Re Sulfides from Zhelos and Tokty-Oi Intrusions (East Sayan, Russia)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
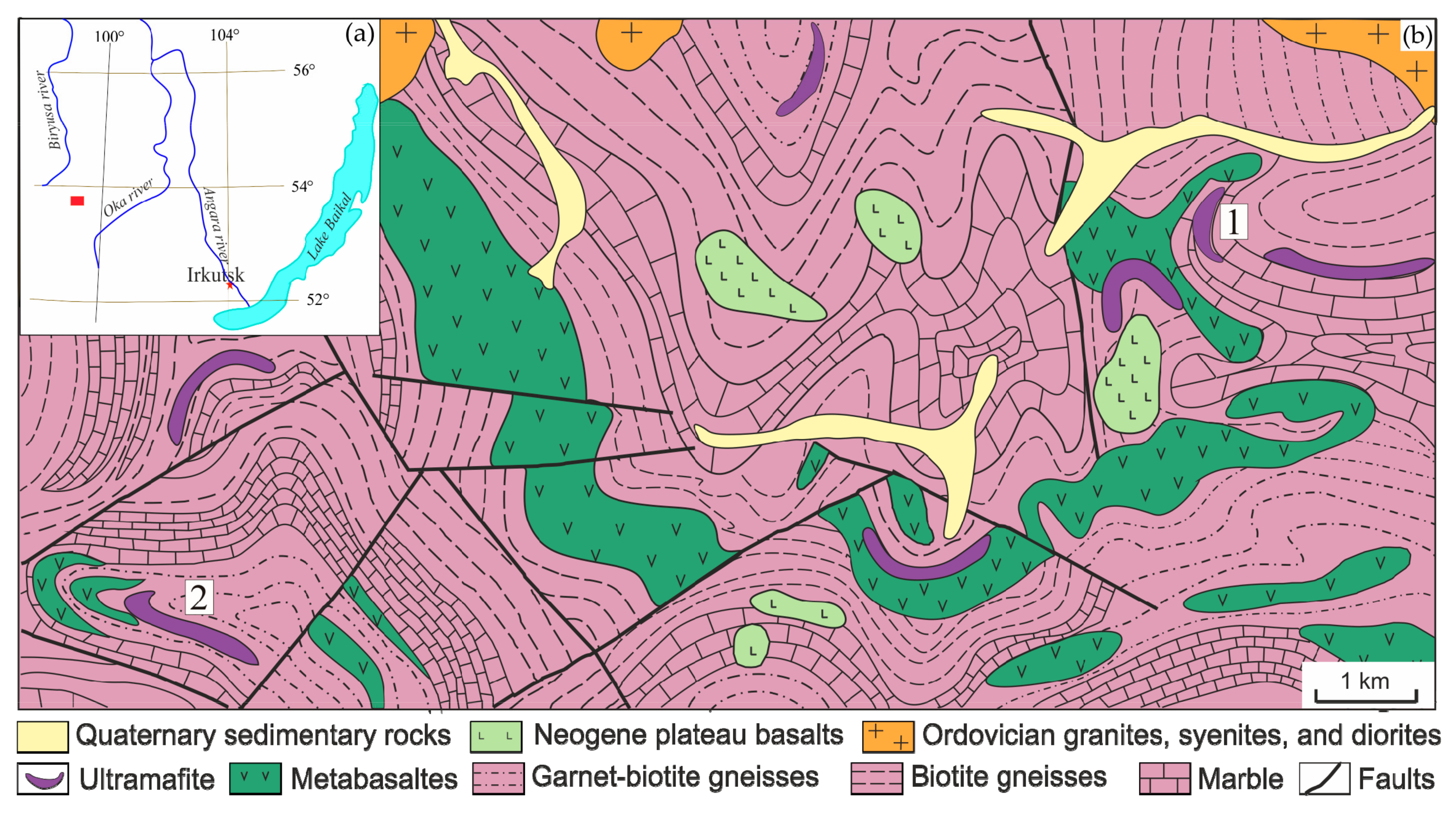
3.1. Geological Background
3.2. Composition of Sulfide Ores
3.3. Samples with Re Sulfide
3.4. Re Sulfide Composition
4. Discussion and Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koide, M.; Hodge, V.; Yang, J.S.; Goldberg, E.D. Determination of rhenium in marine waters and sediments by graphite furnace atomic absorption spectrometry. Anal. Chem. 1987, 59, 1802–1805. [Google Scholar] [CrossRef]
- Lesnov, F.P.; Anoshin, G.N. Correlations between Re and PGE concentrations in rocks, ores, and minerals of mafic-ultramafic associations. Dokl. Earth Sci. 2011, 437, 387–392. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Lednev, A.I. A rhenium–molybdenum–copper sulfide from the Lukkulaisvaara layered intrusion, northern Karelia, Russia. Eur. J. Mineral. 1993, 5, 1227–1233. [Google Scholar] [CrossRef]
- Kojonen, K.K.; Roberts, A.C.; Isomaki, O.P.; Knauf, V.; Johanson, B.; Pakkanen, L. Tarkianite, (Cu,Fe)(Re,Mo)4S8, a new mineral species from the Nitura Mine, Nivala, Finland. Can. Mineral. 2004, 42, 539–544. [Google Scholar] [CrossRef]
- Poplavko, E.M.; Marchukova, I.D.; Zak, S.S. A rhenium mineral in the Dzhezkazgan ore deposit. Dokl. Acad. Nauk SSSR 1962, 146, 433–436. (In Russian) [Google Scholar]
- Häkli, T.A.; Hänninen, E.; Vuorelainen, Y.; Papunen, H. Platinum-group minerals in the Hitura nickel deposit, Finland. Econ. Geol. 1976, 71, 1206–1213. [Google Scholar] [CrossRef]
- Ekstrom, M.; Hálenius, U. A new rhenium rich sulfide from two Swedish localities. Neues Jahrb. Mineral. Mon. 1982, 1, 6–10. [Google Scholar]
- Mitchell, R.H.; Laflamme, J.H.G.; Cabri, L.J. Rhenium sulfide from the Coldwell complex, Northwestern Ontario, Canada. Miner. Mag. 1989, 53, 635–637. [Google Scholar] [CrossRef]
- Marchetto, C.M.L. Platinum-group minerals in the O’Toole (Ni–Cu–Co) deposit, Brazil. Econ. Geol. 1990, 85, 921–927. [Google Scholar] [CrossRef]
- Tarkian, M.; Housley, R.M.; Volborth, A.; Greis, O.; Moh, G.H. Unnamed Re–Mo–Cu sulfide from the Stillwater complex, and crystal chemistry of its synthetic equivalent spinel type (Cu,Fe)(Re,Mo)4S8. Eur. J. Mineral. 1991, 3, 977–982. [Google Scholar] [CrossRef]
- Peltonen, P.; Pakkanen, L.; Johanson, B. Re–Mo–Cu–Os sulphide from the Ekojoki Ni–Cu deposit, SW Finland. Mineral. Petrol. 1995, 52, 257–264. [Google Scholar] [CrossRef]
- Znamensky, W.S.; Korzhinsky, M.A.; Steinberg, G.S.; Tkachenko, S.I.; Yakushev, A.I.; Laputina, U.P.; Bryzgalok, I.A.; Samotoin, N.D.; Magazina, L.O.; Kuzmina, O.V.; et al. Rheniite, ReS2, the natural rhenium disulfide from fumaroles of Kudryavy volcano, Iturup isl., Kurily Islands. Zapiski RMO. 2005, 34, 32–40. (In Russian) [Google Scholar]
- Tessalina, S.G.; Yudovskaya, M.A.; Chaplygin, I.V.; Brick, J.-L.; Capmas, F. Sources of unique rhenium enrichment in fumaroles and sulphides at Kudryavy volcano. Geochim. Cosmochim. Acta 2008, 72, 889–909. [Google Scholar] [CrossRef]
- Lavrov, O.B.; Kulashevich, L.V. The first finds of rhenium minerals in Karelia. Dokl. Earth Sci. 2010, 432, 598–601. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-F.; Prichard, H.M.; Fisher, P.C. The timing and formation of platinum-group minerals from the Creighton Ni–Cu–Platinum-Group element sulfide deposit, Sudbury, Canada: Early crystallization of PGE-rich sulfarsenides. Econ. Geol. 2010, 105, 1071–1096. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G.; Fiorentini, M.L.; Locmelis, M.; Kollegger, P.; Thalhammer, O.A.R. Mineralogical hosts of platinum group elements (PGE) and rhenium in the magmatic Ni–Fe–Cu sulfide deposits of the Ivrea Verbano Zone (Italy): An electron microprobe study. N. Jb. Miner. Abh. 2014, 191, 169–187. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Melfos, V.; Spry, P.G.; Bindi, L.; Kartal, T.; Arikas, K.; Moritz, R.; Ortell, M. Rhenium-rich molybdenite and rheniite in the Pagoni Rachi Mo–Cu–Te–Ag–Au prospect, northern Greece: Implications for the Re geochemistry of porphyry-style Cu–Mo and Mo mineralization. Can. Mineral. 2009, 47, 1013–1036. [Google Scholar] [CrossRef]
- Voudouris, P.C.; Melfos, V.; Spry, P.G.; Bindi, L.; Kartal, T.; Arikas, K.; Moritz, R.; Ortell, M.; Kartal, T. Extremely Re-rich molybdenite from porphyry Cu–Mo–Au prospects in Northeastern Greece: Mode of occurrence, causes of enrichment, and implications for gold exploration. Minerals 2013, 3, 165–191. [Google Scholar] [CrossRef]
- Mekhonoshin, A.S.; Ernst, R.; Söderlund, U.; Hamilton, M.A.; Kolotilina, T.B.; Izokh, A.E.; Polyakov, G.V.; Tolstykh, N.D. Relationship between platinum-bearing ultramafic-mafic intrusions and large igneous provinces (exemplified by the Siberian Craton). Russ. Geol. Geophys. 2016, 57, 822–833. [Google Scholar] [CrossRef]
- Kolotilina, T.B.; Mekhonoshin, A.S.; Orsoev, D.A. PGE distribution in sulfide ores from ultramafic massifs of the central East Sayan Mountains, Southern Siberia, Russia. Geol. Ore Depos. 2016, 58, 20–36. [Google Scholar] [CrossRef]
- Dedeshko M., P.; Satpaeva, T.A.; Fain, E.E. Chemical composition of a rhenium mineral from the Dzhezkazgan ore. Vestn. Akad Nauk Kazakh S.S.R. 1964, 20, 47–53. (In Russian) [Google Scholar]
- Prichard, H.M.; Hutchinson, D.; Fisher, P.C. Petrology and crystallization history of multiphase sulfide droplets in a mafic dike from Uruguay: implications for the origin of Cu–Ni–PGE sulfide deposits. Econ. Geol. 2004, 99, 365–376. [Google Scholar] [CrossRef]
- Sinyakova, E.F.; Kosyakov, V.I. Experimental modeling of zoning in copper–nickel sulfide ores. Dokl. Earth Sci. 2007, 417, 1380–1385. [Google Scholar] [CrossRef]
- Dare, S.A.S.; Barnes, S.-F.; Prichard, H.M. The distribution of platinum group elements (PGE) and other chalcophile elements among sulfides from the Creighton Ni–Cu–PGE sulfide deposit, Sudbury, Canada, and the origin of palladium in pentlandite. Miner. Depos. 2010, 45, 765–793. [Google Scholar] [CrossRef]
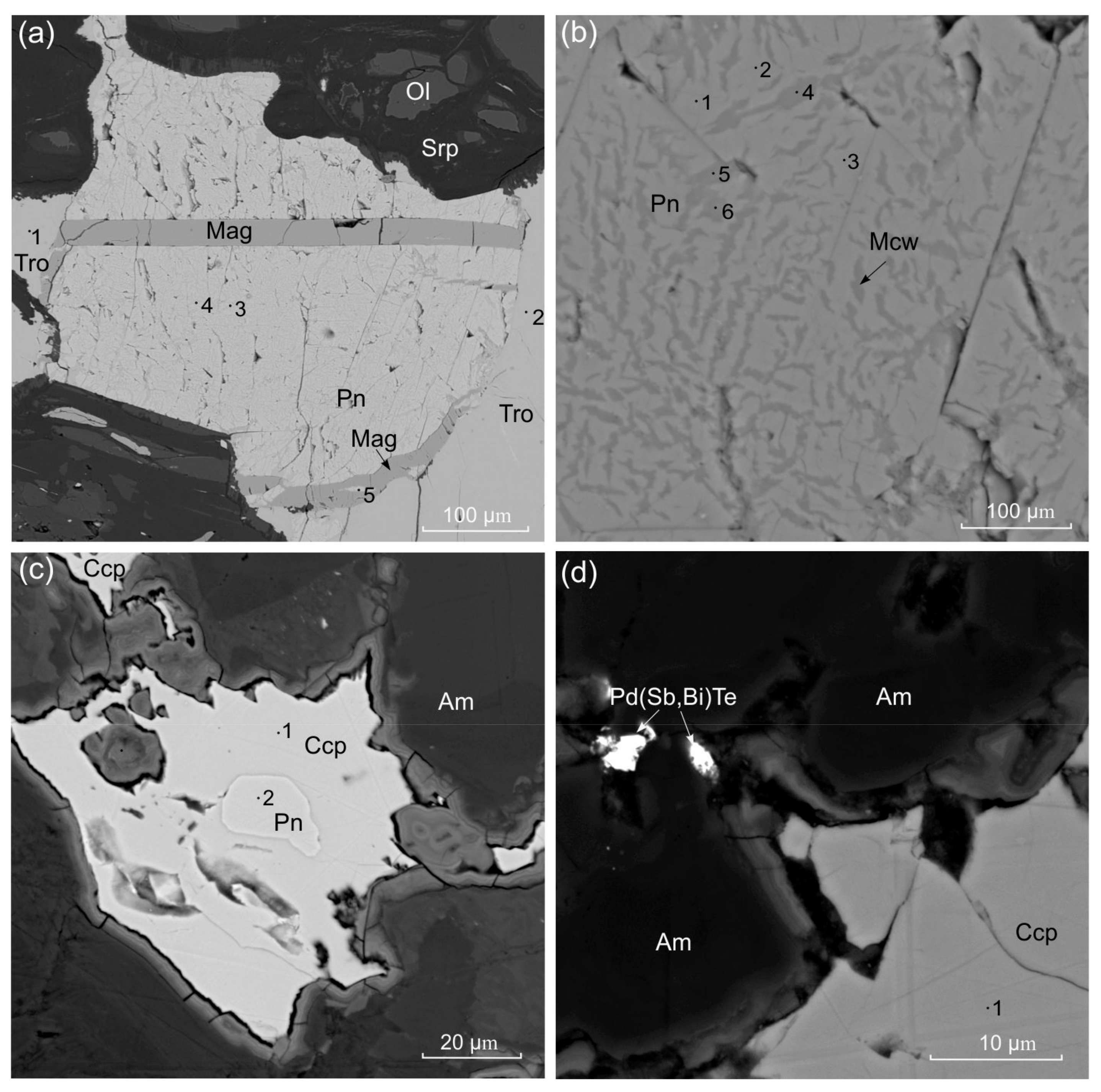
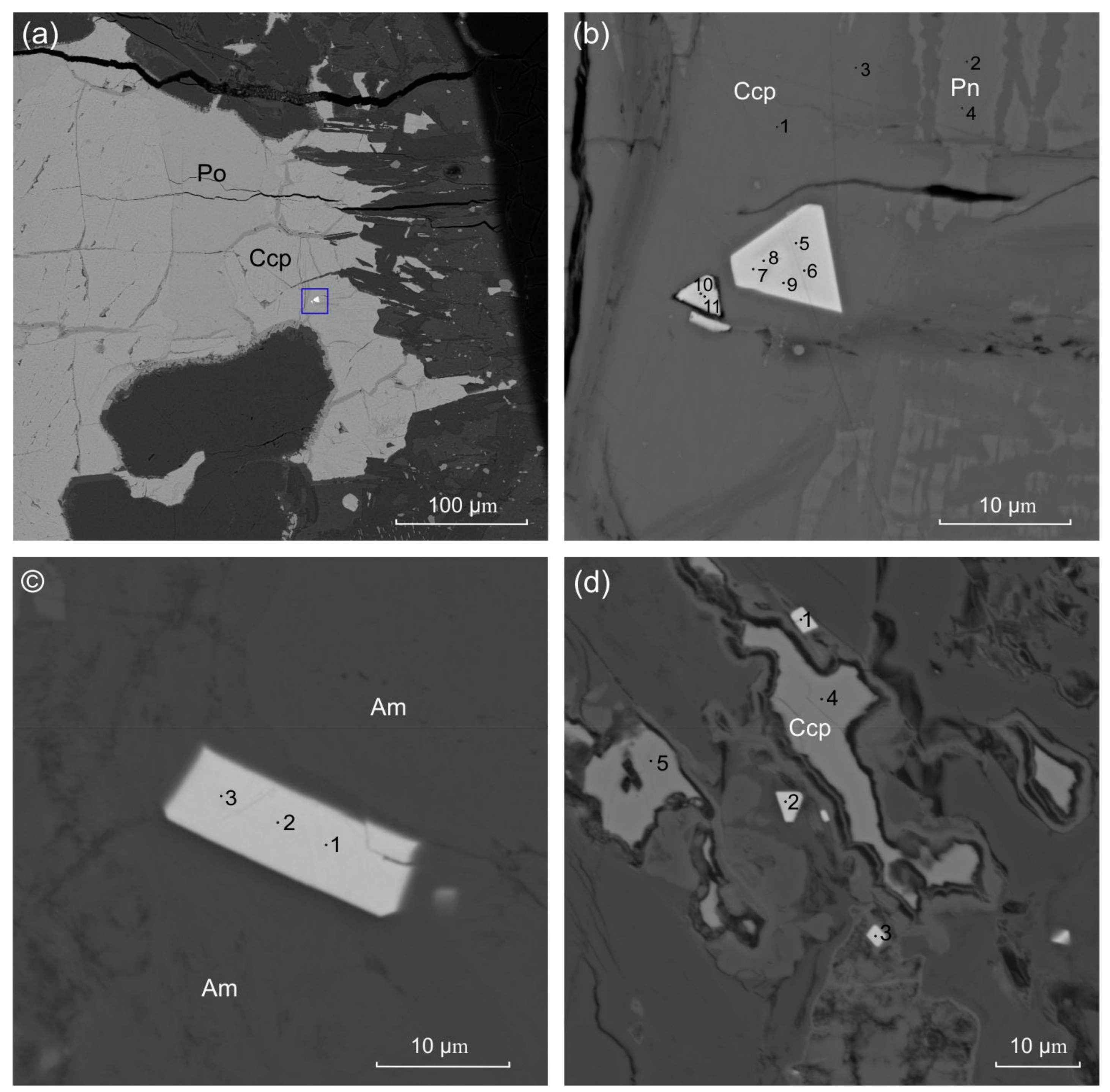

| Sample | Figure | Point | Fe | Co | Ni | Cu | S | Total |
|---|---|---|---|---|---|---|---|---|
| 09-6 | 2(a) | 1 | 64.05 | bdl | bdl | bdl | 36.33 | 100.38 |
| 09-6 | 2(a) | 2 | 63.64 | bdl | bdl | bdl | 36.22 | 99.86 |
| 09-6 | 2(a) | 3 | 38.34 | 0.6 | 27.97 | bdl | 33.52 | 100.43 |
| 09-6 | 2(a) | 4 | 39.2 | 0.98 | 25.77 | bdl | 32.98 | 98.93 |
| 09-6 | 2(a) | 5 | 35.65 | 0.9 | 30.16 | bdl | 33.09 | 99.8 |
| 09-6 | 2(b) | 1 | 35.2 | 0.91 | 30.85 | bdl | 33.34 | 100.3 |
| 09-6 | 2(b) | 2 | 35.52 | 0.78 | 31.02 | bdl | 33.67 | 100.99 |
| 09-6 | 2(b) | 3 | 34.91 | 0.9 | 30.9 | bdl | 33.35 | 100.06 |
| 09-6 | 2(b) | 4 | 49.88 | 0.96 | 11.87 | 0.83 | 35.36 | 98.9 |
| 09-6 | 2(b) | 5 | 49.17 | 0.99 | 11.34 | 1.65 | 35.04 | 98.19 |
| 09-6 | 2(b) | 6 | 44.62 | 1.06 | 17.66 | bdl | 35.01 | 98.35 |
| 06-2 | 2(с) | 1 | 27.51 | 1.97 | 37.27 | bdl | 33.08 | 99.83 |
| 06-2 | 2(с) | 2 | 31.5 | bdl | bdl | 33.56 | 34.55 | 99.62 |
| 06-2 | 2(d) | 1 | 30.47 | bdl | bdl | 34.45 | 34.4 | 99.32 |
| 09-6 | 3(b) | 1 | 31.09 | bdl | bdl | 33.66 | 34.68 | 99.43 |
| 09-6 | 3(b) | 2 | 34.02 | 1.49 | 31.01 | bdl | 33.46 | 99.98 |
| 09-6 | 3(b) | 3 | 31.16 | bdl | 0.84 | 32.74 | 34.99 | 99.73 |
| 09-6 | 3(b) | 4 | 33.68 | 0.93 | 31.41 | bdl | 32.61 | 98.63 |
| 06-2 | 3(d) | 4 | 31.3 | bdl | bdl | 34.2 | 34.28 | 99.78 |
| 06-2 | 3(d) | 5 | 31.13 | bdl | bdl | 33.57 | 34.66 | 99.36 |
| Sample | Figure | Point | Fe | Cu | Os | Mo | Re | S | Total | ΣMe/S1 | Re/Mo1 | Re/Cu1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 09-6 | 3b | 5 | 3.54 | 4.92 | 4.48 | 13.05 | 47.00 | 27.91 | 100.90 | 0.63 | 1.86 | 3.28 |
| 09-6 | 3b | 6 | 3.17 | 4.87 | 3.98 | 12.14 | 48.27 | 27.67 | 100.10 | 0.63 | 2.05 | 3.41 |
| 09-6 | 3b | 7 | 3.42 | 7.22 | 4.93 | 9.38 | 49.76 | 25.32 | 100.03 | 0.72 | 2.73 | 2.37 |
| 09-6 | 3b | 8 | 5.43 | 8.51 | 3.24 | 7.34 | 49.45 | 26.22 | 100.19 | 0.72 | 3.47 | 2.0 |
| 09-6 | 3b | 9 | 2.75 | 5.75 | 3.65 | 9.35 | 51.38 | 27.18 | 100.06 | 0.63 | 2.83 | 3.07 |
| 09-6 | 3b | 10 | 5.56 | 6.15 | 3.39 | 11.84 | 46.60 | 26.49 | 100.03 | 0.71 | 2.03 | 2.60 |
| 09-6 | 3b | 11 | 5.07 | 6.39 | 4.75 | 12.24 | 45.57 | 26.24 | 100.27 | 0.72 | 1.92 | 2.45 |
| 09-6 | 3b | 12 | 15.69 | 13.12 | 2.88 | 6.62 | 32.53 | 28.76 | 99.60 | 0.77 | 2.53 | 0.85 |
| 06-2 | 3c | 1 | 3.82 | 6.99 | bdl | bdl | 57.61 | 31.24 | 99.66 | 0.50 | 2.83 | |
| 06-2 | 3c | 2 | 3.41 | 6.2 | bdl | bdl | 57.81 | 31.89 | 99.32 | 0.47 | 3.20 | |
| 06-2 | 3c | 3 | 4.74 | 6.7 | bdl | bdl | 58.50 | 30.04 | 99.99 | 0.54 | 3.00 | |
| 06-2 | 3d | 1 | 1.77 | 6.36 | bdl | 5.85 | 56.48 | 29.32 | 99.77 | 0.54 | 4.97 | 3.05 |
| 06-2 | 3d | 2 | 1.37 | 5.59 | bdl | 4.89 | 58.39 | 29.13 | 99.37 | 0.52 | 6.15 | 3.59 |
| 06-2 | 3d | 3 | 1.83 | 6.39 | bdl | bdl | 60.20 | 30.97 | 99.39 | 0.47 | 3.24 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolotilina, T.B.; Mekhonoshin, A.S.; Orsoev, D.A. Re Sulfides from Zhelos and Tokty-Oi Intrusions (East Sayan, Russia). Minerals 2019, 9, 479. https://doi.org/10.3390/min9080479
Kolotilina TB, Mekhonoshin AS, Orsoev DA. Re Sulfides from Zhelos and Tokty-Oi Intrusions (East Sayan, Russia). Minerals. 2019; 9(8):479. https://doi.org/10.3390/min9080479
Chicago/Turabian StyleKolotilina, Tatiana B., Aleksey S. Mekhonoshin, and Dmitriy A. Orsoev. 2019. "Re Sulfides from Zhelos and Tokty-Oi Intrusions (East Sayan, Russia)" Minerals 9, no. 8: 479. https://doi.org/10.3390/min9080479







