Creatine Levels in Patients with Phenylketonuria and Mild Hyperphenylalaninemia: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
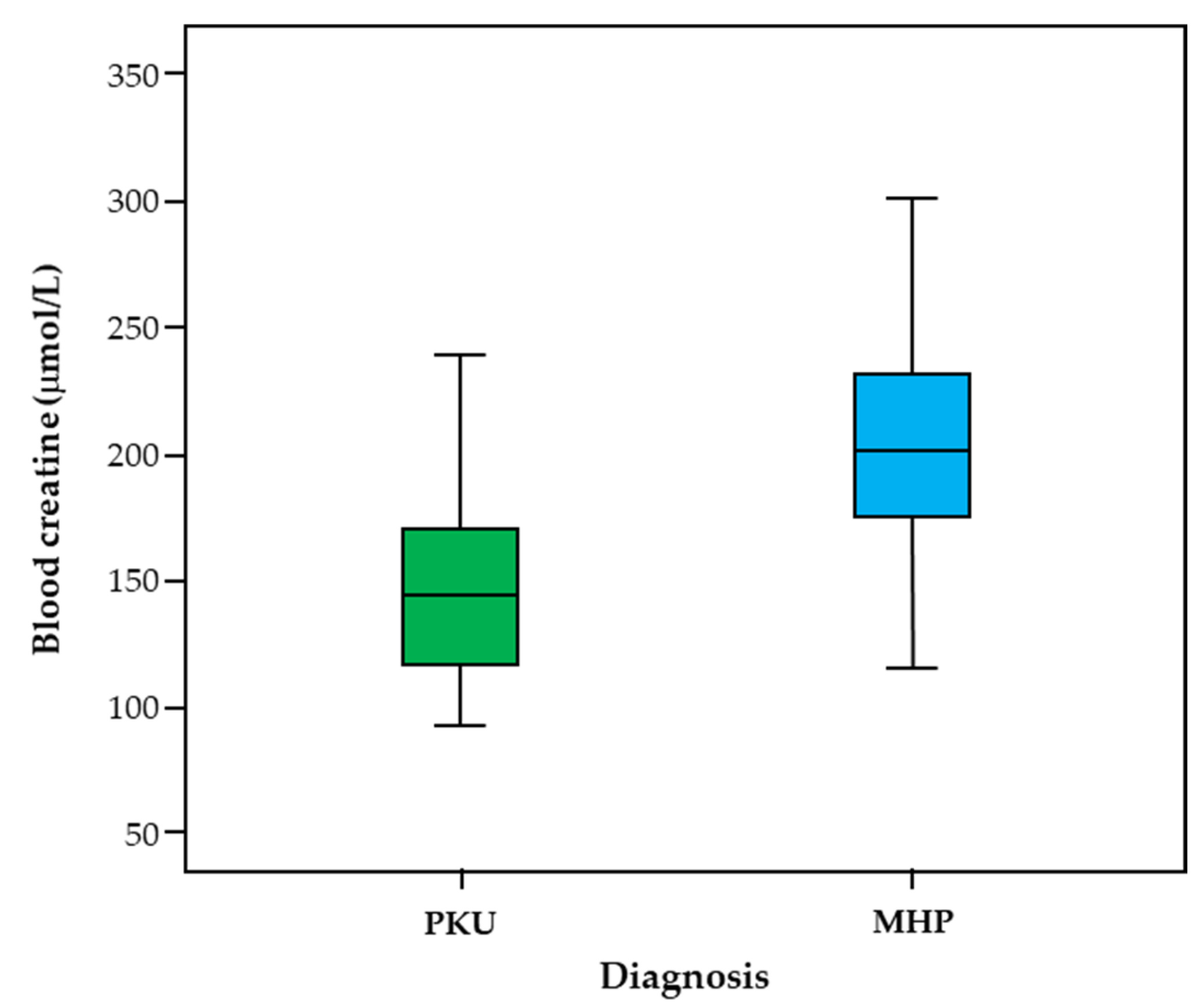
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Walker, J.B. Creatine: Biosynthesis, regulation, and function. Adv. EnzyMol. Relat Areas Mol. Biol. 1979, 50, 177–242. [Google Scholar] [CrossRef]
- Wyss, M.; Kaddurah-Daouk, R. Creatine and creatinine metabolism. Physiol. Rev. 2000, 80, 1107–1213. [Google Scholar] [CrossRef]
- Hultman, E.; Söderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. (1985) 1996, 81, 232–237. [Google Scholar] [CrossRef]
- Green, A.L.; Hultman, E.; Macdonald, I.A.; Sewell, D.A.; Greenhaff, P.L. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol. 1996, 271 Pt 1, E821–R826. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids. 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fondamenti di Biochimica; Zanichelli: Bologna, Italy, 2003. [Google Scholar]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- McGuine, T.A.; Sullivan, J.C.; Bernhardt, D.A. Creatine supplementation in Wisconsin high school athletes. WMJ 2002, 101, 25–30. [Google Scholar] [PubMed]
- Riesberg, L.A.; Weed, S.A.; McDonald, T.L.; Eckerson, J.M.; Drescher, K.M. Beyond muscles: The untapped potential of creatine. Int. Immunopharmacol. 2016, 37, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Schlattner, U.; Klaus, A.; Ramirez Rios, S.; Guzun, R.; Kay, L.; Tokarska-Schlattner, M. Cellular compartmentation of energy metabolism: Creatine kinase microcompartments and recruitment of B-type creatine kinase to specific subcellular sites. Amino Acids 2016, 48, 1751–1774. [Google Scholar] [CrossRef] [PubMed]
- Ydfors, M.; Hughes, M.C.; Laham, R.; Schlattner, U.; Norrbom, J.; Perry, C.G.R. Modelling in vivo creatine/phosphocreatine in vitro reveals divergent adaptations in human muscle mitochondrial respiratory control by ADP after acute and chronic exercise. J. Physiol. 2016, 594, 3127–3140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jagim, A.R.; Kerksick, C.M. Creatine Supplementation in Children and Adolescents. Nutrients 2021, 13, 664. [Google Scholar] [CrossRef]
- Saraiva, A.L.L.; Ferreira, A.P.O.; Silva, L.F.A.; Hoffmann, M.S.; Dutra, F.D.; Furian, A.F.; Oliveira, M.S.; Fighera, M.R.; Royes, L.F. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res. Bull. 2012, 87, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Kim, D.-H.; Meza, C.A.; Ormsbee, M.J.; Hickner, R.C. The Evolving Applications of Creatine Supplementation: Could Creatine Improve Vascular Health? Nutrients 2020, 12, 2834. [Google Scholar] [CrossRef]
- Gaddi, A.V.; Galuppo, P.; Yang, J. Creatine Phosphate Administration in Cell Energy Impairment Conditions: A Summary of Past and Present Research. Heart Lung Circ. 2017, 26, 1026–1035. [Google Scholar] [CrossRef] [Green Version]
- Perasso, L.; Lunardi, G.L.; Risso, F.; Pohvozcheva, A.V.; Leko, M.V.; Gandolfo, C.; Florio, T.; Cupello, A.; Burov, S.V.; Balestrino, M. Protective effects of some creatine derivatives in brain tissue anoxia. Neurochem. Res. 2008, 33, 765–775. [Google Scholar] [CrossRef]
- Marques, E.P.; Wyse, A.T.S. Creatine as a Neuroprotector: An Actor that Can Play Many Parts. Neurotox. Res. 2019, 36, 411–423. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Bougioukas, K.I.; Kapogiannis, D. Effects of creatine supplementation on cognitive function of healthy individuals: A systematic review of randomized controlled trials. Exp. Gerontol. 2018, 108, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef]
- Balestrino, M.; Adriano, E. Beyond sports: Efficacy and safety of creatine supplementation in pathological or paraphysiological conditions of brain and muscle. Med. Res. Rev. 2019, 39, 2427–2459. [Google Scholar] [CrossRef] [PubMed]
- Costabeber, E.; Kessler, A.; Severo Dutra-Filho, C.; de Souza Wyse, A.T.; Wajner, M.; Wannmacher, C.M.D. Hyperphenylalaninemia reduces creatine kinase activity in the cerebral cortex of rats. Int. J. Dev. Neurosci. 2003, 21, 111–116. [Google Scholar] [CrossRef]
- Berti, S.L.; Nasi, G.M.; Garcia, C.; Castro, F.L.; Nunes, M.L.; Rojas, D.B.; Moraes, T.B.; Dutra-Filho, C.S.; Wannmacher, C.M. Pyruvate and creatine prevent oxidative stress and behavioral alterations caused by phenylalanine administration into hippocampus of rats. Metab. Brain Dis. 2012, 27, 79–89. [Google Scholar] [CrossRef]
- Dos Reis, E.A.; Rieger, E.; de Souza, S.S.; Rasia-Filho, A.A.; Wannmacher, C.M.D. Effects of a co-treatment with pyruvate and creatine on dendritic spines in rat hippocampus and posterodorsal medial amygdala in a phenylketonuria animal model. Metab. Brain Dis. 2013, 28, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Adam, M.P.; Ardinger, H.H.; Pagon, R.A.; Wallace, S.E.; Bean, L.J.H.; Mirzaa, G.; Amemiya, A. (Eds.) GeneReviews®; University of Washington: Seattle, WA, USA, 1993. Available online: http://www.ncbi.nlm.nih.gov/books/NBK1116/ (accessed on 30 March 2021).
- Sykut-Cegielska, J.; Gradowska, W.; Mercimek-Mahmutoglu, S.; Stöckler-Ipsiroglu, S. Biochemical and clinical characteristics of creatine deficiency syndromes. Acta Biochim. Pol. 2004, 51, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Nasrallah, F.; Feki, M.; Kaabachi, N. Creatine and creatine deficiency syndromes: Biochemical and clinical aspects. Pediatr. Neurol. 2010, 42, 163–171. [Google Scholar] [CrossRef]
- Braissant, O.; Henry, H.; Béard, E.; Uldry, J. Creatine deficiency syndromes and the importance of creatine synthesis in the brain. Amino Acids 2011, 40, 1315–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockler-Ipsiroglu, S.; van Karnebeek, C.D.M. Cerebral creatine deficiencies: A group of treatable intellectual developmental disorders. Semin. Neurol. 2014, 34, 350–356. [Google Scholar] [CrossRef]
- Longo, N.; Ardon, O.; Vanzo, R.; Schwartz, E.; Pasquali, M. Disorders of creatine transport and metabolism. Am. J. Med. Genet. C Semin. Med. Genet. 2011, 157C, 72–78. [Google Scholar] [CrossRef]
- Ndika, J.D.T.; Johnston, K.; Barkovich, J.A.; Wirt, M.D.; O’Neill, P.; Betsalel, O.T.; Jakobs, C.; Salomons, G.S. Developmental progress and creatine restoration upon long-term creatine supplementation of a patient with arginine:glycine amidinotransferase deficiency. Mol. Genet. Metab. 2012, 106, 48–54. [Google Scholar] [CrossRef]
- Clark, J.F.; Cecil, K.M. Diagnostic methods and recommendations for the cerebral creatine deficiency syndromes. Pediatr. Res. 2015, 77, 398–405. [Google Scholar] [CrossRef]
- Solis, M.Y.; Artioli, G.G.; Otaduy, M.C.G.; Leite, C.D.C.; Arruda, W.; Veiga, R.R.; Gualano, B. Effect of age, diet, and tissue type on PCr response to creatine supplementation. J. Appl. Physiol. 2017, 123, 407–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, B.; Sharma, U.; Balasubramanian, K.; Kalaivani, M.; Kalra, V.; Jagannathan, N.R. Effect of creatine monohydrate in improving cellular energetics and muscle strength in ambulatory Duchenne muscular dystrophy patients: A randomized, placebo-controlled 31P MRS study. Magn. Reson. Imaging 2010, 28, 698–707. [Google Scholar] [CrossRef]
- van de Kamp, J.M.; Pouwels, P.J.W.; Aarsen, F.K.; ten Hoopen, L.W.; Knol, D.L.; de Klerk, J.B.; de Coo, I.F.; Huijmans, J.G.; Jakobs, C.; van der Knaap, M.S.; et al. Long-term follow-up and treatment in nine boys with X-linked creatine transporter defect. J. Inherit. Metab. Dis. 2012, 35, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Söderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. (London) 1992, 83, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet. J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [Green Version]
- Verwied-Jorky, S.; Schiess, S.; Luque, V.; Scaglioni, S.; Vecchi, F.; Martin, F.; Stolarczyk, A.; Koletzko, B.; European Childhood Obesity Project. Methodology for longitudinal assessment of nutrient intake and dietary habits in early childhood in a transnational multicenter study. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 96–102. [Google Scholar] [CrossRef]
- Carducci, C.; Santagata, S.; Leuzzi, V.; Carducci, C.; Artiola, C.; Giovanniello, T.; Battini, R.; Antonozzi, I. Quantitative determination of guanidinoacetate and creatine in dried blood spot by flow injection analysis-electrospray tandem mass spectrometry. Clin. Chim. Acta 2006, 364, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Garwolińska, D.; Namieśnik, J.; Kot-Wasik, A.; Hewelt-Belka, W. Chemistry of Human Breast Milk-A Comprehensive Review of the Composition and Role of Milk Metabolites in Child Development. J. Agric. Food Chem. 2018, 66, 11881–11896. [Google Scholar] [CrossRef] [PubMed]
- Edison, E.E.; Brosnan, M.E.; Aziz, K.; Brosnan, J.T. Creatine and guanidinoacetate content of human milk and infant formulas: Implications for creatine deficiency syndromes and amino acid metabolism. Br. J. Nutr. 2013, 110, 1075–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, J.C.; van Rijn, M.; van Dam, E.; Ahring, K.; Bélanger-Quintana, A.; Dokoupil, K.; Gokmen Ozel, H.; Lammardo, A.M.; Robert, M.; Heidenborg, C.; et al. Weight Management in Phenylketonuria: What Should Be Monitored. Ann. Nutr. Metab. 2016, 68, 60–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazzetta, U. Available online: https://www.gazzettaufficiale.it/do/gazzetta/unione_europea/3/pdfPaginato?numPagina=5&dataPubblicazioneGazzetta=20160404&numeroGazzetta=26&tipoSerie=S2&tipoSupplemento=GU&numeroSupplemento=0&edizione=0&progressivo=0&elenco30giorni=false (accessed on 30 March 2021).
- Allen, P.J. Creatine metabolism and psychiatric disorders: Does creatine supplementation have therapeutic value? Neurosci. Biobehav. Rev. 2012, 36, 1442–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| PKU Subjects | MHP Subjects | |
|---|---|---|
| Diagnosis | PKU (Phe ≥ 360 μmol/L) | PKU (Phe < 360 μmol/L) |
| Subjects (n) | 25 | 35 |
| Dietary treatment | Yes | No |
| Gender (n, m/f) | 13/12 | 18/17 |
| Mean age (years) ± SD | 6.89 0.81 | 6.82 0.85 |
| PKU Subjects (n = 25) | MHP Subjects (n = 35) | ||||
|---|---|---|---|---|---|
| Anthropometric Parameters | Mean (SD) | Median (Min–Max) | Mean (SD) | Median (Min–Max) | p† |
| Weight (kg) | 23.28 (5.16) | 24.0 (16.0–35.0) | 23.34 (4.50) | 23.0 (15.0–35.0) | 0.960 |
| Height (cm) | 121.28 (7.53) | 123.0 (109.0–139.0) | 120.54 (6.94) | 119.0 (105.0–134.0) | 0.697 |
| BMI (kg/m2) | 15.2 (1.91) | 15.0 (12.0–19.0) | 15.68 (2.37) | 15.0 (13.0–24.0) | 0.402 |
| z-score (weight) | 0.05 (1.20) | −0.20 (−1.79–2.08) | 0.33 (1.24) | 0.49 (−1.75–4.42) | 0.381 |
| z-score (height) | 0.05 (0.98) | −0.12 (−1.53–2.40) | 0.13 (1.05) | 0.21 (−2.41–1.98) | 0.760 |
| z-score (BMI) | 0.0 (1.19) | 0.32 (−2.45–1.83) | 0.28 (1.35) | −0.09 (−1.97–5.16) | 0.399 |
| PKU Subjects (n = 25) | MHP Subjects (n = 35) | ||||
|---|---|---|---|---|---|
| Nutritional Intake | Mean (SD) | Median (Min–Max) | Mean (SD) | Median (Min-Max) | p† |
| Caloric intake (kcal/day) | 1480.52 (291.72) | 1442.0 (969.0–2189.0) | 1564.68 (182.31) | 1576.0 (1002.0–2069.0) | 0.105 |
| Carbohydrate intake (g/day) | 225.36 (52.30) | 227.0 (138.0–343.0) | 230.25 (26.03) | 234.0 (154.0–284.0) | 0.626 |
| Fat intake (g/day) | 47.72 (15.26) | 46.0 (24.0–81.0) | 44.74 (7.56) | 44.00 (25.0–71.0) | 0.405 |
| Total protein intake (g) | 36.88 (6.62) | 40.0 (22.0–47.0) | 59.45 (7.06) | 61.00 (36.0–70.0) | <0.001 * |
| Animal-derived protein intake (g) | 2.40 (2.43) | 2.0 (0.0–9.0) | 32.94 (5.27) | 33.00 (13.0–45.0) | <0.001 * |
| Protein intake (% of caloric intake) | 9.8 (2.62) | 10.0 (5.0–17.0) | 14.82 (1.27) | 15.00 (11.0–18.0) | <0.001 * |
| Phe intake (mg/day) | 594.36 (405.8) | 500.0 (141.0–1975.0) | 3002.80 (348.36) | 3074.0 (1849.0–3539.0) | <0.001 * |
| Creatine ** (µmol/L) | 151.64 (41.27) | 145.0 (94.0–241.0) | 208.51 (48.39) | 202.00 (115.0–322.0) | <0.001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verduci, E.; Carbone, M.T.; Fiori, L.; Gualdi, C.; Banderali, G.; Carducci, C.; Leuzzi, V.; Biasucci, G.; Zuccotti, G.V. Creatine Levels in Patients with Phenylketonuria and Mild Hyperphenylalaninemia: A Pilot Study. Life 2021, 11, 425. https://doi.org/10.3390/life11050425
Verduci E, Carbone MT, Fiori L, Gualdi C, Banderali G, Carducci C, Leuzzi V, Biasucci G, Zuccotti GV. Creatine Levels in Patients with Phenylketonuria and Mild Hyperphenylalaninemia: A Pilot Study. Life. 2021; 11(5):425. https://doi.org/10.3390/life11050425
Chicago/Turabian StyleVerduci, Elvira, Maria Teresa Carbone, Laura Fiori, Claudia Gualdi, Giuseppe Banderali, Claudia Carducci, Vincenzo Leuzzi, Giacomo Biasucci, and Gian Vincenzo Zuccotti. 2021. "Creatine Levels in Patients with Phenylketonuria and Mild Hyperphenylalaninemia: A Pilot Study" Life 11, no. 5: 425. https://doi.org/10.3390/life11050425







