscopic Study of Mineralogical Composition of Iron-Manganese Nodules (Pacific and Indian Oceans). Int. J. Exp. Spectrosc. Tech. 2017, 2, 2–12. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R., Eds.; W. De Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]
- Julien, C.; Massot, M.; Baddour-Hadjean, R.; Franger, S.; Bach, S.; Pereira-Ramos, P.J. Raman spectra of birnessite manganese dioxides. Solid State Ion. 2003, 159, 345–356. [Google Scholar] [CrossRef]
- Julien, C. Local cationic environment in lithium nickel-cobalt oxides used as cathode materials for lithium batteries. Solid State Ion. 2000, 136, 887–896. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef]
- Chukanov, N.V.; Chervonnyi, A.D. Infrared Spectroscopy of Minerals and Related Compounds; Springer: Cham, Switzerland, 2016; p. 1109. [Google Scholar]
- Oeser, M.; Weyer, S.; Horn, I.; Schuth, S. High-precision fe and mg isotope ratios of silicate reference glasses determined in situ by femtosecond LA-MC-ICP-MS and by solution nebulisation MC-ICP-MS. Geostand. Geoanal. Res. 2014, 38, 311–328. [Google Scholar] [CrossRef]
- Lazarov, M.; Horn, I. Matrix and energy effects during in-situ determination of Cu isotope ratios by ultraviolet-femtosecond laser ablation multicollector inductively coupled plasma mass spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2015, 111, 64–73. [Google Scholar] [CrossRef]
- Collinet, M.; Charlier, B.; Namur, O.; Oeser, M.; Médard, E.; Weyer, S. Crystallization history of enriched shergottites from Fe and Mg isotope fractionation in olivine megacrysts. Geochim. Cosmochim. Acta 2017, 207, 277–297. [Google Scholar] [CrossRef]
- Neave, D.A.; Shorttle, O.; Oeser, M.; Weyer, S.; Kobayashi, K. Mantle-derived trace element variability in olivines and their melt inclusions. Earth Planet. Sci. Lett. 2018, 483, 90–104. [Google Scholar] [CrossRef] [Green Version]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A New Geochemical Database for Reference Materials and Isotopic Standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610-617 Glasses Following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jackson, S. LAMTRACE Data Reduction Software for LA-ICP-MS. In Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada Short Course Series; Mineralogical Association of Canada: Vancouver, BC, Canada, 2008; pp. 305–307. [Google Scholar]
- Lipin, B.R.; McKay, G.A. Geochemistry and Mineralogy of Rare Earth Elements; Mineralogicas Society of America: Washington, DC, USA, 1989. [Google Scholar]
- Kane, W.S.; Cardwell, P.H. Winning of Metal Values from Ore Utilizing Recycled Acid Leaching Agent. U.S. Patent 3923615A, 2 December 1974. [Google Scholar]
- Jana, R.K.; Singh, D.D.N.; Roy, S.K. Hydrochloric Acid Leaching of Sea Nodules with Methanol and Ethanol Addition. Mater. Trans. JIM 1993, 34, 593–598. [Google Scholar] [CrossRef] [Green Version]
- Mulè, G.; Burlet, C.; Vanbrabant, Y. Automated curve fitting and unsupervised clustering of manganese oxide Raman responses. J. Raman Spectrosc. 2017, 48, 1665–1675. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Jiang, X.; Sa, R.; Zhou, L.; Huang, Y.; Liu, Y.; Li, X.; Lu, R.; Wang, C. The effect of Fe-Mn minerals and seawater interface and enrichment mechanism of ore-forming elements of polymetallic crusts and nodules from the South China Sea. Acta Oceanol. Sin. 2017, 36, 34–46. [Google Scholar] [CrossRef]
- Cui, H.; Feng, X.; Tan, W.; He, J.; Hu, R.; Liu, F. Synthesis of todorokite-type manganese oxide from Cu-buserite by controlling the pH at atmospheric pressure. Microporous Mesoporous Mater. 2009, 117, 41–47. [Google Scholar] [CrossRef]
- Al-Sagheer, F.A.; Zaki, M.I. Synthesis and surface characterization of todorokite-type microporous manganese oxides: Implications for shape-selective oxidation catalysts. Microporous Mesoporous Mater. 2004, 67, 43–52. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust, Its Composition and Evolution: An Examination of the Geochemical Record Preserved in Sedimentary Rocks; Blackwell Scientific: Hoboken, NJ, USA, 1985. [Google Scholar]
- Baturin, G.N.; Dubinchuk, V.T. Mineralogy and chemistry of ferromanganese crusts from the Atlantic Ocean. Geochem. Int. 2011, 49, 578–593. [Google Scholar] [CrossRef]
- Yeo, I.A.; Dobson, K.; Josso, P.; Pearce, R.B.; Howarth, S.A.; Lusty, P.A.J.; le Bas, T.P.; Murton, B.J. Assessment of the Mineral Resource Potential of Atlantic Ferromanganese Crusts Based on Their Growth History. Microstruct. Texture Miner. 2018, 8, 327. [Google Scholar] [CrossRef]
- Benites, M.; Millo, C.; Hein, J.; Nath, B.N.; Murton, B.; Galante, D.; Jovane, L. Integrated Geochemical and Morphological Data Provide Insights into the Genesis of Ferromanganese Nodules. Minerals 2018, 8, 488. [Google Scholar] [CrossRef]
- Hein, J.R.; Spinardi, F.; Okamoto, N.; Mizell, K.; Thorburn, D.; Tawake, A. Critical metals in manganese nodules from the Cook Islands EEZ, abundances and distributions. Ore Geol. Rev. 2015, 68, 97–116. [Google Scholar] [CrossRef]
- Frazer, J.Z.; Fisk, M.B. Geological factors related to characteristics of sea-floor manganese nodule deposits. Deep Sea Res. Part A Oceanogr. Res. Pap. 1981, 28, 1533–1551. [Google Scholar] [CrossRef]
- Koschinsky, A. Heavy metal distributions in Peru Basin surface sediments in relation to historic, present and disturbed redox environments. Deep Sea Res. Part II Top. Stud. Oceanogr. 2001, 48, 3757–3777. [Google Scholar] [CrossRef]
- Halbach, P.; Scherhag, C.; Hebisch, U.; Marchig, V. Geochemical and mineralogical control of different genetic types of deep-sea nodules from the Pacific Ocean. Min. Depos. 1981, 16, 59–84. [Google Scholar] [CrossRef]
- Friedrich, G.; Kunzendorf, H.; Plüger, W.L. Geochemical investigations of deep-sea manganese nodules from the Pacific on board RV “Valdivia“. In Papers on the Origin and Distribution of Manganese Nodules in the Pacific and Prospects for Their Exploration; Morgenstein, M., Ed.; University of Hawaii and IDOE-NSF: Valdivia, Chile, 1973; pp. 32–43. [Google Scholar]
- Friedrich, G.H.W.; Kunzendorf, H.; Plüger, W.L. Ship-borne geochemical investigations of deep-sea manganese-nodule deposits in the Pacific using a radioisotope energy-dispersive X-ray system. J. Geochem. Explor. 1974, 3, 303–317. [Google Scholar] [CrossRef]
- Koschinsky, A.; Stascheit, A.; Bau, M.; Halbach, P. Effects of phosphatization on the geochemical and mineralogical composition of marine ferromanganese crusts. Geochim. Cosmochim. Acta 1997, 61, 4079–4094. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on ferromanganese crust composition and reconnaissance resource potential, Ninetyeast Ridge, Indian Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Rodrı́guez-Losada, J.A.; Martinez-Frias, J.; Bustillo, M.A.; Delgado, A.; Hernandez-Pacheco, A.; de Krauss, J.V. The hydrothermally altered ankaramite basalts of Punta Poyata (Tenerife, Canary Islands). J. Volcanol. Geotherm. Res. 2000, 103, 367–376. [Google Scholar] [CrossRef]
- Schiffman, P.; Staudigel, H. Hydrothermal alteration of a seamount complex on La Palma, Canary Islands: Implications for metamorphism in accreted terranes. Geology 1994, 22, 151–154. [Google Scholar] [CrossRef]
- Lindblom, S.; Gérard, M. Textural and fluid inclusion evidence for hydrothermal activity in the volcaniclastic apron of Gran Canaria. In Proceedings of the Ocean Drilling Program, Scientific Results; Weaver, P.P.E., Schmincke, H.-U., Firth, J.V., Eds.; Texas A&M University: College Station, TX, USA, 1998; Volume 157, pp. 429–439. [Google Scholar] [CrossRef]
- Schmincke, H.-U.; Krastel, S.; Hansteen, T.; Sumita, M. Preliminary Results, Leg M43/1, Rock Sampling and Description. In DECOS/OMEX II, Cruise No. 43; METEOR-Berichte: Hamburg, Germany, 2000. [Google Scholar]
- van den Bogaard, P. The origin of the Canary Island Seamount Province-New ages of old seamounts. Sci. Rep. 2013, 3, 2107. [Google Scholar] [CrossRef] [PubMed]
- Hannington, M.D.; Petersen, S.; Santana-Casiano, M.; Fraile-Nuez, E.; Klischies, M.; Lange, S.; Hissman, K.; Schauer, J.; Striewski, P.; Anderson, M.O.; et al. POS-494 Leg 2 Cruise Report: Assessment of Ongoing Magmatic-Hydrothermal Discharge of the El Hierro Submarine Volcano, Canary Islands by the Submersible JAGO; RV POSEIDON. GEOMAR Helmholtz Centre for Ocean Research Kiel, Instituto de Oceanografia y Cambio Global, Instituto Español de Oceanografía: Kiel, Germany, 2016; p. 88. [Google Scholar] [CrossRef]
- Donoghue, E.; Troll, V.R.; Harris, C.; O’Halloran, A.; Walter, T.R.; Torrado, F.J.P. Low-temperature hydrothermal alteration of intra-caldera tuffs, Miocene Tejeda caldera, Gran Canaria, Canary Islands. J. Volcanol. Geotherm. Res. 2008, 176, 551–564. [Google Scholar] [CrossRef]
- González, F.J.; Rincón-Tomás, B.; Somoza, L.; Hein, J.R.; Medialdea, T.; Madureira, P.; Reyes, J.; Hoppert, M.; Reitner, J. Fe-rich mineralized microbes from hydrothermal vents at Tagoro submarine volcano, El Hierro Island (central east Atlantic). In Proceedings of the 113th Annual GSA Cordilleran Section Meeting-2017, Honolulu, HI, USA, 23–25 May 2017. [Google Scholar]
- Somoza, L.; González, F.J.; Barker, S.J.; Madureira, P.; Medialdea, T.; de Ignacio, C.; Lourenço, N.; León, R.; Vázquez, J.T.; Palomino, D. Evolution of submarine eruptive activity during the 2011-2012 El Hierro event as documented by hydroacoustic images and remotely operated vehicle observations. Geochem. Geophys. Geosyst. 2017, 18, 3109–3137. [Google Scholar] [CrossRef]
- Muiños, S.B.; Hein, J.R.; Frank, M.; Monteiro, J.H.; Gaspar, L.; Conrad, T.; Pereira, H.G.; Abrantes, F. Deep-sea Fe-Mn Crusts from the Northeast Atlantic Ocean: Composition and Resource Considerations. Mar. Georesour. Geotechnol. 2013, 31, 40–70. [Google Scholar] [CrossRef]
- Brandt, P.; Hormann, V.; Körtzinger, A.; Visbeck, M.; Krahmann, G.; Stramma, L.; Lumpkin, R.; Schmid, C. Changes in the Ventilation of the Oxygen Minimum Zone of the Tropical North Atlantic. J. Phys. Oceanogr. 2010, 40, 1784–1801. [Google Scholar] [CrossRef]
- Brandt, P.; Greatbatch, R.J.; Claus, M.; Didwischus, S.-H.; Hormann, V.; Funk, A.; Hahn, J.; Krahmann, G.; Fischer, J.; Körtzinger, A. Ventilation of the equatorial Atlantic by the equatorial deep jets. J. Geophys. Res. Ocean. 2012, 117, C12. [Google Scholar] [CrossRef]
- Eltayeb, M.A.H.; Injuk, J.; Maenhaut, W.; van Grieken, R.E. Elemental Composition of mineral aerosol generated from Sudan Sahara Sand. J. Atmos. Chem. 2001, 40, 247–273. [Google Scholar] [CrossRef]
- Muhs, D.R.; Budahn, J.; Skipp, G.; Prospero, J.M.; Patterson, D.; DeAnna Patterson, E. Geochemical and mineralogical evidence for Sahara and Sahel dust additions to quaternary soils on Lanzarote, eastern Canary Islands, Spain. Terra Nov. 2010, 22, 399–410. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P.; Hein, J.R.; Mangini, A. Ferromanganese crusts as indicators for paleoceanographic events in the NE Atlantic. Geol. Rundsch. 1996, 85, 567–576. [Google Scholar] [CrossRef]
- Usui, A.; Nishi, K.; Sato, H.; Nakasato, Y.; Thornton, B.; Kashiwabara, T.; Tokumaru, A.; Sakaguchi, A.; Yamaoka, K.; Kato, S.; et al. Continuous growth of hydrogenetic ferromanganese crusts since 17 Myr ago on Takuyo-Daigo Seamount, NW Pacific, at water depths of 800–5500 m. Ore Geol. Rev. 2017, 87, 71–87. [Google Scholar] [CrossRef]
- Schlitzer, R.; Anderson, R.F.; Dodas, E.M.; Lohan, M.; Geibert, W.; Tagliabue, A.; Bowie, A.; Jeandel, C.; Maldonado, M.T.; Landing, W.M.; et al. The GEOTRACES Intermediate Data Product 2017. Chem. Geol. 2018, 493, 210–223. [Google Scholar] [CrossRef]
- Grousset, F.E.; Parra, M.; Bory, A.; Martinez, P.; Bertrand, P.; Shimmield, G.; Ellam, R.M. Saharan wind regimes traced by the Sr-Nd isotopic composition of subtropical Atlantic sediments: Last glacial maximumvs today. Quat. Sci. Rev. 1998, 17, 395–409. [Google Scholar] [CrossRef]
- Grousset, F.E.; Biscaye, P.E. Tracing dust sources and transport patterns using Sr, Nd and Pb isotopes. Chem. Geol. 2005, 222, 149–167. [Google Scholar] [CrossRef]
- Blum, N.; Halbach, P.; Münch, U. Geochemistry and mineralogy of alkali basalts from Tropic Seamount, Central Atlantic Ocean. Mar. Geol. 1996, 136, 1–19. [Google Scholar] [CrossRef]
- Presentación Parcial de Datos e Información Sobre los Límites de la Plataforma Continental de España al Oeste de las Islas Canarias, Conforme a la Parte VI y el Anexo II de la Convención de las Naciones Unidas Sobre el Derecho del Mar; Instituto Geológico y Minero de España (IGME): Madrid, Spain, 2015; Volume 5.
- Mehegan, J.M.; Robinson, P.T.; Delaney, J.R. Secondary Mineralization and Hydrothermal Alteration in the Reydarfjordur Drill Core, Eastern Iceland. J. Geophys. Res. Solid Earth 1982, 87, 6511–6524. [Google Scholar] [CrossRef]
- Dekov, V.M.; Savelli, C. Hydrothermal activity in the SE Tyrrhenian Sea: An overview of 30 years of research. Mar. Geol. 2004, 204, 161–185. [Google Scholar] [CrossRef]
- Bau, M. Controls on the fractionation of isovalent trace elements in magmatic and aqueous systems: Evidence from Y/Ho, Zr/Hf, and lanthanide tetrad effect. Contrib. Mineral. Petrol. 1996, 123, 323–333. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Somoza, L.; Medialdea, T.; Lunar, R. Hydrothermal input on the formation of Fe-Mn crusts from Canary Islands Seamount Province: Geochemical and Fe isotopic studies. In preparation.
- Dubinin, A.V.; Uspenskaya, T.Y.; Gavrilenko, G.M.; Rashidov, V.A. Geochemistry and genesis of Fe-Mn mineralization in island arcs in the west Pacific Ocean. Geochem. Int. 2008, 46, 1206–1227. [Google Scholar] [CrossRef]
- Koschinsky, A.; Heinrich, L.; Boehnke, K.; Cohrs, J.C.; Markus, T.; Shani, M.; Singh, P.; Stegen, K.S.; Werner, W. Deep-sea mining: Interdisciplinary research on potential environmental, legal, economic, and societal implications. Integr. Environ. Assess. Manag. 2018, 14, 672–691. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.C.; Wilder, T. Recovery of Metal Values from Manganese Nodules. US Patent 3788841A, 1 January 1974. [Google Scholar]
- Agarwal, J.C.; Beecher, N.; Davies, D.S.; Hubred, G.L.; Kakaria, V.K.; Kust, R.N. Processing of ocean nodules: A technical and economic review. JOM 1976, 28, 24–31. [Google Scholar] [CrossRef]
- Han, K.N.; Fuerstenau, D.W. Acid leaching of ocean manganese nodules at elevated temperatures. Int. J. Miner. Process. 1975, 2, 163–171. [Google Scholar] [CrossRef]
- Acharya, S.; Anand, S.; Das, S.C.; Das, R.P.; Jena, P.K. Ammonia leaching of ocean nodules using various reductants. Erzmetall 1989, 42, 66–73. [Google Scholar]
- Mishra, D.; Srivastava, R.R.; Sahu, K.K.; Singh, T.B.; Jana, R.K. Leaching of roast-reduced manganese nodules in NH3–(NH4)2CO3 medium. Hydrometallurgy 2011, 109, 215–220. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Filippidis, A.; Pontikes, Y. The Rare Earth Elements Potential of Greek Bauxite Active Mines in the Light of a Sustainable REE Demand. J. Sustain. Metall. 2018, 5, 20–47. [Google Scholar] [CrossRef]
- Kanungo, S.B.; Jena, P.K. Reduction leaching of manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1988, 21, 41–58. [Google Scholar] [CrossRef]
- Sridhar, R.; Jones, W.E.; Warner, J.S. Extraction of copper, nickel and cobalt from sea nodules. JOM 1976, 28, 32–37. [Google Scholar] [CrossRef]
- Mohwinkel, D.; Kleint, C.; Koschinsky, A. Phase associations and potential selective extraction methods for selected high-tech metals from ferromanganese nodules and crusts with siderophores. Appl. Geochem. 2014, 43, 13–21. [Google Scholar] [CrossRef]
- Spickermann, R. Rare Earth Content of Manganese Nodules in the Lockheed Martin Clarion-Clipperton Zone Exploration Areas. In Proceedings of the Offshore Technology Conference, Houston, TX, USA, 30 April–3 May 2012. [Google Scholar]
- Papavasileiou, K. Near future REE resources for Europe-The new frontier of Marine exploration, mining and processing. In Proceedings of the 1st European Rare Earth Resources Conference, At Milos, Greece, 4–7 September 2014; pp. 410–427. [Google Scholar]
- Lugovskaya, I.G.; Dubinchuk, V.T.; Baturin, G.N. Composition of technological sample of ferromanganese crusts from seamounts and products of its processing. Lithol. Miner. Resour. 2007, 42, 515–522. [Google Scholar] [CrossRef]
- Manceau, A.; Drits, V.A.; Silvester, E.; Bartoli, C.; Lanson, B. Structural mechanism of Co2+oxidation by the phyllomanganate buserite. Am. Mineral. 1997, 82, 1150–1175. [Google Scholar] [CrossRef]
- Manceau, A.; Lanson, B.; Drits, V.A. Structure of heavy metal sorbed birnessite. Part III: Results from powder and polarized EXAFS spectroscopy. Geochim. Cosmochim. Acta 2002, 66, 2639–2663. [Google Scholar] [CrossRef]
- Manceau, A.; Gorshkov, A.I.; Drits, V.A. Structural chemistry of Mn, Fe, Co, and Ni in manganese hydrous oxides: Part II. Information from EXAFS spectroscopy and electron and X-ray diffraction. Am. Mineral. 1992, 77, 1144–1157. [Google Scholar]
- Kashiwabara, T.; Takahashi, Y.; Tanimizu, M. A XAFS study on the mechanism of isotopic fractionation of molybdenum during its adsorption on ferromanganese oxides. Geochem. J. 2009, 43, e31–e36. [Google Scholar] [CrossRef] [Green Version]
- Kashiwabara, T.; Takahashi, Y.; Marcus, M.A.; Uruga, T.; Tanida, H.; Terada, Y.; Usui, A. Tungsten species in natural ferromanganese oxides related to its different behavior from molybdenum in oxic ocean. Geochim. Cosmochim. Acta 2013, 106, 364–378. [Google Scholar] [CrossRef]
- Sahu, K.K.; Agarwal, S.; Mishra, D.; Agrawal, A.; Randhawa, N.S.; Godiwalla, K.M.; Jana, R.K. Nickel, Cobalt and Copper Recovery from Sea Nodules by Direct Smelting Process. In Ni-Co 2013; Springer International Publishing: Cham, Switzerland, 2013; pp. 291–298. [Google Scholar]
- Balintova, M.; Petrilakova, A. Study of pH Influence on Selective Precipitation of Heavy Metals from Acid Mine Drainage. Chem. Eng. Trans. 2011, 25, 345–350. [Google Scholar]
- González, F.J. Nódulos y Costras de Hierro-Manganeso en el Golfo de Cádiz y la Antártida: Génesis e Implicaciones Paleoceanográficas; Publicaciones del Instituto Geológico y Minero de España: Madrid, Spain, 2008. [Google Scholar]











| Sample | Seamount | Sample Localization | Latitude | Longitude | Water Depth (m) | Fe-Mn Crust Thickness (mm) |
|---|---|---|---|---|---|---|
| DR02-10 | Echo | North flank | 25°29,62′ N | 19°23,47′ W | 1890 | 38 |
| DR07-9 | The Paps | West flank | 25°57,18′ N | 20°21,73′ W | 1860 | 90 |
| DR16-14 | Tropic | East flank | 23°52,91′ N | 20°37,07′ W | 1719 | 140 |
| 107-11H | Tropic | West flank | 23°54,70′ N | 20°46,73′ W | 1716 | 5 |
| Crust | Major | Moderate | Minor |
|---|---|---|---|
| DR02-10 | δ-MnO2 | Goethite | 10Å Mn-Ox., Quartz, Feldspar |
| DR07-9 | δ-MnO2 | Goethite | 10Å Mn–Ox., Quartz, carbonate-fluorapatite (CFA), Feldspar, phyllosilicates |
| DR16-14 | δ-MnO2 | Goethite | |
| 107-11H | CFA | Goethite, Hematite, Quartz, Magnetite, Ilmenite | δ-MnO2, smectite |
| Element | DR02-10 | DR07-9 | DR16-14 | 107-11H |
|---|---|---|---|---|
| Fe (wt. %) | 26.4 | 21.9 | 27.2 | 38.1 |
| Mn | 17.5 | 15.6 | 20.6 | 1.44 |
| Ti | 0.96 | 0.81 | 1.18 | 0.20 |
| Si | 5.13 | 9.70 | 2.63 | 1.87 |
| Al | 1.99 | 3.29 | 1.64 | 0.71 |
| Ca | 2.34 | 2.56 | 3.30 | 10,2 |
| K | 0.42 | 0.81 | 0.35 | 0.21 |
| Mg | 1.50 | 2.20 | 1.54 | 0.40 |
| P | 0.57 | 0.56 | 0.60 | 3.99 |
| Na | 1.63 | 1.50 | 1.63 | 0.40 |
| LOI | 22.9 | 20.7 | 23.9 | 12.3 |
| Mn/Fe | 0.67 | 0.72 | 0.75 | 0.04 |
| Co (µg/g) | 4355 | 4416 | 8347 | 497 |
| Ni | 2483 | 4430 | 2481 | 398 |
| V | 1187 | 878 | 1266 | 319 |
| Cu | 595 | 1016 | 463 | 178 |
| Mo | 453 | 381 | 648 | 52 |
| Zn | 613 | 683 | 649 | 187 |
| Pb | 1874 | 1420 | 1868 | 193 |
| Te | 42 | 38 | 64 | 5 |
| Tl | 68 | 121 | 130 | 14 |
| Ba | 1658 | 1328 | 1998 | 139 |
| As | 439 | 352 | 489 | 146 |
| Se | 37 | 28 | 40 | 7 |
| Cd | 2.2 | 2.3 | 1.9 | <D.L. (0.7) |
| Sb | 59 | 59 | 89 | 12 |
| Cr | 26 | 54 | 30 | 35 |
| Be | 14 | 11 | 12 | 4 |
| Th | 54 | 48 | 81 | 5 |
| U | 16 | 13 | 12 | 4 |
| Y | 217 | 164 | 179 | 115 |
| La | 342 | 253 | 426 | 70 |
| Ce | 1717 | 1343 | 2230 | 154 |
| Pr | 73 | 56 | 97 | 10 |
| Nd | 305 | 232 | 392 | 44 |
| Sm | 63 | 48 | 79 | 8.5 |
| Eu | 15.2 | 11.8 | 18.8 | 2.1 |
| Gd | 73 | 55 | 83 | 12 |
| Tb | 10.5 | 7.9 | 11.7 | 1.6 |
| Dy | 63 | 47 | 66 | 11 |
| Ho | 12.3 | 9 | 12.2 | 2.7 |
| Er | 35 | 25 | 33 | 8.2 |
| Tm | 4.9 | 3.5 | 4.6 | 1.2 |
| Yb | 31 | 22 | 28 | 8.2 |
| Lu | 4.8 | 3.4 | 4.1 | 1.3 |
| Element | DR02-10 n = 136 | DR07-9 n = 256 | DR16-14 n = 364 | 107-11H n = 184 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Min. | Max. | ± 2σ | Mean | Min. | Max. | ± 2σ | Mean | Min. | Max | ± 2σ | Mean | Min | Max. | ±2σ | |
| Mn | 21 | 1.11 | 46 | 1.13 | 24.6 | 0.35 | 49 | 1.28 | 17 | 0.10 | 28 | 0.70 | 17 | 0.01 | 38 | 1.21 |
| Fe | 21 | 0.44 | 47 | 1.58 | 19.5 | 0.37 | 50 | 1.38 | 25 | 0.16 | 53 | 0.88 | 28 | 4.27 | 59 | 1.47 |
| Si | 1.89 | 0.28 | 7.49 | 0.13 | 1.96 | 0.01 | 23 | 0.31 | 1.79 | 0.11 | 10.5 | 0.11 | 1.59 | 0.09 | 3.38 | 0.08 |
| Al | 1.14 | 0.39 | 3.06 | 0.05 | 1.47 | 0.02 | 4.56 | 0.09 | 0.76 | 0.00 | 4.8 | 0.05 | 1.45 | 0.12 | 3.97 | 0.09 |
| Ca | 3.02 | 0.50 | 17 | 0.44 | 1.87 | 0.07 | 2.92 | 0.08 | 2.12 | 0.00 | 5.43 | 0.09 | 2.44 | 0.23 | 30 | 0.38 |
| K | 0.22 | 0.06 | 1.01 | 0.04 | 0.24 | 0.01 | 2.04 | 0.04 | 0.15 | 0.01 | 1.74 | 0.02 | 0.11 | 0.00 | 0.56 | 0.01 |
| Na | 0.55 | 0.18 | 1.46 | 0.06 | 0.35 | 0.02 | 1.22 | 0.03 | 0.21 | 0.00 | 0.64 | 0.01 | 0.22 | 0.01 | 0.40 | 0.01 |
| Mg | 1.56 | 1.14 | 3.03 | 0.06 | 1.91 | 0.06 | 6.19 | 0.15 | 1.15 | 0.00 | 2.32 | 0.02 | 1.58 | 0.12 | 6.34 | 0.12 |
| P | 0.14 | 0.00 | 0.25 | 0.01 | 0.17 | 0.00 | 0.30 | 0.01 | 0.14 | 0.00 | 0.38 | 0.01 | 0.28 | 0.02 | 3.30 | 0.04 |
| Ti | 0.53 | 0.00 | 1.13 | 0.04 | 0.43 | 0.00 | 1.01 | 0.45 | 0.55 | 0.00 | 1.35 | 0.02 | 0.88 | 0.05 | 6.10 | 0.09 |
| Co | 0.44 | 0.00 | 0.84 | 0.03 | 0.51 | 0.00 | 1.68 | 0.04 | 0.64 | 0.00 | 1.36 | 0.03 | 0.64 | 0.00 | 1.60 | 0.05 |
| Ni | 0.31 | 0.03 | 1.11 | 0.03 | 1.08 | 0.00 | 5.49 | 0.17 | 0.21 | 0.00 | 0.50 | 0.01 | 0.36 | 0.00 | 2.31 | 0.05 |
| Cu | 0.07 | 0.00 | 0.44 | 0.01 | 0.25 | 0.00 | 1.10 | 0.03 | 0.05 | 0.00 | 0.27 | 0.00 | 0.13 | 0.00 | 0.56 | 0.01 |
| V | 0.12 | 0.01 | 0.21 | 0.01 | 0.11 | 0.01 | 0.48 | 0.01 | 0.15 | 0.00 | 0.32 | 0.01 | 0.15 | 0.01 | 0.32 | 0.01 |
| S | 0.27 | 0.02 | 0.65 | 0.02 | 0.12 | 0.00 | 0.27 | 0.01 | 0.16 | 0.00 | 0.42 | 0.01 | 0.20 | 0.01 | 0.40 | 0.01 |
| Cl | 0.22 | 0.02 | 0.67 | 0.02 | 0.09 | 0.00 | 0.59 | 0.01 | 0.13 | 0.01 | 1.25 | 0.01 | 0.07 | 0.00 | 0.44 | 0.01 |
| Mo | 0.04 | 0.00 | 0.08 | 0.00 | 0.04 | 0.00 | 0.08 | 0.00 | 0.05 | 0.00 | 0.12 | 0.00 | 0.03 | 0.00 | 0.07 | 0.00 |
| Ba | 0.19 | 0.00 | 0.30 | 0.01 | 0.20 | 0.01 | 5.73 | 0.06 | 0.21 | 0.00 | 0.34 | 0.01 | 0.21 | 0.00 | 0.84 | 0.01 |
| Ce | 0.18 | 0.00 | 0.31 | 0.01 | 0.18 | 0.00 | 0.33 | 0.01 | 0.23 | 0.01 | 0.43 | 0.01 | 0.19 | 0.00 | 0.39 | 0.01 |
| Element | Li | Al | Ca | Mn | Fe | Mn/Fe | Co | Ni | Cu | Zn | As | Nb | Mo | Te | Y | Ce | ΣREY | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Reflectivity Laminae | ||||||||||||||||||
| DR02-10 | Mean | 29.7 | 0.7 | 2.9 | 16 | 20 | 0.85 | 4056 | 2524 | 521 | 518 | 649 | 168 | 385 | 69 | 202 | 1550 | 2369 |
| Min. | 21.5 | 0.5 | 2.0 | 12 | 16 | 0.42 | 2250 | 2060 | 154 | 373 | 453 | 62 | 261 | 52 | 169 | 893 | 1743 | |
| Max. | 53.9 | 1.0 | 4.0 | 18 | 28 | 1.08 | 5730 | 3110 | 845 | 635 | 747 | 411 | 485 | 103 | 238 | 2340 | 3040 | |
| ± 2σ | 11.9 | 0.2 | 0.7 | 2 | 4 | 0.22 | 1232 | 359 | 223 | 102 | 101 | 135 | 76 | 18 | 24 | 589 | 482 | |
| DR07-9 | Mean | 14.4 | 0.7 | 2.4 | 20 | 23 | 0.97 | 6151 | 3827 | 1341 | 1076 | 631 | 67 | 742 | 58 | 136 | 1771 | 2738 |
| Min. | 8.1 | 0.6 | 1.9 | 17 | 14 | 0.60 | 3390 | 2660 | 847 | 548 | 493 | 47 | 442 | 1 | 97 | 1360 | 2240 | |
| Max. | 39.8 | 0.9 | 3.0 | 27 | 31 | 1.87 | 10,300 | 5910 | 2160 | 1690 | 878 | 82 | 1010 | 91 | 269 | 2220 | 3197 | |
| ± 2σ | 5.9 | 0.1 | 0.2 | 2 | 3 | 0.25 | 1255 | 619 | 231 | 255 | 74 | 8 | 94 | 19 | 31 | 186 | 226 | |
| DR16-14 | Mean | <D.L. | 0.4 | 2.6 | 18 | 21 | 0.88 | 6695 | 2090 | 389 | 647 | 623 | 74 | 555 | 86 | 225 | 1868 | 3445 |
| Min. | <D.L. | 0.3 | 2.1 | 14 | 18 | 0.67 | 5960 | 1460 | 357 | 550 | 556 | 63 | 490 | 65 | 198 | 1600 | 2931 | |
| Max. | <D.L. | 0.4 | 3.0 | 21 | 24 | 1.09 | 7560 | 2690 | 433 | 837 | 688 | 87 | 597 | 95 | 253 | 2060 | 3790 | |
| ± 2σ | <D.L. | 0.0 | 0.3 | 3 | 3 | 0.20 | 645 | 533 | 35 | 132 | 53 | 10 | 45 | 14 | 25 | 210 | 404 | |
| 107-11H | Mean | 63.3 | 1.2 | 2.6 | 23 | 23 | 1.03 | 13,583 | 5390 | 1717 | 1443 | 815 | 197 | 592 | 161 | 207 | 2727 | 3868 |
| Min. | 16.6 | 0.9 | 1.6 | 17 | 18 | 0.68 | 8380 | 4280 | 1450 | 1190 | 676 | 121 | 403 | 105 | 147 | 1940 | 2747 | |
| Max. | 102 | 1.4 | 3.4 | 30 | 35 | 1.52 | 17,700 | 7080 | 2230 | 1830 | 1170 | 234 | 870 | 208 | 254 | 3580 | 4888 | |
| ± 2σ | 21.9 | 0.2 | 0.6 | 4 | 4 | 0.21 | 2305 | 772 | 252 | 158 | 123 | 31 | 125 | 24 | 32 | 489 | 606 | |
| High Reflectivity Fibrous Laminae | ||||||||||||||||||
| DR02-10 | Mean | 6.60 | 0.4 | 11.1 | 34 | 0 | 79.80 | 223 | 6528 | 2088 | 1248 | 19 | 7 | 574 | 3 | 17 | 19 | 73 |
| Min. | 292. | 0.3 | 7.0 | 32 | 0 | 46.93 | 177 | 5470 | 1340 | 442 | 14 | 4 | 457 | 2 | 9 | 11 | 58 | |
| Max. | 913. | 0.6 | 18.2 | 36 | 1 | 106.96 | 309 | 7920 | 2790 | 2210 | 23 | 10 | 699 | 5 | 27 | 25 | 95 | |
| ± 2σ | 209.2 | 0.1 | 4.4 | 2 | 0 | 19.29 | 56 | 975 | 518 | 655 | 3 | 2 | 84 | 1 | 6 | 5 | 13 | |
| DR07-9 | Mean | 197. | 0.5 | 1.4 | 42 | 1 | 63.50 | 1306 | 27933 | 11256 | 2603 | 42 | 4 | 492 | 1 | 15 | 70 | 158 |
| Min. | .80 | 0.1 | 0.7 | 20 | 0 | 19.70 | 438 | 6830 | 4960 | 1080 | 17 | 1 | 243 | 0 | 5 | 22 | 62 | |
| Max. | 287. | 1.4 | 2.7 | 63 | 2 | 152.38 | 2350 | 60000 | 21000 | 5480 | 84 | 10 | 755 | 3 | 50 | 192 | 270 | |
| ± 2σ | 65.5 | 0.4 | 0.5 | 13 | 0 | 34.96 | 573 | 16328 | 4451 | 1168 | 17 | 2 | 170 | 1 | 12 | 45 | 61 | |
| High Reflectivity Poor Fibrous Laminae | ||||||||||||||||||
| DR07-9 | Mean | 197.1 | 1.2 | 1.2 | 39 | 5 | 9.32 | 5679 | 34,038 | 7258 | 1917 | 188 | 17 | 429 | 10 | 33 | 201 | 369 |
| Min. | 71.7 | 0.8 | 0.9 | 30 | 3 | 2.64 | 2080 | 13,500 | 5150 | 834 | 90 | 7 | 227 | 0 | 13 | 84 | 151 | |
| Max. | 408.0 | 2.0 | 1.7 | 64 | 12 | 15.03 | 15500 | 65,100 | 12600 | 3340 | 358 | 37 | 632 | 16 | 56 | 273 | 496 | |
| ± 2σ | 72.0 | 0.3 | 0.2 | 8 | 2 | 3.02 | 3446 | 12,160 | 1778 | 633 | 67 | 6 | 95 | 4 | 9 | 43 | 75 | |
| Fe-Rich Laminae | ||||||||||||||||||
| DR16-14 | Mean | <D.L. | 0.8 | 0.6 | 1 | 34 | 0.03 | 708 | 304 | 650 | 1125 | 1108 | 91 | 64 | 39 | 118 | 297 | 696 |
| Min. | <D.L. | 0.7 | 0.5 | 0 | 31 | 0.01 | 242 | 194 | 560 | 1000 | 1060 | 78 | 41 | 30 | 104 | 208 | 635 | |
| Max. | <D.L. | 1.0 | 0.7 | 1 | 37 | 0.04 | 951 | 414 | 749 | 1230 | 1160 | 104 | 94 | 52 | 127 | 351 | 775 | |
| ± 2σ | <D.L. | 0.1 | 0.1 | 0 | 3 | 0.01 | 326 | 89 | 87 | 99 | 49 | 14 | 23 | 9 | 11 | 65 | 70 | |
| 107-11H | Mean | 28.7 | 1.1 | 1.0 | 5 | 39 | 0.13 | 1103 | 1624 | 1144 | 657 | 1209 | 300 | 157 | 196 | 163 | 1469 | 2192 |
| Min. | 9.6 | 0.9 | 0.5 | 1 | 31 | 0.02 | 270 | 375 | 777 | 538 | 933 | 216 | 26 | 141 | 77 | 476 | 728 | |
| Max. | 51.0 | 1.4 | 1.3 | 10 | 51 | 0.31 | 1850 | 3940 | 1800 | 815 | 1570 | 472 | 254 | 245 | 192 | 1990 | 2843 | |
| ± 2σ | 17.8 | 0.2 | 0.3 | 3 | 6 | 0.10 | 564 | 1219 | 347 | 99 | 203 | 91 | 78 | 38 | 43 | 532 | 755 | |
| Near Substrate Rock Fe-Rich Laminae | ||||||||||||||||||
| 107-11H | Mean | 6.1 | 1.2 | 0.5 | 1 | 39 | 0.02 | 191 | 366 | 1033 | 904 | 1508 | 293 | 43 | 138 | 85 | 467 | 749 |
| Min. | 4.0 | 1.2 | 0.4 | 0 | 33 | 0.01 | 71 | 201 | 561 | 571 | 1190 | 252 | 16 | 65 | 75 | 127 | 347 | |
| Max. | 7.6 | 1.3 | 0.6 | 2 | 43 | 0.05 | 457 | 710 | 1260 | 1330 | 1900 | 335 | 93 | 180 | 95 | 837 | 1173 | |
| ± 2σ | 1.7 | 0.1 | 0.1 | 1 | 4 | 0.02 | 175 | 232 | 313 | 364 | 290 | 44 | 36 | 50 | 9 | 286 | 334 | |
| Altered Substrate Rock | ||||||||||||||||||
| 107-11H | Mean | 3.8 | 0.5 | 1.1 | 0 | 26 | 0.00 | 38 | 185 | 427 | 225 | 440 | 60 | 15 | 17 | 44 | 22 | 131 |
| Min. | 3.2 | 0.2 | 0.3 | 0 | 17 | 0.00 | 11 | 122 | 144 | 97 | 121 | 12 | 8 | 3 | 20 | 6 | 51 | |
| Max. | 4.5 | 1.2 | 1.9 | 0 | 31 | 0.01 | 68 | 274 | 991 | 495 | 1130 | 170 | 19 | 53 | 62 | 68 | 209 | |
| ± 2σ | 1.0 | 0.4 | 0.7 | 0 | 6 | 0.00 | 29 | 66 | 374 | 179 | 456 | 73 | 5 | 23 | 19 | 30 | 70 | |
| BULK | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn (%) | Fe | Co (μg/g) | Ni | Cu | V | Mo | Y | La | Ce | |
| DR02-10 | 18 | 26 | 4355 | 2483 | 595 | 1187 | 453 | 217 | 342 | 1717 |
| DR07-9 | 16 | 22 | 4416 | 4430 | 1016 | 878 | 381 | 164 | 253 | 1343 |
| DR16-14 | 21 | 27 | 8347 | 2481 | 463 | 1266 | 648 | 179 | 426 | 2230 |
| Hydrometallurgical Method | ||||||||||
| DR02-10 | 14 | 15 | 4720 | 2100 | 443 | 1005 | 285 | 192 | 271 | 1483 |
| DR07-9 | 12 | 11 | 3708 | 2330 | 685 | 512 | 98 | 118 | 176 | 1056 |
| DR16-14 | 17 | 15 | 5213 | 1732 | 230 | 1044 | 271 | 159 | 334 | 1709 |
| Recovery Rate ±10 (%) | ||||||||||
| DR02-10 | 81 | 58 | 108 | 85 | 74 | 85 | 63 | 88 | 79 | 86 |
| DR07-9 | 75 | 49 | 84 | 53 | 68 | 58 | 26 | 72 | 70 | 79 |
| DR16-14 | 81 | 57 | 63 | 70 | 50 | 83 | 42 | 89 | 78 | 77 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, E.; González, F.J.; Kuhn, T.; Madureira, P.; Wegorzewski, A.V.; Mirao, J.; Medialdea, T.; Oeser, M.; Miguel, C.; Reyes, J.; et al. Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods. Minerals 2019, 9, 439. https://doi.org/10.3390/min9070439
Marino E, González FJ, Kuhn T, Madureira P, Wegorzewski AV, Mirao J, Medialdea T, Oeser M, Miguel C, Reyes J, et al. Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods. Minerals. 2019; 9(7):439. https://doi.org/10.3390/min9070439
Chicago/Turabian StyleMarino, Egidio, Francisco Javier González, Thomas Kuhn, Pedro Madureira, Anna V. Wegorzewski, Jose Mirao, Teresa Medialdea, Martin Oeser, Catarina Miguel, Jesús Reyes, and et al. 2019. "Hydrogenetic, Diagenetic and Hydrothermal Processes Forming Ferromanganese Crusts in the Canary Island Seamounts and Their Influence in the Metal Recovery Rate with Hydrometallurgical Methods" Minerals 9, no. 7: 439. https://doi.org/10.3390/min9070439





