Analysis of the Application Potential of Coffee Oil as an Ilmenite Flotation Collector
Abstract
:1. Introduction
2. Materials and Methods
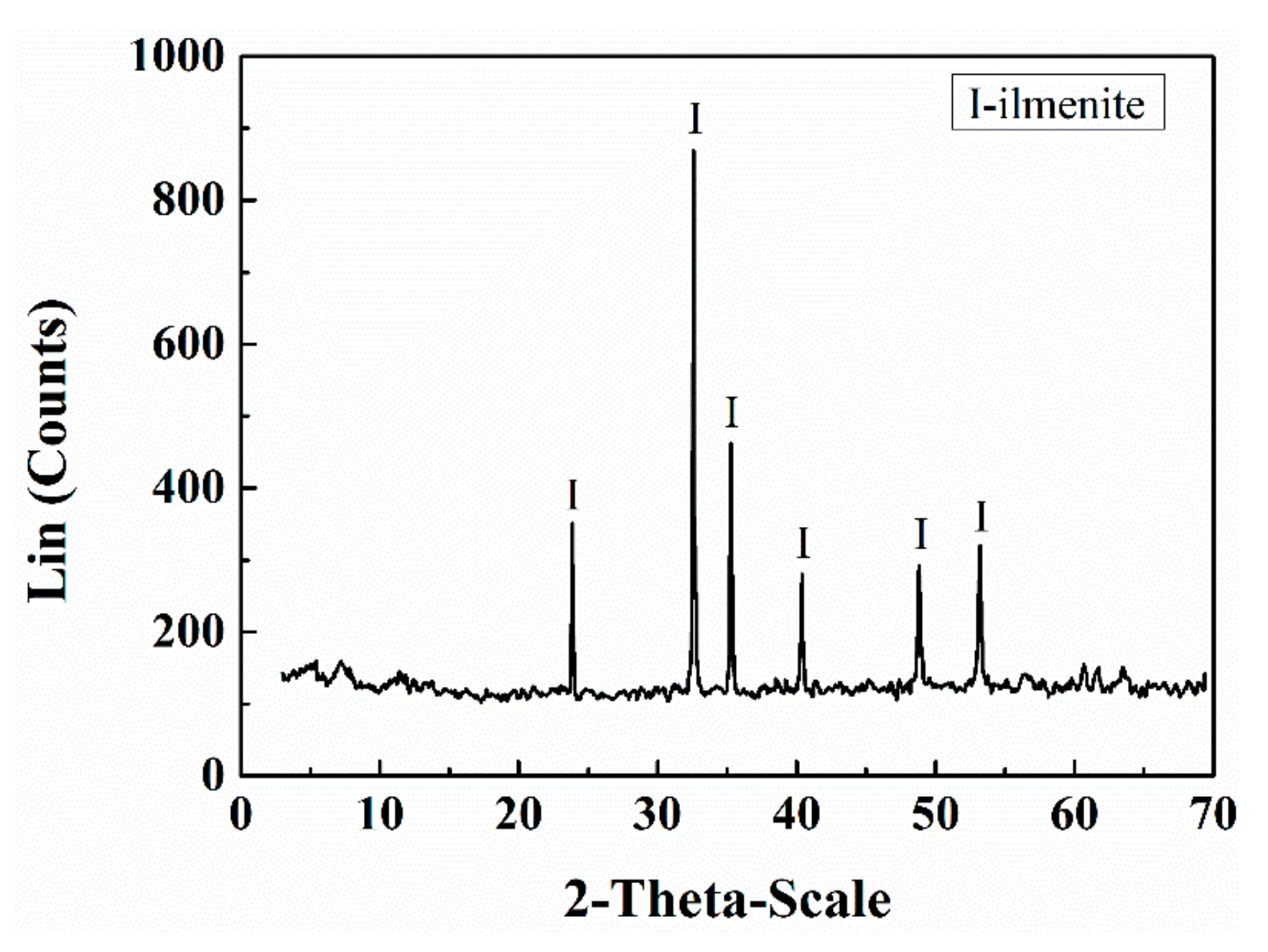
2.1. Materials and Reagents
2.2. Methods
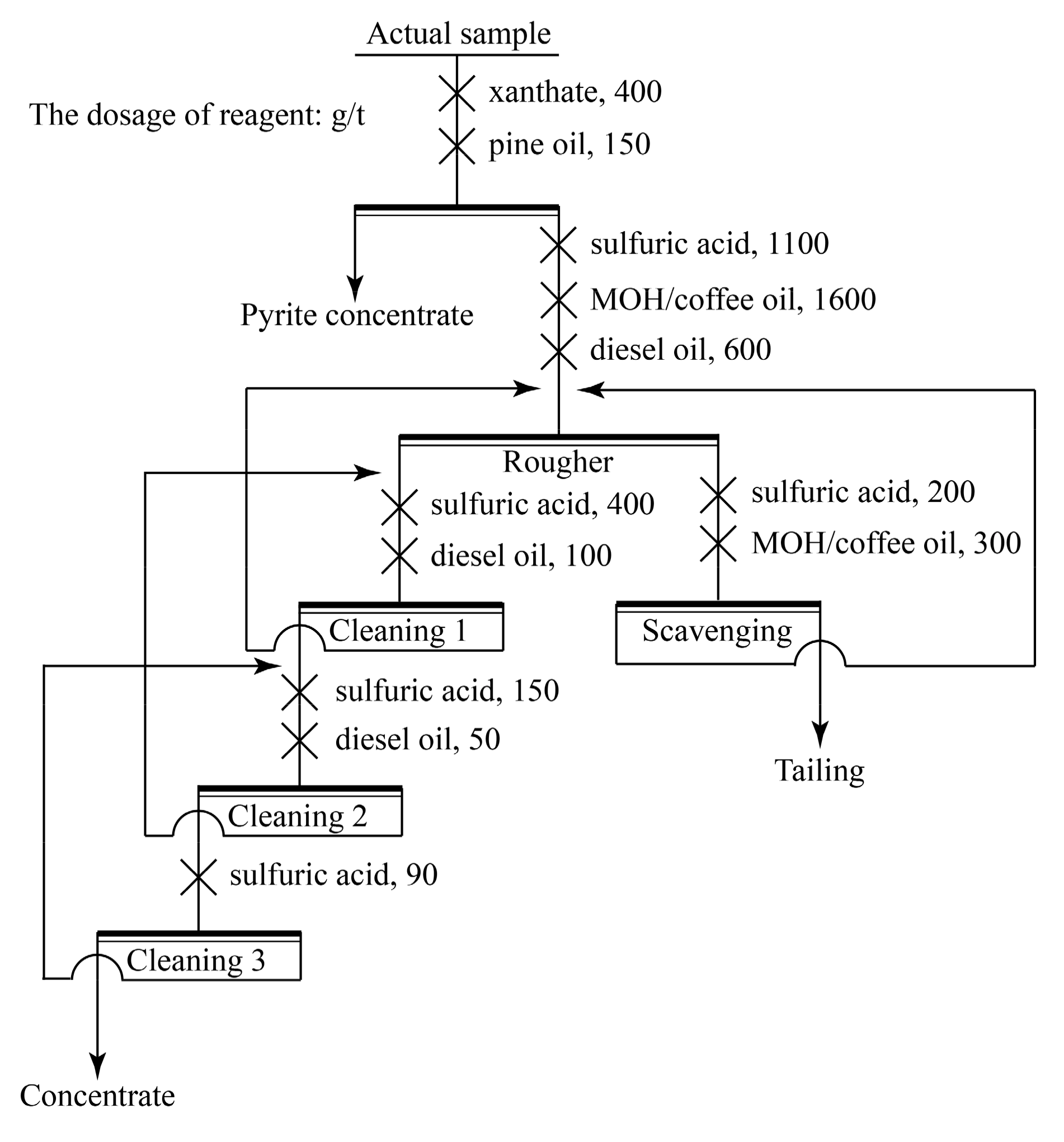
2.2.1. Actual Ilmenite Flotation
2.2.2. Zeta Potential Measurements
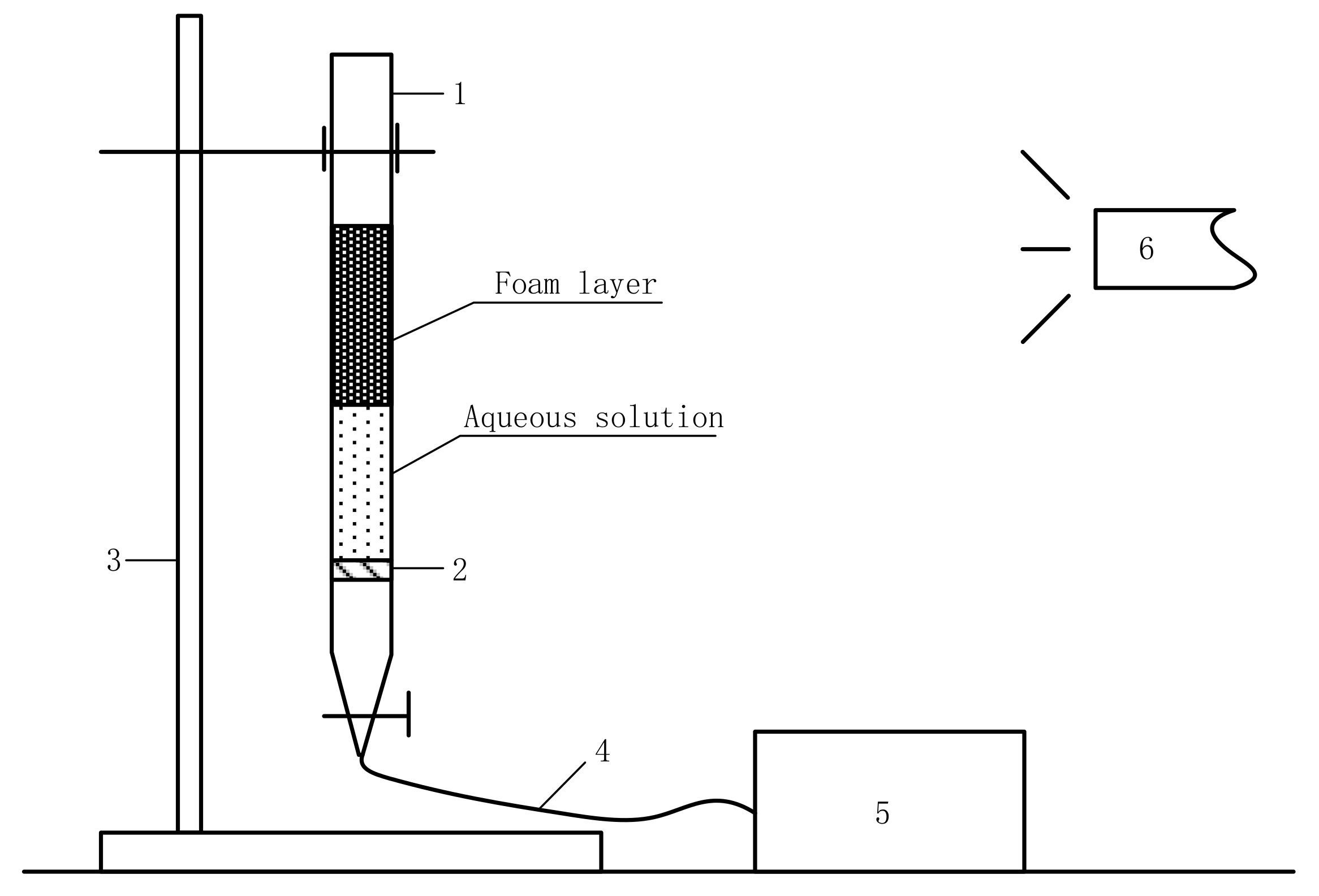
2.2.3. Foam Property Measurements
3. Results and Discussion
3.1. Zeta Potential Measurements
3.2. Foam Property Measurements
3.3. Actual Ilmenite Flotation
4. Conclusions
- (1)
- In neutral pulp solution (pH 6.7), both MOH and coffee oil can produce strong chemical adsorption on the ilmenite surface. Their solutions have excellent foaming properties and the foam is very stable with little difference between the two.
- (2)
- In strong acid slurry solution (pH 2.8), the adsorption capacity of coffee oil on the ilmenite surface is much stronger than that of MOH and the adsorption is mainly electrostatic. The foaming performance of the coffee oil solution is also much stronger than that of the MOH solution.
- (3)
- In laboratory experiments, it is possible to replace MOH with coffee oil as a collector for ilmenite flotation, and the grade (46.83%) and recovery rate (90.22%) of TiO2 in the concentrate product meet the experimental requirements. In addition, coffee oil has obvious advantages in environmental protection and further optimization.
Author Contributions
Funding
Conflicts of Interest
References
- Barbosa, M.D.G.; Scholz, M.B.D.; Kitzberger, C.S.G.; Benassi, M.D. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Lee, J. Emotions elicited while drinking coffee: A cross-cultural comparison between Korean and Chinese consumers. Food Qual. Prefer. 2019, 76, 160–168. [Google Scholar] [CrossRef]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Coffee oil as a natural surfactant. Food Chem. 2019, 295, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Kamil, M.; Ramadan, K.M.; Awad, O.I.; Ibrahim, T.K.; Inayat, A.; Ma, X. Environmental impacts of biodiesel production from waste spent coffee grounds and its implementation in a compression ignition engine. Sci. Total Environ. 2019, 675, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Obruca, S.; Petrik, S.; Benesova, P.; Svoboda, Z.; Eremka, L.; Marova, I. Utilization of oil extracted from spent coffee grounds for sustainable production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.G.; Ferreira, N.J.; Oliveira, A.L.; Cabral, F.A.; Meirelles, A.J.A. High pressure phase equilibrium of the crude green coffee oil-CO2-ethanol system and the oil bioactive compounds. J. Supercrit. Fluids 2018, 133, 49–57. [Google Scholar] [CrossRef]
- Mueanmas, C.; Nikhom, R.; Petchkaew, A.; Iewkittayakorn, J.; Prasertsit, K. Extraction and esterification of waste coffee grounds oil as non-edible feedstock for biodiesel production. Renew. Energy 2019, 133, 1414–1425. [Google Scholar] [CrossRef]
- McNutt, J.; He, Q. Spent coffee grounds: A review on current utilization. J. Ind. Eng. Chem. 2019, 71, 78–88. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simoes, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Xuan, S.H.; Lee, N.H.; Park, S.N. Atractyligenin, a terpenoid isolated from coffee silverskin, inhibits cutaneous photoaging. J. Photochem. Photobiol. B Biol. 2019, 194, 166–173. [Google Scholar] [CrossRef]
- Thligene, N.; Mezzapesa, G.N.; Mondelli, D.; Trani, A.; Veronico, P.; Melillo, M.T.; Dumontet, S.; Miano, T.; Sasanelli, N. Effect of coffee silver skin and brewers’ spent grain in the control of root-knot nematodes. Helminthologia 2019, 56, 30–41. [Google Scholar] [CrossRef]
- Wong, T.H.T.; Sui, Z.; Rangan, A.; Louie, J.C.Y. Discrepancy in socioeconomic status does not fully explain the variation in diet quality between consumers of different coffee types. Eur. J. Nutr. 2018, 57, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Cacciotti, I.; Mori, S.; Cherubini, V.; Nanni, F. Eco-sustainable systems based on poly(lactic acid), diatomite and coffee grounds extract for food packaging. Int. J. Biol. Macromol. 2018, 112, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Sun, W.; Hu, Y. The flotation and adsorption of mixed collectors on oxide and silicate minerals. Adv. Colloid Interface Sci. 2017, 250, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xia, W. Role of particle shape in the floatability of mineral particle: An overview of recent advances. Powder Technol. 2017, 317, 104–116. [Google Scholar] [CrossRef]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef]
- Souto de Medeiros, A.R.; Magalhaes Baltar, C.A. Importance of collector chain length in flotation of fine particles. Miner. Eng. 2018, 122, 179–184. [Google Scholar] [CrossRef]
- Nakhaei, F.; Irannajad, M. Reagents types in flotation of iron oxide minerals: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 89–124. [Google Scholar] [CrossRef]
- He, T.; Li, H.; Jin, J.; Peng, Y.; Wang, Y.; Wan, H. Improving fine molybdenite flotation using a combination of aliphatic hydrocarbon oil and polycyclic aromatic hydrocarbon. Results Phys. 2019, 12, 1050–1055. [Google Scholar] [CrossRef]
- Lu, J.; Tong, Z.; Yuan, Z.; Li, L. Investigation on flotation separation of chalcopyrite from arsenopyrite with a novel collector: N-Butoxycarbonyl-O-Isobutyl Thiocarbamate. Miner. Eng. 2019, 137, 118–123. [Google Scholar] [CrossRef]
- Huang, Z.; Cheng, C.; Liu, Z.; Zeng, H.; Feng, B.; Zhong, H.; Luo, W.; Hu, Y.; Guo, Z.; He, G.; et al. Utilization of a new Gemini surfactant as the collector for the reverse froth flotation of phosphate ore in sustainable production of phosphate fertilizer. J. Clean. Prod. 2019, 221, 108–112. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, X.; He, Y.; Lu, C.; Gu, Q. Three-dimensional multiple-relaxation-time lattice Boltzmann models for single-phase and solid-liquid phase-change heat transfer in porous media at the REV scale. Appl. Therm. Eng. 2019, 152, 319–337. [Google Scholar] [CrossRef]
- Wang, D.; Jiao, F.; Qin, W.; Wang, X. Effect of surface oxidation on the flotation separation of chalcopyrite and galena using sodium humate as depressant. Sep. Sci. Technol. 2018, 53, 961–972. [Google Scholar] [CrossRef]
- Nie, X.; Feng, S.; Shudu, Z.; Quan, G. Simulation study on the dynamic ventilation control of single head roadway in high-altitude mine based on thermal comfort. Adv. Civ. Eng. 2019, 2019, 12. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z.; Lai, X. Adsorption mechanism of lead ions at ilmenite/water interface and its influence on ilmenite flotability. J. Ind. Eng. Chem. 2017, 53, 285–293. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Liu, Y.; Han, Y. Flotation separation of ilmenite from titanaugite using mixed collectors. Sep. Sci. Technol. 2016, 51, 1840–1846. [Google Scholar] [CrossRef]
- Fan, G.; Liu, J.; Cao, Y.; Huo, T. Optimization of fine ilmenite flotation performed in a cyclonic-static micro-bubble flotation column. Physicochem. Probl. Miner. Process. 2014, 50, 823–834. [Google Scholar]
- Huang, X.; Zhu, T.; Duan, W.; Liang, S.; Li, G.; Xiao, W. Comparative studies on catalytic mechanisms for natural chalcopyrite-induced Fenton oxidation: Effect of chalcopyrite type. J. Hazard. Mater. 2020, 381, 120998. [Google Scholar] [CrossRef]
- Xiao, W.; Cao, P.; Liang, Q.; Zhang, E.; Wang, J. Synergistic adsorption mechanism of styryl phosphoric acid and nonyl alcohol on the rutile surface and effects on flotation. Can. Metall. Q. 2019, 58, 19–27. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, Y.; Yang, J.; Ren, Y.; Yang, W.; Huang, X.; Zhang, L. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface. Langmuir 2019, 35, 9239–9245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Cao, P.; Liang, Q.; Huang, X.; Li, K.; Zhang, Y.; Qin, W.; Qiu, G.; Wang, J. Adsorption behavior and mechanism of Bi(III) ions on rutile-water interface in the presence of nonyl hydroxamic acid. Trans. Nonferrous Met. Soc. China 2018, 28, 348–355. [Google Scholar] [CrossRef]
- Xiao, W.; Fang, C.; Wang, J.; Liang, Q.; Cao, P.; Wang, X.; Zhang, L.; Qiu, G.; Hu, J. The role of EDTA on rutile flotation using Al3+ ions as an activator. Rsc Adv. 2018, 8, 4872–4880. [Google Scholar] [CrossRef]
- Koopal, L.K.; Lee, E.M.; Böhmer, M.R. Adsorption of cationic and anionic surfactants on charged metal oxide surfaces. J. Colloid Interface Sci. 1995, 170, 85–97. [Google Scholar] [CrossRef]
- Acharya, S.; Nayak, A. Separation of D2EHPA and M2EHPA. Hydrometallurgy 1988, 19, 309–320. [Google Scholar] [CrossRef]
- Xiao, W.; Jiao, F.; Zhao, H.B.; Qin, W.Q.; Qiu, G.Z.; Wang, J. Adsorption Structure and Mechanism of Styryl Phosphoric Acid at the Rutile-Water Interface. Minerals 2018, 8, 14. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Fang, S.; Lu, Z.; Ma, C.; Sun, W.; Hu, Y. Anisotropic surface chemistry properties and adsorption behavior of silicate mineral crystals. Adv. Colloid Interface Sci. 2018, 256, 340–351. [Google Scholar] [CrossRef]
- Kosior, D.; Zawala, J.; Krasowska, M.; Malysa, K. Influence of n-octanol and alpha-terpineol on thin film stability and bubble attachment to hydrophobic surface. Phys. Chem. Chem. Phys. 2013, 15, 2586–2595. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y.; Wang, Y.; Xu, M.; Wang, D.; Li, C. Effect of compound collector and blending frother on froth stability and flotation performance of oxidized coal. Powder Technol. 2017, 305, 166–173. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Evans, G.M. The liquid flow force on a particle in the bubble-particle interaction in flotation. J. Colloid Interface Sci. 2002, 246, 100–104. [Google Scholar] [CrossRef]
- Phan, C.A.; Nguyen, A.V.; Evans, G.A. Combining hydrodynamics and molecular kinetics to predict dewetting between a small bubble and a solid surface. J. Colloid Interface Sci. 2006, 29, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Krasowska, M.; Malysa, K. Kinetics of bubble collision and attachment to hydrophobic solids: I. Effect of surface roughness. Int. J. Miner. Process. 2007, 8, 205–216. [Google Scholar] [CrossRef]
- Koh, P.T.L.; Schwarz, M.P. CFD modelling of bubble–particle collision rates and efficiencies in a flotation cell. Miner. Eng. 2003, 16, 1055–1059. [Google Scholar] [CrossRef]


| Sample | TiO2 | FeO | S | SiO2 | Al2O3 | CaO | MgO | Other |
|---|---|---|---|---|---|---|---|---|
| Single | 49.97 | 41.28 | - | 1.68 | 0.51 | 0.13 | 4.26 | 2.17 |
| Actual | 21.68 | 19.45 | 0.85 | 25.65 | 4.68 | 11.12 | 5.39 | 11.18 |
| Composition | Content (%) |
|---|---|
| Palmitic acid (C16:0) | 21.4 |
| Stearic acid (C18:0) | 4.2 |
| Oleic acid (C18:1) | 5.6 |
| Linoleic acid (C18:2) | 26.2 |
| Arachidic acid (C20:0) | 1.3 |
| Polyphenolic compound | About 1.1 |
| Condition∗ | Number | ζ (mV) | Δζ (mV) | Г (mol/L) | |
|---|---|---|---|---|---|
| Coffee oil pH 2.8 | 1 | 19.13 | –33.63 | kC exp(0.00262) | –6.48 |
| 2 | –16.50 | –52.85 | kC exp(0.00412) | –10.20 | |
| 3 | –33.72 | –17.22 | kC exp(0.00134) | –3.32 | |
| Coffee oil pH 6.7 | 4 | –11.59 | –21.31 | kC exp(0.00083) | –2.06 |
| 5 | –32.90 | –30.72 | kC exp(0.00119) | –2.96 | |
| 6 | –42.31 | –9.41 | kC exp(0.00037) | –0.91 | |
| MOH pH 2.8 | 7 | 18.77 | –7.66 | kC exp(0.00058) | –1.48 |
| 8 | 11.11 | –14.63 | kC exp(0.00114) | –2.82 | |
| 9 | 4.14 | –6.97 | kC exp(0.00054) | –1.34 | |
| MOH pH 6.7 | 10 | –11.17 | –34.15 | kC exp(0.00133) | –3.30 |
| 11 | –45.32 | –37.28 | kC exp(0.00145) | –3.60 | |
| 12 | –48.45 | –3.13 | kC exp(0.00012) | –0.30 |
| Collector. | Concentrates | Tailings | Dosage of Collector (g/t) | Dosage of Sulfuric Acid (g/t) | ||
|---|---|---|---|---|---|---|
| β | ε | β | ε | |||
| MOH | 47.37 | 88.50 | 4.19 | 11.50 | 2418 | 1904 |
| Coffee oil | 46.83 | 90.22 | 3.64 | 9.78 | 2146 | 2167 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Xiao, W.; Ma, X.; Li, J.; Chen, L.; Yao, H. Analysis of the Application Potential of Coffee Oil as an Ilmenite Flotation Collector. Minerals 2019, 9, 505. https://doi.org/10.3390/min9090505
Wang S, Xiao W, Ma X, Li J, Chen L, Yao H. Analysis of the Application Potential of Coffee Oil as an Ilmenite Flotation Collector. Minerals. 2019; 9(9):505. https://doi.org/10.3390/min9090505
Chicago/Turabian StyleWang, Sen, Wei Xiao, Xiao Ma, Jiuzhou Li, Lijuan Chen, and Hui Yao. 2019. "Analysis of the Application Potential of Coffee Oil as an Ilmenite Flotation Collector" Minerals 9, no. 9: 505. https://doi.org/10.3390/min9090505





