Bio-Precipitation of Calcium and Magnesium Ions through Extracellular and Intracellular Process Induced by Bacillus Licheniformis SRB2
Abstract
:1. Introduction
2. Materials and Methods
2.1. 16S rDNA-Based Identification of Bacteria
2.2. Preparation of the Liquid Bacterial Seed of SRB2 Strain
2.3. Physiological and Biochemical Characteristics of SRB2 Bacteria
2.3.1. Morphology, Gram Staining, and Ammonia Test
2.3.2. Growth Curve and pH Changes
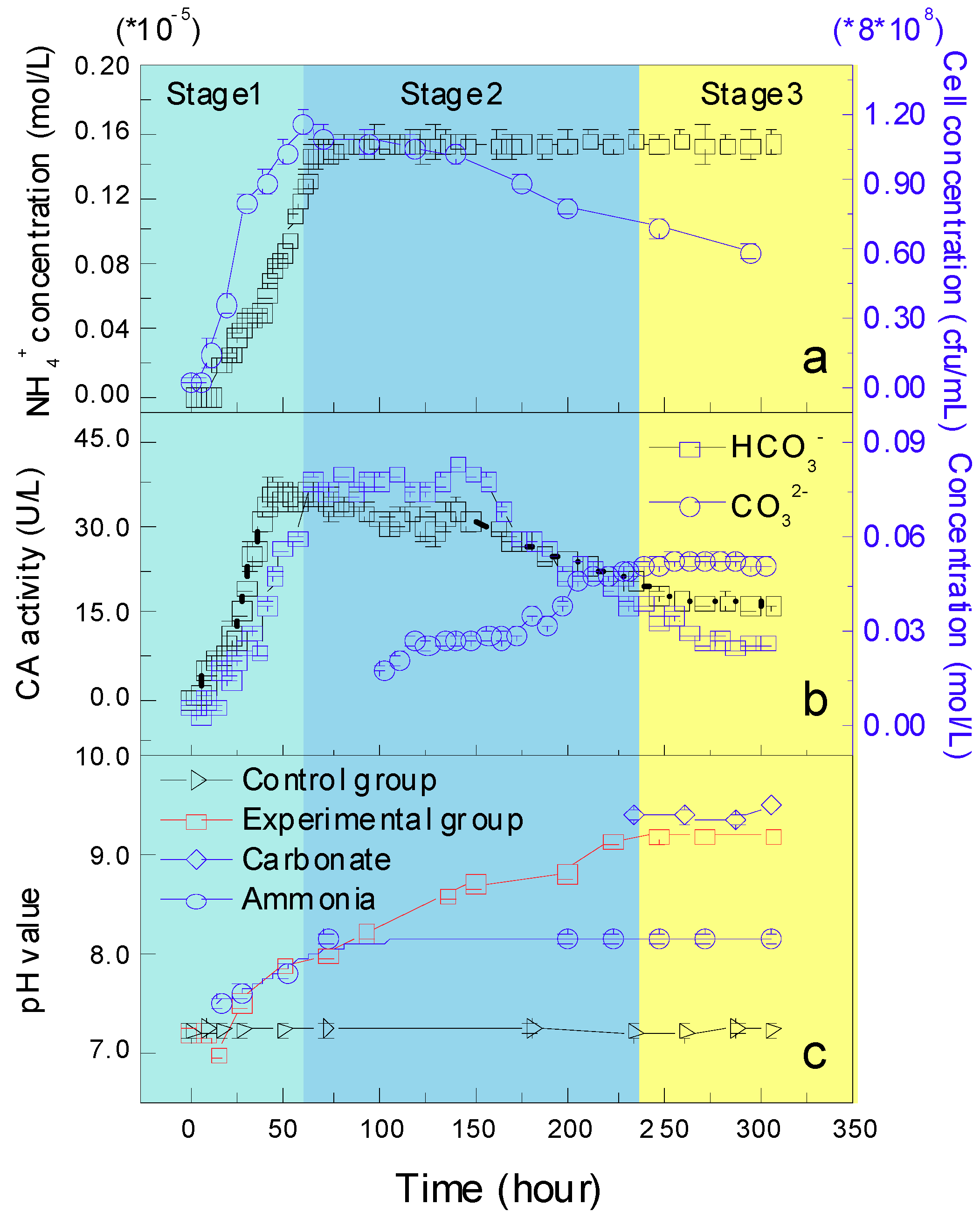
2.3.3. Ammonium Concentration and pH Based on Ammonium
2.3.4. Carbonic Anhydrase Activity
2.3.5. Bicarbonate and Carbonate Ions, and pH Values
2.4. Medium Used to Induce Biominerals
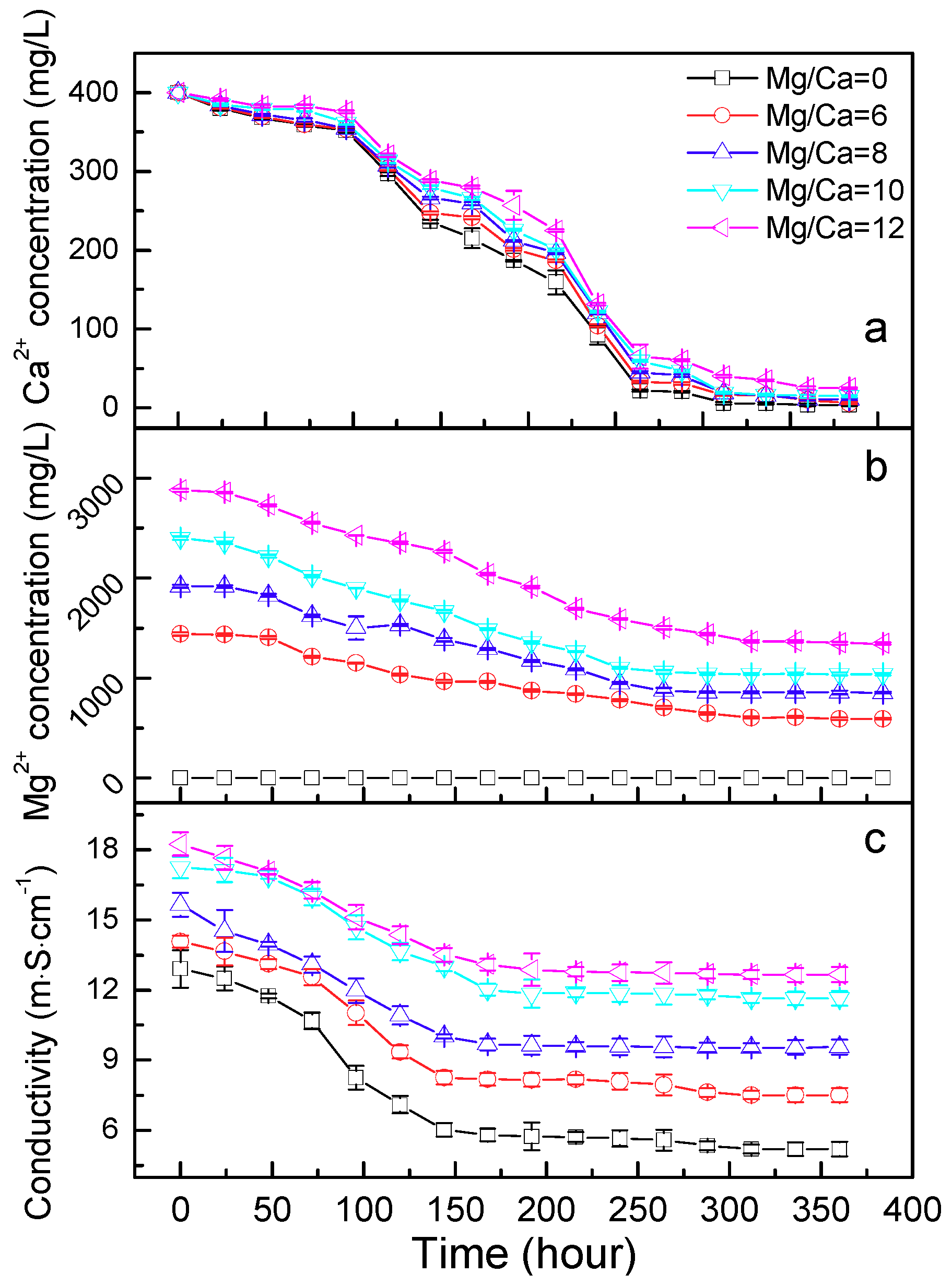
2.5. Ca2+ and Mg2+ Concentration and the Electroconductivity
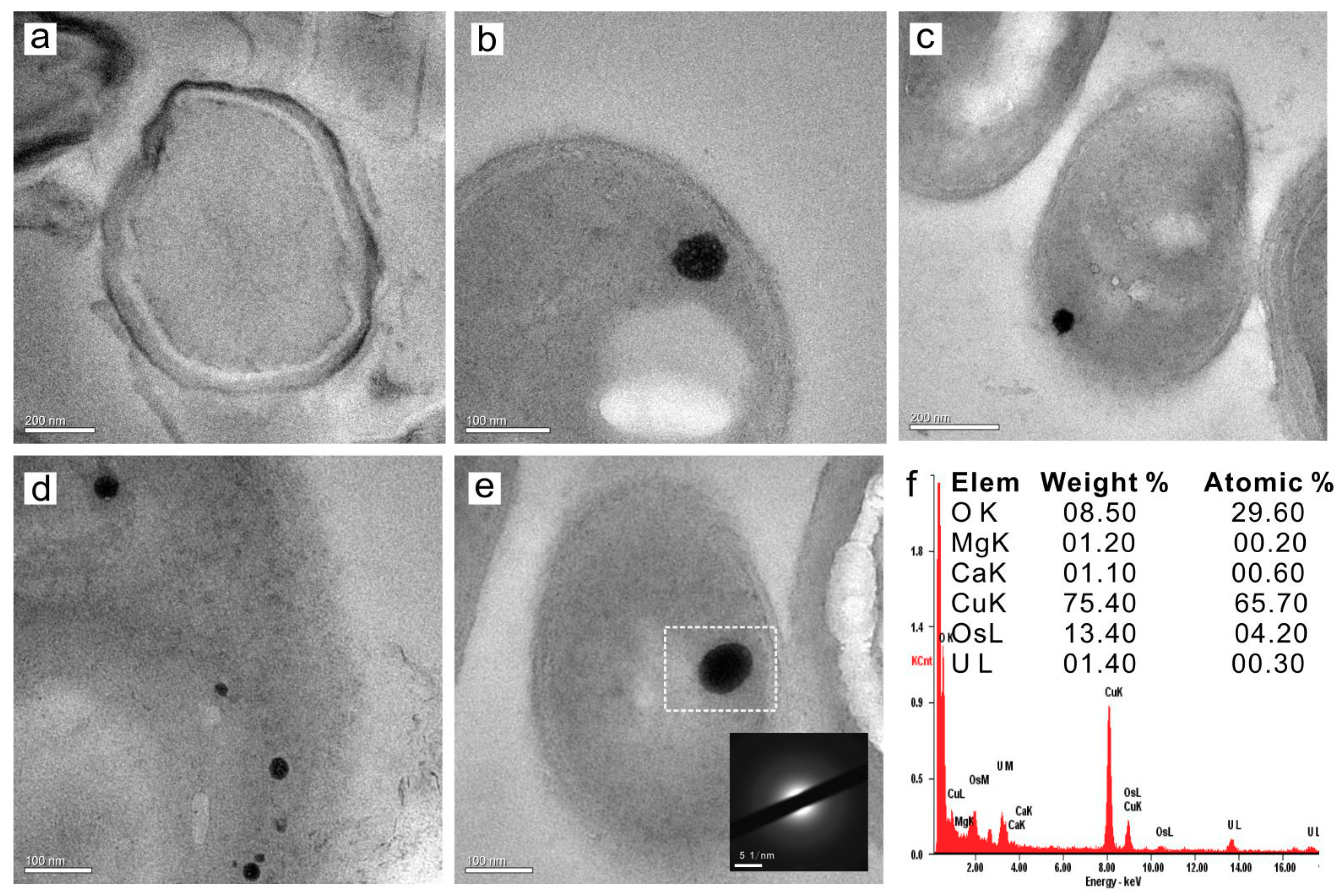
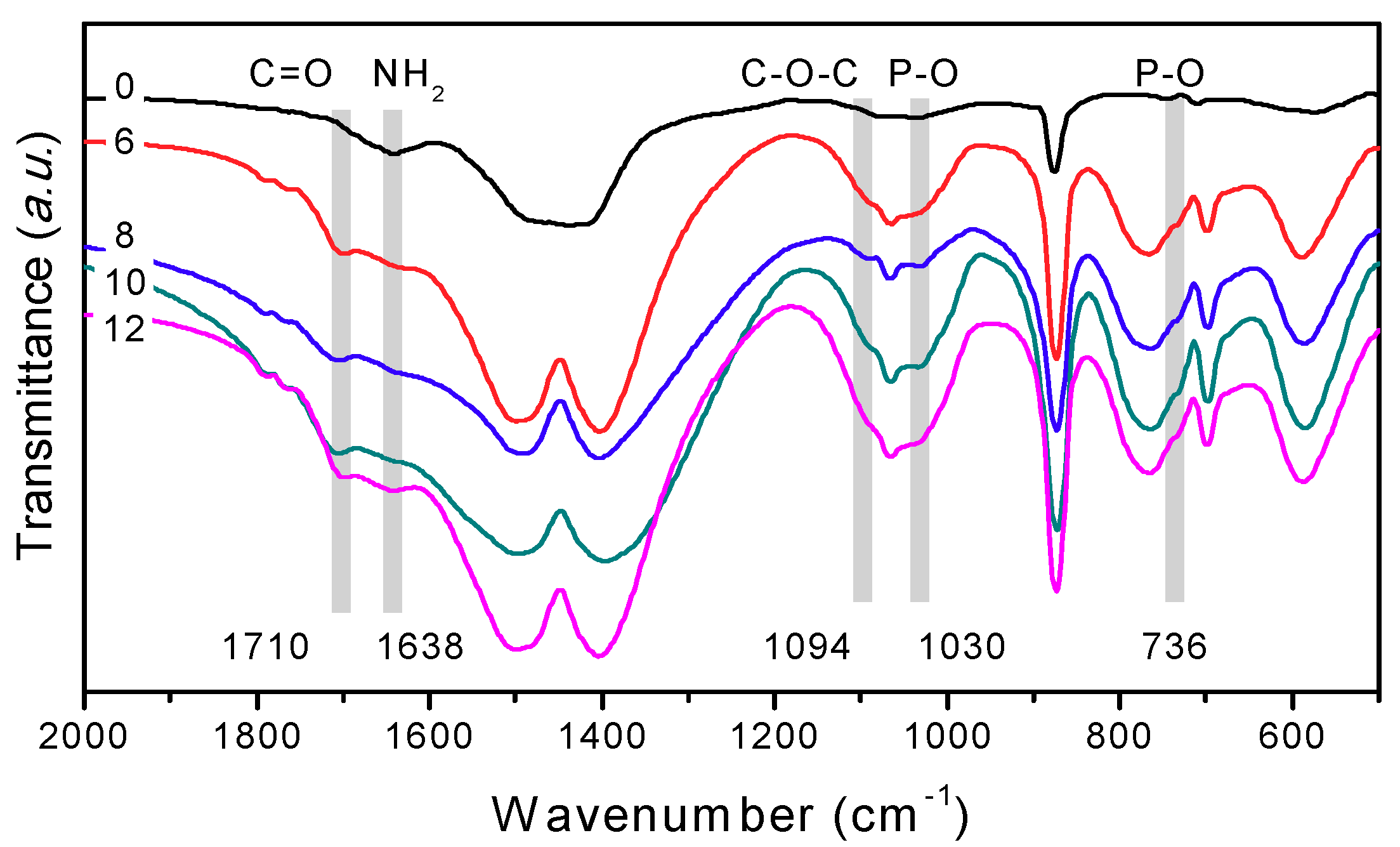
2.6. Characterization of Minerals
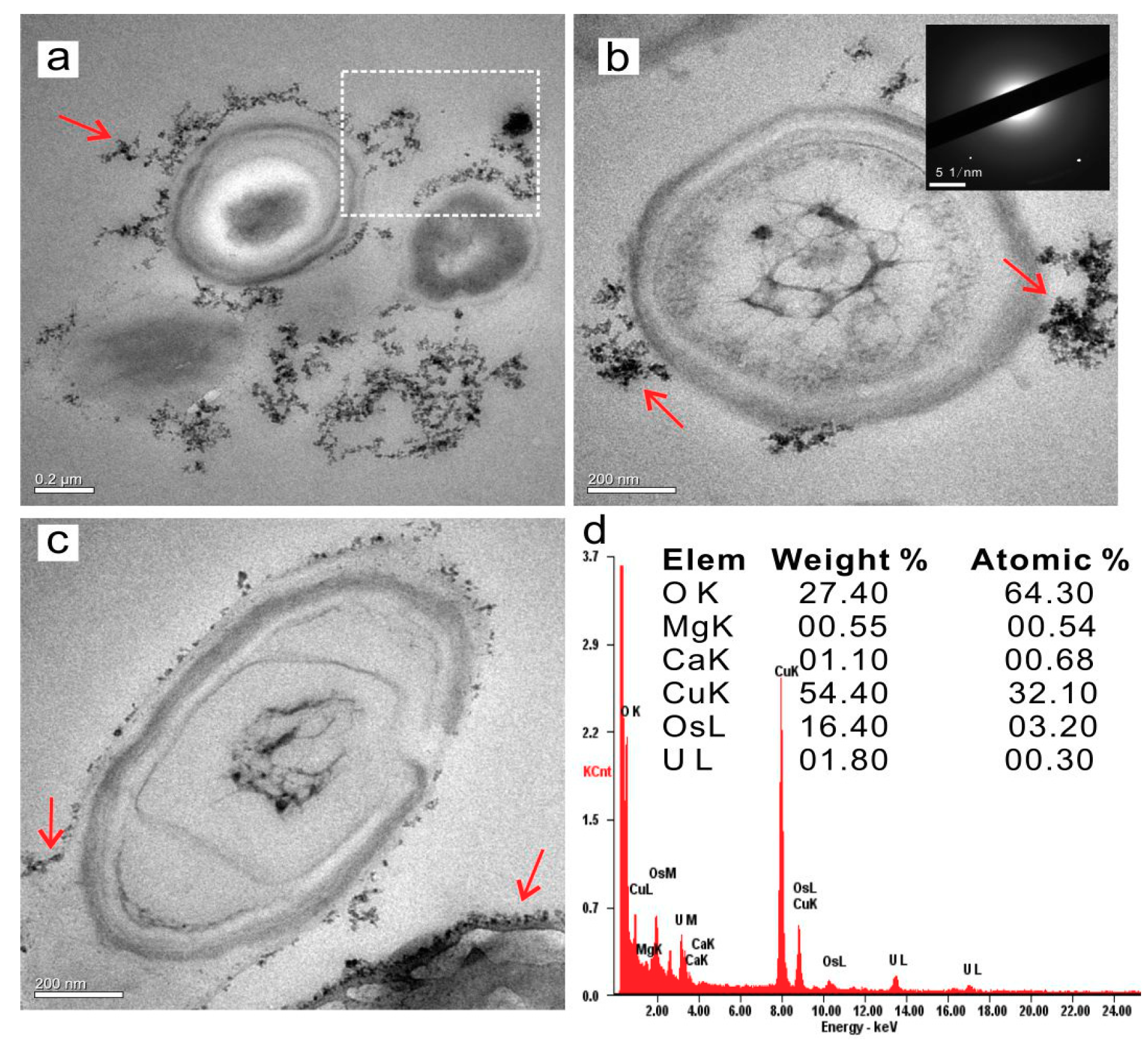
2.7. EPS and Ultrathin Slices of SRB2
3. Results and Discussion
3.1. 16S rDNA Identification
3.2. Physiological and Biochemical Characteristics of SRB2 Bacteria
3.2.1. Morphology, Gram Staining, and Ammonia Test
3.2.2. Growth Curve, CA Activity, the Concentration of NH4+, HCO3− and CO32− Ions, and pH Changes
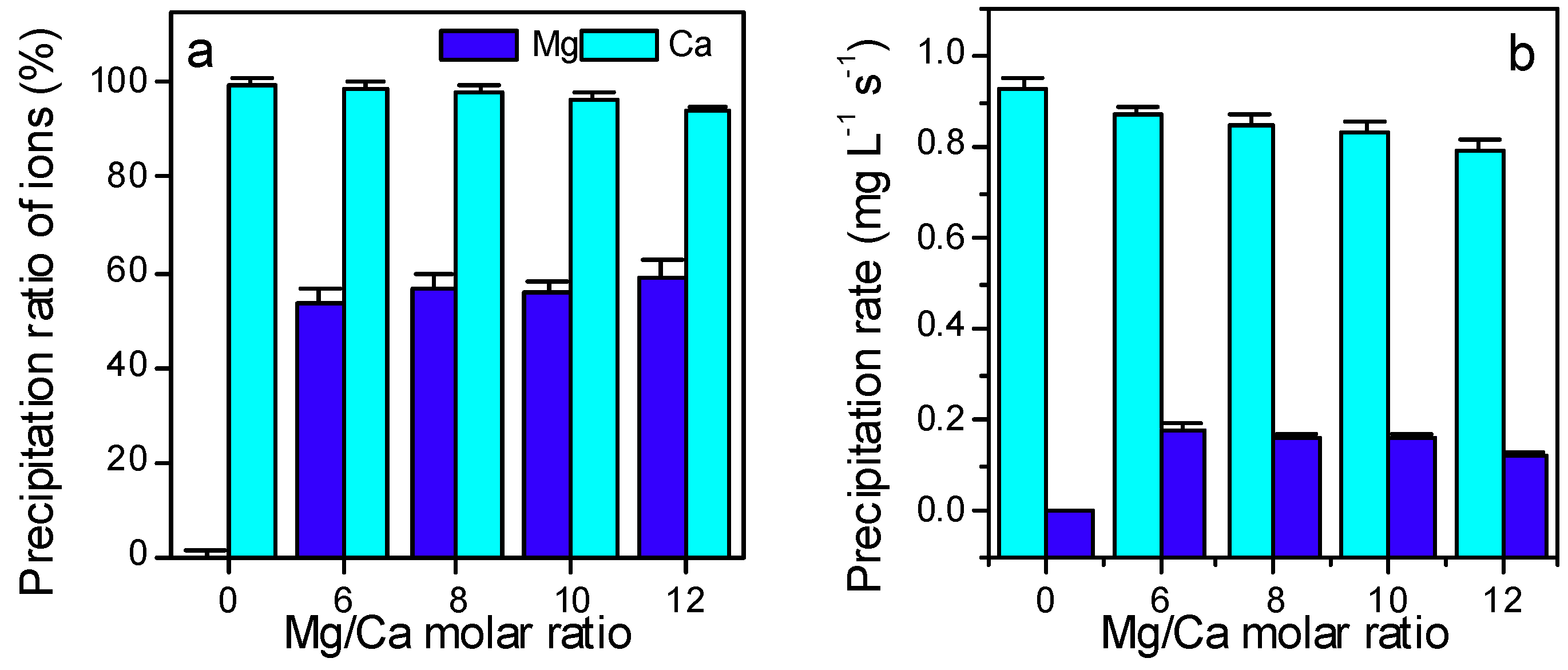
3.3. Ca2+ and Mg2+ Concentration, and the Electroconductivity
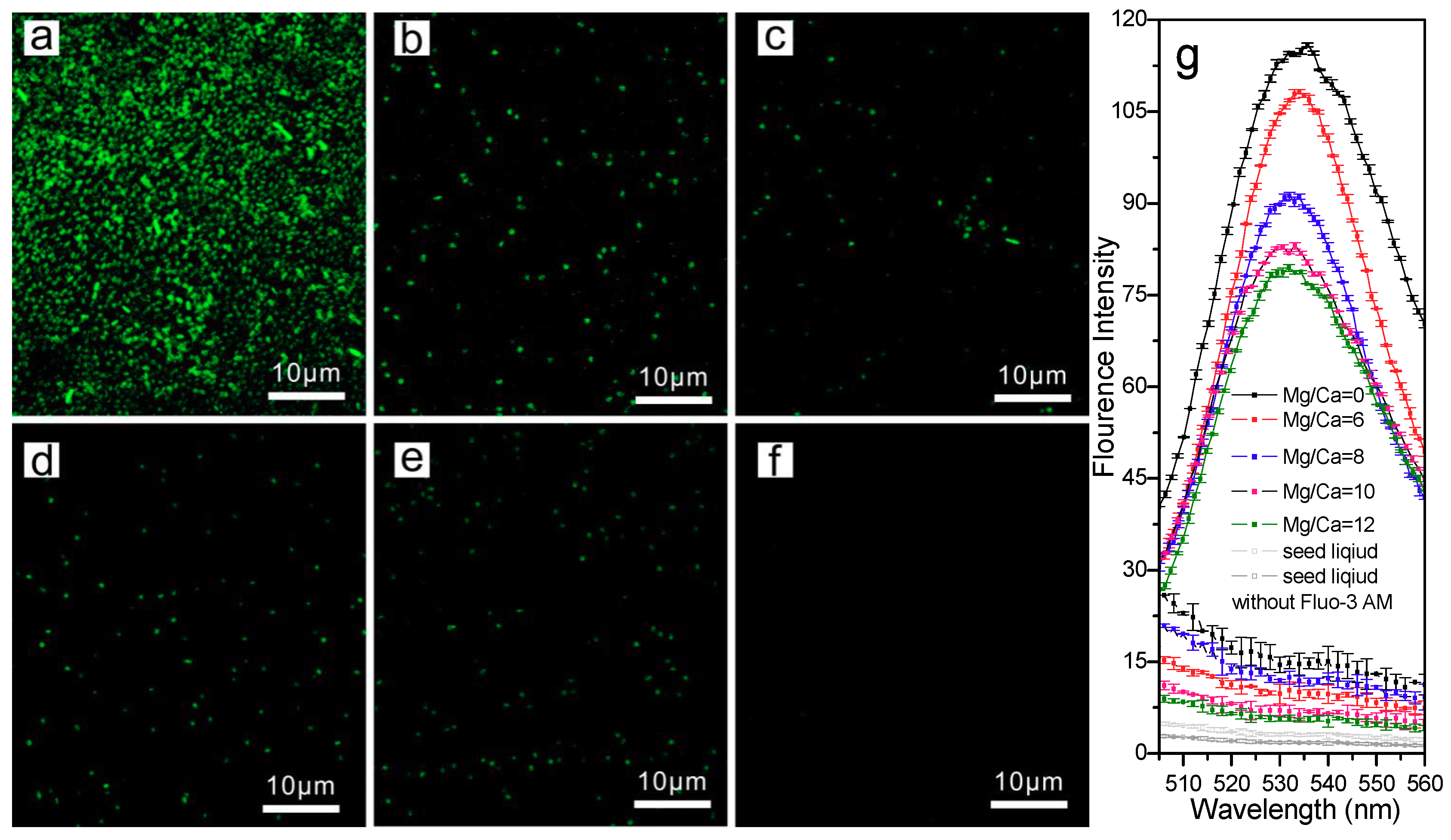
3.4. The Extra- and Intra- Cellular Precipitation of Ca2+ and Mg2+ Ions, and Biominerals Induced by SRB2
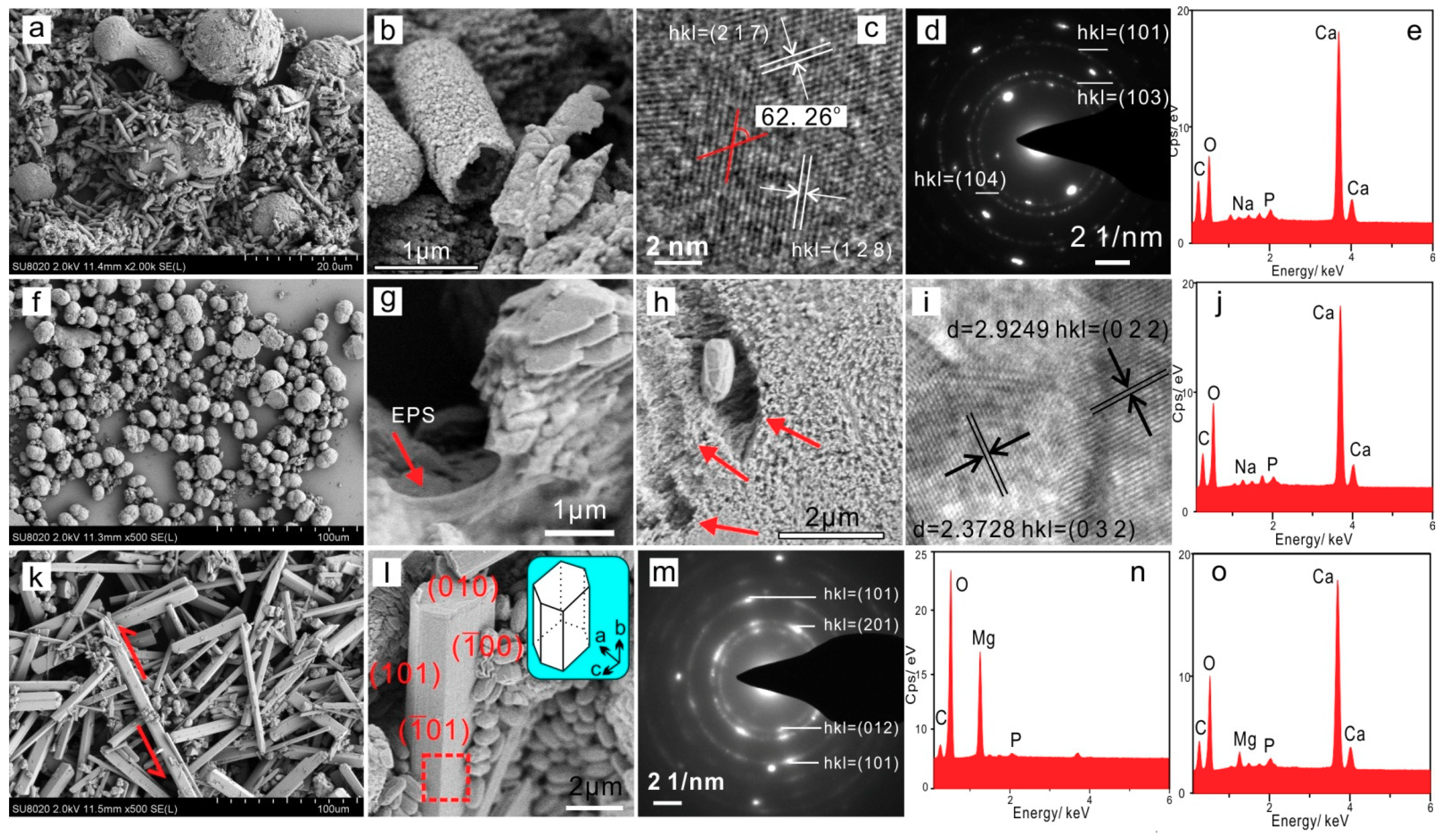
3.4.1. Extracellular Biominerals
3.4.2. Intracellular Amorphous Inclusions
3.5. The Mechanism of Ca2+, Mg2+ Bio-Precipitation Induced by SRB2
3.5.1. Carbon Source for Bio-Precipitation
3.5.2. Nucleation Sites of Extracellular Biominerals
3.5.3. Precipitation Controlled by Amino Acids and Organic Functional Groups
4. Implications for Microbial Desalination and Biomineralization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Harvie, C.E.; Eugster, H.P.; Weare, J.H. Mineral equilibria in the six-component seawater system, Na-K-Mg-Ca-SO4-Cl-H2O at 25°C. II: Compositions of the saturated solutions. Geochim. Cosmochim. Acta 1982, 46, 1603–1618. [Google Scholar] [CrossRef]
- Valladares Linares, R.; Li, Z.; Yangali-Quintanilla, V.; Ghaffour, N.; Amy, G.; Leiknes, T.; Vrouwenvelder, J.S. Life cycle cost of a hybrid forward osmosis—Low pressure reverse osmosis system for seawater desalination and wastewater recovery. Water Res. 2016, 88, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.; Cisternas, L.A.; Rivas, M. Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile). Desalination 2017, 405, 1–9. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The Future of Seawater Desalination: Energy, Technology, and the Environment. Science 2011, 333, 712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhong, L.; Fu, X.; Zhao, Z. Managing Scarce Water Resources in China’s Coal Power Industry. Environ. Manag. 2016, 57, 1188–1203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Chen, D.; Wang, Q.; Zhang, Z. Seawater desalination in China: Retrospect and prospect. Chem. Eng. J. 2014, 242, 404–413. [Google Scholar] [CrossRef]
- Zarzo, D.; Campos, E.; Terrero, P. Spanish experience in desalination for agriculture. Desalin. Water Treat. 2013, 51, 53–66. [Google Scholar] [CrossRef]
- Amy, G.; Ghaffour, N.; Li, Z.; Francis, L.; Linares, R.V.; Missimer, T.; Lattemann, S. Membrane-based seawater desalination: Present and future prospects. Desalination 2017, 401, 16–21. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Valero, F.; Arbós, R. Desalination of brackish river water using Electrodialysis Reversal (EDR): Control of the THMs formation in the Barcelona (NE Spain) area. Desalination 2010, 253, 170–174. [Google Scholar] [CrossRef]
- Ge, Q.; Wang, P.; Wan, C.; Chung, T.-S. Polyelectrolyte-Promoted Forward Osmosis–Membrane Distillation (FO–MD) Hybrid Process for Dye Wastewater Treatment. Environ. Sci. Technol. 2012, 46, 6236–6243. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.; Koschikowski, J.; Wieghaus, M. Desalination using membrane distillation: Experimental studies on full scale spiral wound modules. J. Membr. Sci. 2011, 375, 104–112. [Google Scholar] [CrossRef]
- Chen, X.; Xia, X.; Liang, P.; Cao, X.; Sun, H.; Huang, X. Stacked Microbial Desalination Cells to Enhance Water Desalination Efficiency. Environ. Sci. Technol. 2011, 45, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Hakizimana, J.N.; Gourich, B.; Vial, C.; Drogui, P.; Oumani, A.; Naja, J.; Hilali, L. Assessment of hardness, microorganism and organic matter removal from seawater by electrocoagulation as a pretreatment of desalination by reverse osmosis. Desalination 2016, 393, 90–101. [Google Scholar] [CrossRef]
- Ping, Q.; Huang, Z.; Dosoretz, C.; He, Z. Integrated experimental investigation and mathematical modeling of brackish water desalination and wastewater treatment in microbial desalination cells. Water Res. 2015, 77, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Ping, Q.; Zhang, C.; Chen, X.; Zhang, B.; Huang, Z.; He, Z. Mathematical Model of Dynamic Behavior of Microbial Desalination Cells for Simultaneous Wastewater Treatment and Water Desalination. Environ. Sci. Technol. 2014, 48, 13010–13019. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yan, H.; Zhao, H.; Zhou, S.; Han, M.; Meng, X.; Zhang, Y.; Zhao, Y.; Sun, B.; Yao, C.; et al. Bio-precipitation of Calcite with Preferential Orientation Induced by Synechocystis sp. PCC6803. Geomicrobiol. J. 2014, 31, 884–899. [Google Scholar] [CrossRef]
- Kiskira, K.; Papirio, S.; van Hullebusch, E.D.; Esposito, G. Fe(II)-mediated autotrophic denitrification: A new bioprocess for iron bioprecipitation/biorecovery and simultaneous treatment of nitrate-containing wastewaters. Int. Biodeterior. Biodegrad. 2017, 119, 631–648. [Google Scholar] [CrossRef]
- Silva-Castro, G.A.; Uad, I.; Gonzalez-Martinez, A.; Rivadeneyra, A.; Gonzalez-Lopez, J.; Rivadeneyra, M.A. Bioprecipitation of Calcium Carbonate Crystals by Bacteria Isolated from Saline Environments Grown in Culture Media Amended with Seawater and Real Brine. Biomed Res. Int. 2015, 2015, 12. [Google Scholar] [CrossRef]
- Minas, K.; Karunakaran, E.; Bond, T.; Gandy, C.; Honsbein, A.; Madsen, M.; Amezaga, J.; Amtmann, A.; Templeton, M.R.; Biggs, C.A.; et al. Biodesalination: An emerging technology for targeted removal of Na+ and Cl− from seawater by cyanobacteria. Desalin. Water Treat. 2015, 55, 2647–2668. [Google Scholar] [CrossRef]
- Jacobson, K.S.; Drew, D.M.; He, Z. Efficient salt removal in a continuously operated upflow microbial desalination cell with an air cathode. Bioresour. Technol. 2011, 102, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Huang, X.; Liang, P.; Xiao, K.; Zhou, Y.; Zhang, X.; Logan, B.E. A New Method for Water Desalination Using Microbial Desalination Cells. Environ. Sci Technol. 2009, 43, 7148–7152. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Zhao, Y.; Yan, H.; Zhao, H.; Han, M.; Sun, B.; Meng, R.; Zhuang, D.; Li, D.; Gao, J.W.; et al. The characterization of intracellular and extracellular biomineralization induced by Synechocystis sp. PCC6803 cultured under low Mg/Ca ratios conditions. Geomicrobiol. J. 2017, 34, 362–373. [Google Scholar] [CrossRef]
- Han, Z.; Zhao, Y.; Yan, H.; Zhao, H.; Han, M.; Sun, B.; Sun, X.; Hou, F.; Sun, H.; Han, L.J.; et al. Struvite precipitation induced by a novel sulfate-reducing bacterium Acinetobacter calcoaceticus SRB4 isolated from river sediment. Geomicrobiol. J. 2015, 32, 868–877. [Google Scholar] [CrossRef]
- Falagán, C.; Yusta, I.; Sánchez-España, J.; Johnson, D.B. Biologically-induced precipitation of aluminium in synthetic acid mine water. Miner. Eng. 2017, 106, 79–85. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Miner. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Pentecost, A. Significance of the biomineralizing microniche in a lyngbya (cyanobacterium) travertine. Geomicrobiol. J. 1995, 13, 213–222. [Google Scholar] [CrossRef]
- Chough, S.K.; Lee, H.S.; Woo, J.; Chen, J.; Choi, D.K.; Lee, S.-B.; Kang, I.; Park, T.-Y.; Han, Z. Cambrian stratigraphy of the North China Platform: Revisiting principal sections in Shandong Province, China. Geosci. J. 2010, 14, 235–268. [Google Scholar] [CrossRef]
- Van Lith, Y.; Warthmann, R.; Vasconcelos, C.; McKenzie, J.A. Sulphate-reducing bacteria induce low-temperature Ca-dolomite and high Mg-calcite formation. Geobiology 2003, 1, 71–79. [Google Scholar] [CrossRef]
- Kajander, E.O.; Çiftçioglu, N. Nanobacteria: An alternative mechanism for pathogenic intra- and extracellular calcification and stone formation. Proc. Natl. Acad. Sci. USA 1998, 95, 8274. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Biomineralization of calcium carbonates and their engineered applications: A review. Front. Microbiol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Warren, L.A.; Maurice, P.A.; Parmar, N.; Ferris, F.G. Microbially Mediated Calcium Carbonate Precipitation: Implications for Interpreting Calcite Precipitation and for Solid-Phase Capture of Inorganic Contaminants. Geomicrobiol. J. 2001, 18, 93–115. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, Z.; Liu, J.; Hu, S. Flora characteristics of Chenier Wetland in Bohai Bay and biogeographic relations with adjacent wetlands. Front. Earth Sci. 2017, 11, 620–628. [Google Scholar] [CrossRef]
- Chen, Y.; Steele-MacInnis, M.; Ge, Y.; Zhou, Z.; Zhou, Y. Synthetic saline-aqueous and hydrocarbon fluid inclusions trapped in calcite at temperatures and pressures relevant to hydrocarbon basins: A reconnaissance study. Mar. Pet. Geol 2016, 76, 88–97. [Google Scholar] [CrossRef]
- Cheng, B.; Wang, T.G.; Chen, Z.; Chang, X.; Yang, F. Biodegradation and possible source of Silurian and Carboniferous reservoir bitumens from the Halahatang sub-depression, Tarim Basin, NW China. Mar. Pet. Geol. 2016, 78, 236–246. [Google Scholar] [CrossRef]
- Guo, J.; Zhai, P.; Wu, L.; Cribb, M.; Li, Z.; Ma, Z.; Wang, F.; Chu, D.; Wang, P.; Zhang, J. Diurnal variation and the influential factors of precipitation from surface and satellite measurements in Tibet. Int. J. Clim. 2014, 34, 2940–2956. [Google Scholar] [CrossRef]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M.E. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, J.; Chough, S.K. Paleoenvironmental implications of an extensive maceriate microbialite bed in the Furongian Chaomidian Formation, Shandong Province, China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 621–632. [Google Scholar] [CrossRef]
- Yang, R.; Fan, A.; Han, Z.; Chi, N.; Han, Y. Characteristics and genesis of microbial lumps in the Maozhuang Stage (Cambrian Series 2), Shandong Province, China. Sci. China Earth Sci. 2013, 56, 494–503. [Google Scholar] [CrossRef]
- Li, F.; Jing, X.; Zou, C.; Zhang, H.; Xiang, F. Facies analysis of the Callovian–Oxfordian carbonates in the northeastern Amu Darya Basin, southeastern Turkmenistan. Mar. Pet. Geol. 2017, 88, 359–380. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Y.; Feng, Q.; Lin, J.; Zhou, D.; Wang, D. Sedimentary evidence of Early–Late Permian mid-latitude continental climate variability, southern Bogda Mountains, NW China. Palaeogeogr. Palaeoclim. Palaeoecol. 2007, 252, 239–258. [Google Scholar] [CrossRef]
- Spadafora, A.; Perri, E.; Mckenzie, J.A.; Vasconcelos, C. Microbial biomineralization processes forming modern Ca:Mg carbonate stromatolites. Sedimentology 2010, 57, 27–40. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Yao, Y.; Duan, Y. High salinity facilitates dolomite precipitation mediated by Haloferax volcanii DS52. Earth Planet. Sci. Lett 2017, 472, 197–205. [Google Scholar] [CrossRef]
- Qiu, X.; Yao, Y.; Wang, H.; Shen, A.; Zhang, J. Halophilic Archaea Mediate the Formation of Proto-Dolomite in Solutions With Various Sulfate Concentrations and Salinities. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Yu, W.; Zhao, H.; Zhao, Y.; Tucker, E.M.; Yan, H. The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2. Minerals 2018, 8, 594. [Google Scholar] [CrossRef]
- Suzuki, M.; Nagasawa, H.; Kogure, T. Synthesis and Structure of Hollow Calcite Particles. Cryst. Growth Des. 2006, 6, 2004–2006. [Google Scholar] [CrossRef]
- Daskalakis, M.I.; Magoulas, A.; Kotoulas, G.; Katsikis, I.; Bakolas, A.; Karageorgis, A.P.; Mavridou, A.; Doulia, D.; Rigas, F. Cupriavidus metallidurans biomineralization ability and its application as a bioconsolidation enhancer for ornamental marble stone. Appl. Microbiol. Biotechnol. 2014, 98, 6871–6883. [Google Scholar] [CrossRef] [PubMed]
- Tourney, J.; Ngwenya, B.T. The role of bacterial extracellular polymeric substances in geomicrobiology. Chem. Geol. 2014, 386, 115–132. [Google Scholar] [CrossRef]
- Han, Z.; Gao, X.; Zhao, H.; Tucker, E.M.; Zhao, Y.; Bi, Z.; Pan, J.; Wu, G.; Yan, H. Extracellular and Intracellular Biomineralization Induced by Bacillus licheniformis DB1-9 at Different Mg/Ca Molar Ratios. Minerals 2018, 8, 585. [Google Scholar] [CrossRef]
- Han, Z.; Li, D.; Zhao, H.; Yan, H.; Li, P. Precipitation of Carbonate Minerals Induced by the Halophilic Chromohalobacter Israelensis under High Salt Concentrations: Implications for Natural Environments. Minerals 2017, 7, 95. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. Calcium carbonate crystallization in the presence of glutamic acid. J. Cryst. Growth 2001, 222, 293–297. [Google Scholar] [CrossRef]
- Malkaj, P.; Dalas, E. Calcium Carbonate Crystallization in the Presence of Aspartic Acid. Cryst. Growth Des. 2004, 4, 721–723. [Google Scholar] [CrossRef]
- Xie, A.-J.; Shen, Y.-H.; Zhang, C.-Y.; Yuan, Z.-W.; Zhu, X.-M.; Yang, Y.-M. Crystal growth of calcium carbonate with various morphologies in different amino acid systems. J. Cryst Growth 2005, 285, 436–443. [Google Scholar] [CrossRef]
- Wolf, S.E.; Loges, N.; Mathiasch, B.; Panthöfer, M.; Mey, I.; Janshoff, A.; Tremel, W. Phase Selection of Calcium Carbonate through the Chirality of Adsorbed Amino Acids. Angew. Chem. Int. Ed. 2007, 46, 5618–5623. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallization of monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Ma, P.; Wang, C.; Lv, D.; Li, Y.; Yi, L. Controls on deposition of aquatic and terrestrial organic matter in the lacustrine Namling–Oiyug basin (Oligocene–Miocene, southern Tibet). Int. J. Coal Geol. 2015, 149, 108–117. [Google Scholar] [CrossRef]
- Yang, R.; van Loon, A.J.; Jin, X.; Jin, Z.; Han, Z.; Fan, A.; Liu, Q. From divergent to convergent plates: Resulting facies shifts along the southern and western margins of the Sino-Korean Plate during the Ordovician. J. Geodyn. 2018. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.; Slowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2017, 65. [Google Scholar] [CrossRef]
- Chen, J.; Chough, S.K.; Lee, J.H.; Han, Z. Sequence-stratigraphic comparison of the upper Cambrian Series 3 to Furongian succession between the Shandong region, China and the Taebaek area, Korea: high variability of bounding surfaces in an epeiric platform. Geosci. J. 2012, 16, 357–379. [Google Scholar] [CrossRef]
- Zhang, X.L.; Feng, Q.; Sun, P.; LI, W. Characteristics of High-Gamma-Ray Sandstone Reservoirs for Yanchang Formation in Ordos Basin. Chin. J. Geophys. 2010, 53, 154–163. [Google Scholar] [CrossRef]
- Qiu, X.; Liu, C.; Mao, G.; Deng, Y.; Wang, F.; Wang, J. Late Triassic tuff intervals in the Ordos basin, Central China: Their depositional, petrographic, geochemical characteristics and regional implications. J. Asian Earth Sci 2014, 80, 148–160. [Google Scholar] [CrossRef]
- Lv, D.; Chen, J. Depositional environments and sequence stratigraphy of the Late Carboniferous−Early Permian coal-bearing successions (Shandong Province, China): Sequence development in an epicontinental basin. J. Asian Earth Sci. 2014, 79, 16–30. [Google Scholar] [CrossRef]
- Fan, A.; Yang, R.; Van Loon, A.J.; Yin, W.; Han, Z.; Zavala, C. Classification of gravity-flow deposits and their significance for unconventional petroleum exploration, with a case study from the Triassic Yanchang Formation (southern Ordos Basin, China). J. Asian Earth Sci. 2018, 161, 57–73. [Google Scholar] [CrossRef]
- Yin, S.; Lv, D.; Jin, L.; Ding, W. Experimental analysis and application of the effect of stress on continental shale reservoir brittleness. J. Geophys. Eng. 2018, 15, 478–494. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yang, R.; Song, M.; Lenhardt, N.; Wang, X.; Zhang, X.; Yang, S.; Wang, J.; Cao, H. Characteristics, controls and geological models of hydrocarbon accumulation in the Carboniferous volcanic reservoirs of the Chunfeng Oilfield, Junggar Basin, northwestern China. Mar. Pet. Geol. 2018, 94, 65–79. [Google Scholar] [CrossRef] [Green Version]
- Bian, X.; Wang, Z.-F.; Ding, G.-Q.; Cao, Y.-P. Compressibility of cemented dredged clay at high water content with super-absorbent polymer. Eng. Geol. 2016, 208, 198–205. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.G.; Li, Q.; Cheng, B.; Tao, X. Geochemistry and possible origin of petroleum in Palaeozoic reservoirs from Halahatang Depression. J. Asian Earth Sci. 2013, 74, 129–141. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, X.; Chen, N.; Yang, C.; Wang, Q. Evaluation of reservoir connectivity using whole-oil gas chromatographic fingerprint technology: A case study from the Es33 reservoir in the Nanpu Sag, China. Pet. Sci. 2012, 9, 290–294. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Lu, M. Microbially enhanced oil recovery at simulated reservoir conditions by use of engineered bacteria. J. Pet. Sci. Eng. 2011, 78, 233–238. [Google Scholar] [CrossRef]
- Lv, D.; Li, Z.; Chen, J.; Liu, H.; Guo, J.; Shang, L. Characteristics of the Permian coal-formed gas sandstone reservoirs in Bohai Bay Basin and the adjacent areas, North China. J. Pet. Sci. Eng. 2011, 78, 516–528. [Google Scholar] [CrossRef]
- Ding, H.-P.; Xin, G.-Q.; Chen, K.-C.; Zhang, M.; Liu, Q.; Hao, J.; Liu, H.-G. Silver dendritic nanostructures formed at the solid/liquid interface via electroless deposition. Colloid. Surf. A Physicochem. Eng. Asp. 2010, 353, 166–171. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barabesi, C.; Galizzi, A.; Mastromei, G.; Rossi, M.; Tamburini, E.; Perito, B.J.J.o.b. Bacillus subtilis gene cluster involved in calcium carbonate biomineralization. J. Bacteriol. 2007, 189, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170. [Google Scholar] [CrossRef] [PubMed]
- Mass, T.; Giuffre, A.J.; Sun, C.-Y.; Stifler, C.A.; Frazier, M.J.; Neder, M.; Tamura, N.; Stan, C.V.; Marcus, M.A.; Gilbert, P.U.P.A. Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. USA 2017, 114, 7670–7678. [Google Scholar] [CrossRef] [PubMed]
- Herbaud, M.-L.; Guiseppi, A.; Denizot, F.; Haiech, J.; Kilhoffer, M.-C. Calcium signalling in Bacillus subtilis. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 1998, 1448, 212–226. [Google Scholar] [CrossRef] [Green Version]
- Van Dijk-Salkinoja, M.S.; Stoof, T.J.; Planta, R.J. The Distribution of Polysomes, Ribosomes and Ribosomal Subunits in Exponential-Phase Cells of Bacillus licheniformis. Eur. J. Biochem. 1970, 12, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tucker, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y.; et al. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: Further insights into biotic and abiotic calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Bontognali, T.R.; McKenzie, J.A.; Warthmann, R.J.; Vasconcelos, C.J.T.N. Microbially influenced formation of Mg-calcite and Ca-dolomite in the presence of exopolymeric substances produced by sulphate-reducing bacteria. Terra. Nova. 2014, 26, 72–77. [Google Scholar] [CrossRef]
- Sancho-Tomás, M.; Fermani, S.; Reggi, M.; García-Ruiz, J.M.; Gómez-Morales, J.; Falini, G. Polypeptide effect on Mg2+ hydration inferred from CaCO3 formation: A biomineralization study by counter-diffusion. Cryst. Eng. Comm. 2016, 18, 3265–3272. [Google Scholar] [CrossRef]
- Han, Z.; Meng, R.; Yan, H.; Zhao, H.; Han, M.; Zhao, Y.; Sun, B.; Sun, Y.; Wang, J.; Zhuang, D.J.C.; et al. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonate. Evaporite. 2017, 32, 561–575. [Google Scholar] [CrossRef]
- Stocks-Fischer, S.; Galinat, J.K.; Bang, S.S. Microbiological precipitation of CaCO3. Soil Biol. Biochem. 1999, 31, 1563–1571. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonates: The geological record of calcified bacterial–algal mats and biofilms. Sedimentology 2000, 47, 179–214. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawe, R.A. Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem. Geol. 2000, 163, 129–138. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Wu, G.; Zhou, J.; Yin, J.; Zhang, H.; Zhang, X.; et al. Mechanism of Biomineralization Induced by Bacillus subtilis J2 and Characteristics of the Biominerals. Minerals 2019, 9, 218. [Google Scholar] [CrossRef]




| Mg/Ca | Biomineral | Abiotic Mineral | Beef Extract | Tryptone | NaHCO3 | Na2CO3 | Air [78] |
|---|---|---|---|---|---|---|---|
| 0 | −16.54 ± 0.12 | −13.25 ± 0.22 | −18.14 ± 0.11 | −21.52 ± 0.13 | −14.25 ± 0.09 | −13.36 ± 0.12 | −8 |
| 6 | −17.55 ± 0.05 | - | |||||
| 8 | −17.38 ± 0.08 | - | |||||
| 10 | −18.01 ± 0.13 | - | |||||
| 12 | −16.12 ± 0.62 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Yan, H.; Zhou, J.; Tucker, M.E.; Han, M.; Zhao, H.; Mao, G.; Zhao, Y.; Han, Z. Bio-Precipitation of Calcium and Magnesium Ions through Extracellular and Intracellular Process Induced by Bacillus Licheniformis SRB2. Minerals 2019, 9, 526. https://doi.org/10.3390/min9090526
Zhao Y, Yan H, Zhou J, Tucker ME, Han M, Zhao H, Mao G, Zhao Y, Han Z. Bio-Precipitation of Calcium and Magnesium Ions through Extracellular and Intracellular Process Induced by Bacillus Licheniformis SRB2. Minerals. 2019; 9(9):526. https://doi.org/10.3390/min9090526
Chicago/Turabian StyleZhao, Yanyang, Huaxiao Yan, Jingxuan Zhou, Maurice E. Tucker, Mei Han, Hui Zhao, Guangzhou Mao, Yifan Zhao, and Zuozhen Han. 2019. "Bio-Precipitation of Calcium and Magnesium Ions through Extracellular and Intracellular Process Induced by Bacillus Licheniformis SRB2" Minerals 9, no. 9: 526. https://doi.org/10.3390/min9090526





