Beneficial Effects of Pulmonary Vasodilators on Pre-Capillary Pulmonary Hypertension in Patients with Chronic Kidney Disease on Hemodialysis
Abstract
:1. Introduction
2. Methods
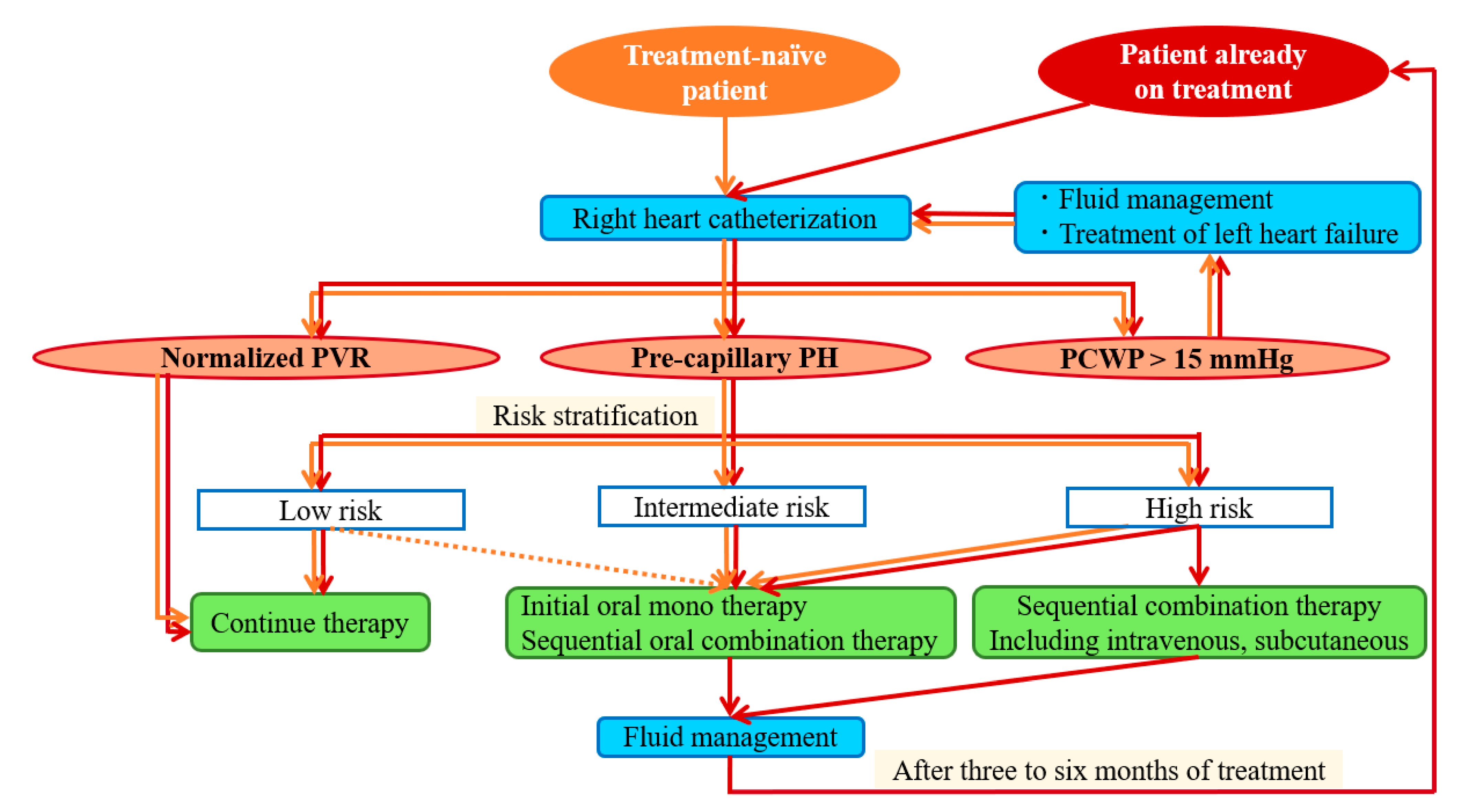
2.1. Prevailing Treatment Strategy for Hemodialysis Patients with Pre-Capillary PH
2.2. Statistical Analysis
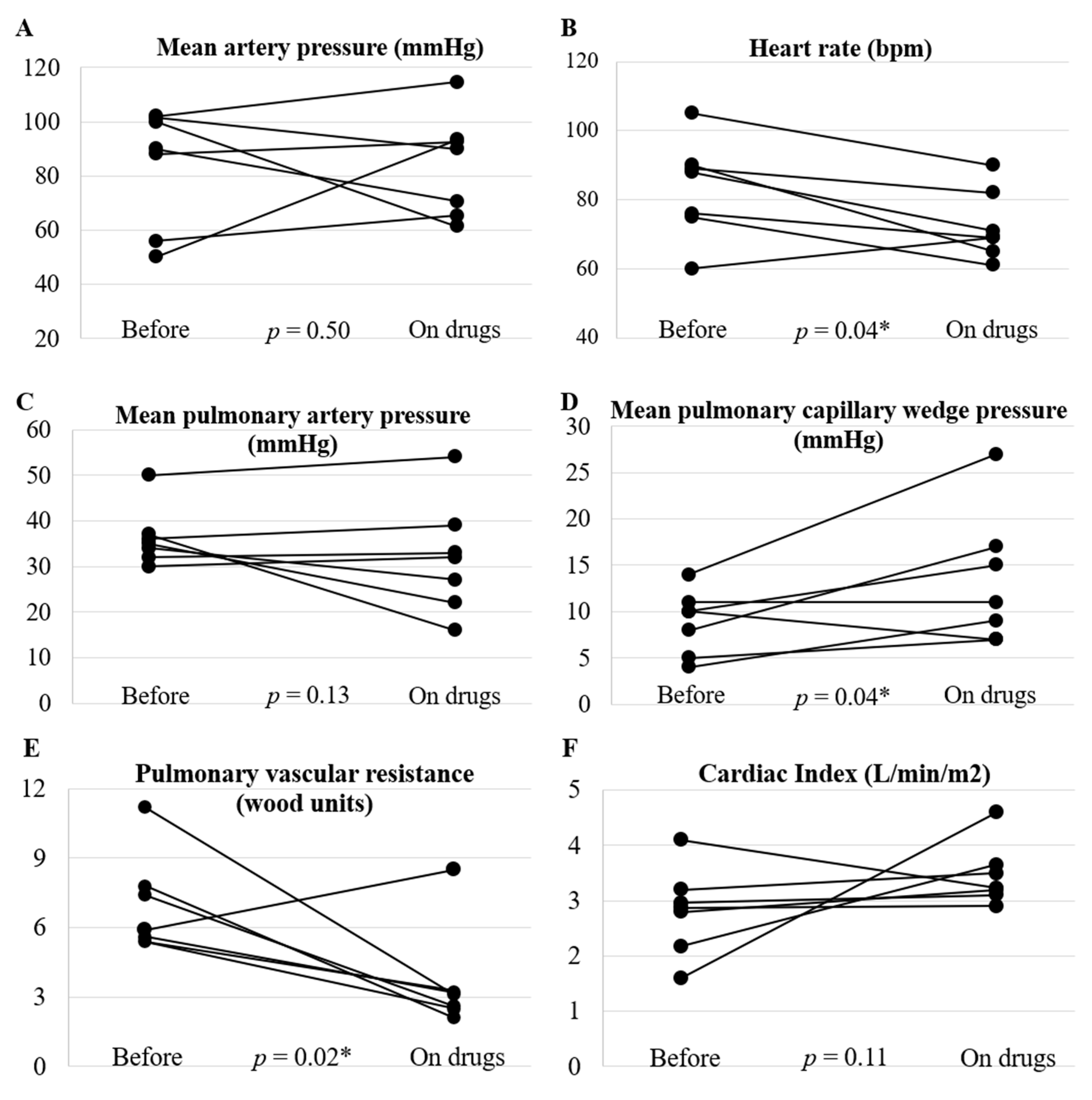
3. Results
4. Discussion
4.1. Effects of Pulmonary Vasodilators on Hemodynamics and Exercise Tolerance
4.2. Comorbid Left Ventricular Diastolic Dysfunction in Patients on Hemodialysis Is a Pitfall in Treating Existing Pre-Capillary PH
4.3. Treatment Algorithm for Pre-Capillary PH in Patients on Hemodialysis
5. Limitation
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanafusa, N.; Abe, M.; Joki, N.; Hoshino, J.; Kikuchi, K.; Goto, S.; Kanda, E.; Taniguchi, M.; Nakai, S.; Naganuma, T.; et al. Annual dialysis data report, JSDT renal data registry. J. Jpn. Soc. Dial. Ther. 2020, 54, 611–657. [Google Scholar]
- United States Renal Data System. 2019 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2019. [Google Scholar]
- Ramasubbu, K.; Deswal, A.; Herdejurgen, C.; Aguilar, D.; Frost, A.E. A prospective echocardiographic evaluation of pulmonary hypertension in chronic hemodialysis patients in the United States: Prevalence and clinical significance. Int. J. Gen. Med. 2010, 3, 279–286. [Google Scholar] [PubMed] [Green Version]
- Abdelwhab, S.; Elshinnawy, S. Pulmonary hypertension in chronic renal failure patients. Am. J. Nephrol. 2008, 28, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Sise, M.E.; Courtwright, A.M.; Channick, R.N. Pulmonary hypertension in patients with chronic and end-stage kidney disease. Kidney Int. 2013, 84, 682–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yigla, M.; Nakhoul, F.; Sabag, A.; Tov, N.; Gorevich, B.; Abassi, Z.; Reisner, S. Pulmonary hypertension in patients with end-stage renal disease. Chest 2003, 123, 1577–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolignano, D.; Rastelli, S.; Agarwal, R.; Fliser, D.; Massy, Z.; Ortiz, A.; Wiecek, A.; Martinez-Castelao, A.; Covic, A.; Goldsmith, D.; et al. Pulmonary hypertension in CKD. Am. J. Kidney Dis. 2013, 61, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Kawar, B.; Ellam, T.; Jackson, C.; Kiely, D.G. Pulmonary hypertension in renal disease: Epidemiology, potential mechanisms and implications. Am. J. Nephrol. 2013, 37, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Acarturk, G.; Albayrak, R.; Melek, M.; Yuksel, S.; Uslan, I.; Atli, H.; Colbay, M.; Unlu, M.; Fidan, F.; Asci, Z.; et al. The relationship between arteriovenous fistula blood flow rate and pulmonary artery pressure in hemodialysis patients. Int. Urol. Nephrol. 2008, 40, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Unal, A.; Tasdemir, K.; Oymak, S.; Duran, M.; Kocygit, I.; Oguz, F.; Tokgoz, B.; Sipahioglu, M.H.; Utas, C.; Oymak, O. The long-term effects of arteriovenous fistula creation on the development of pulmonary hypertension in hemodialysis patients. Hemodial. Int. 2010, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Yigla, M.; Banderski, R.; Azzam, Z.S.; Reisner, S.A.; Nakhoul, F. Arterio-venous access in end-stage renal disease patients and pulmonary hypertension. Ther. Adv. Respir. Dis. 2008, 2, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Nakhoul, F.; Yigla, M.; Gilman, R.; Reisner, S.A.; Abassi, Z. The pathogenesis of pulmonary hypertension in haemodialysis patients via arterio-venous access. Nephrol. Dial. Transplant. 2005, 20, 1686–1692. [Google Scholar] [CrossRef] [PubMed]
- Pabst, S.; Hammerstingl, C.; Hundt, F.; Gerhardt, T.; Grohé, C.; Nickenig, G.; Woitas, R.; Skowasch, D. Pulmonary hypertension in patients with chronic kidney disease on dialysis and without dialysis: Results of the PEPPER-study. PLoS ONE 2012, 7, e35310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, J.M.; Assad, T.R.; Xu, M.; Birdwell, K.A.; Farber-Eger, E.; Wells, Q.S.; Hemnes, A.R.; Brittain, E.L. Pulmonary hypertension in patients with chronic kidney disease: Invasive hemodynamic etiology and outcomes. Pulm. Circ. 2017, 7, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yigla, M.; Fruchter, O.; Aharonson, D.; Yanay, N.; Reisner, S.A.; Lewin, M.; Nakhoul, F. Pulmonary hypertension is an independent predictor of mortality in hemodialysis patients. Kidney Int. 2009, 75, 969–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmonston, D.L.; Parikh, K.S.; Rajagopal, S.; Shaw, L.K.; Abraham, D.; Grabner, A.; Sparks, M.A.; Wolf, M. Pulmonary hypertension subtypes and mortality in CKD. Am. J. Kidney Dis. 2020, 75, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Simonneau, G.; Montani, D.; Celermajer, D.S.; Denton, C.P.; Gatzoulis, M.A.; Krowka, M.; Williams, P.G.; Souza, R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801913. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Humbert, M.; Vachiery, J.-L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [PubMed]
- Watanabe, T.; Abe, K.; Horimoto, K.; Hosokawa, K.; Ohtani, K.; Tsutsui, H. Subcutaneous treprostinil was effective and tolerable in a patient with severe pulmonary hypertension associated with chronic kidney disease on hemodialysis. Heart Lung 2017, 46, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., III; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galiè, N.; Channick, R.N.; Frantz, R.P.; Grünig, E.; Jing, Z.C.; Moiseeva, O.; Preston, I.R.; Pulido, T.; Safdar, Z.; Tamura, Y.; et al. Risk stratification and medical therapy of pulmonary artery hypertension. Eur. Respir. J. 2019, 53, 1801889. [Google Scholar] [CrossRef] [PubMed]

| Patient Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| Patient Characteristics | |||||||
| Age, years | 52 | 73 | 61 | 78 | 62 | 83 | 56 |
| Sex | Male | Female | Female | Male | Male | Male | Female |
| Body mass index, kg/m2 | 26.5 | 19.0 | 17.2 | 22.8 | 17.4 | 19.3 | 19.0 |
| Kidney Disease and Hemodialysis | |||||||
| Primary kidney disease | Diabetic nephropathy | Chronic glomerulonephritis | Lupus nephritis | IgA nephropathy | Horseshoe kidney | Diabetic nephropathy | Glycogen storage disease |
| Duration of dialysis, years | 6 | 4 | 24 | 14 | 19 | 6 | 3 |
| Past Medical History and Comorbidity | |||||||
| Prior pulmonary thromboembolism | No | No | No | No | No | No | No |
| Prior myocardial infarction | No | No | No | No | No | No | No |
| Coronary artery disease | No | No | No | Yes | No | Yes | No |
| Valvular heart disease | No | No | No | No | No | No | No |
| Intracardiac shunt | No | No | No | No | No | No | No |
| Atrial fibrillation/Atrial flutter | No | No | No | No | No | Yes | No |
| Interstitial lung disease | No | No | No | No | No | No | No |
| Chronic obstructive pulmonary disease | No | No | No | No | No | No | No |
| Hypertension | Yes | Yes | Yes | No | Yes | No | Yes |
| Diabetes mellitus | Yes | No | No | No | No | Yes | No |
| Echocardiographic Parameters | |||||||
| LVEF, % | 69 | 75 | 74 | 62 | 70 | 66 | 61 |
| E/e’ | 16.8 | 19.5 | 31.5 | 9.4 | 17.8 | 14.9 | 18.6 |
| e’, cm/sec | 4.3 | 5.1 | 4.8 | 3.9 | 4.5 | 5.2 | 3.6 |
| Left atrial volume index, mL/m2 | N/A | N/A | 53.0 | 41.5 | 37.6 | N/A | 47.0 |
| Tricuspid regurgitant velocity, m/s | 3.8 | 3.2 | 3.8 | 3.5 | 3.8 | 3.7 | 4.3 |
| Treatment | |||||||
| Pulmonary vasodilator | Bosentan 62.5 mg b.i.d., Sildenafil 10 mg t.i.d. | Macitentan 10 mg q.d., Sildenafil 20 mg t.i.d. | Sildenafil 10 mg t.i.d. | Selexipag 1.0 mg b.i.d. | Treprostinil (subcutaneous) 50 ng/kg/min, Sildenafil 10 mg t.i.d., Bosentan 62.5 mg b.i.d. | Selexipag 2.0 mg b.i.d. | Ambrisentan 5.0 mg q.d., Sildenafil 10 mg b.i.d., Selexipag 0.8 mg b.i.d. |
| Observation period from baseline to post-treatment evaluation, months | 8 | 7 | 6 | 6 | 45 | 6 | 108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimuro, K.; Hosokawa, K.; Abe, K.; Masaki, K.; Imakiire, S.; Sakamoto, T.; Tsutsui, H. Beneficial Effects of Pulmonary Vasodilators on Pre-Capillary Pulmonary Hypertension in Patients with Chronic Kidney Disease on Hemodialysis. Life 2022, 12, 780. https://doi.org/10.3390/life12060780
Kimuro K, Hosokawa K, Abe K, Masaki K, Imakiire S, Sakamoto T, Tsutsui H. Beneficial Effects of Pulmonary Vasodilators on Pre-Capillary Pulmonary Hypertension in Patients with Chronic Kidney Disease on Hemodialysis. Life. 2022; 12(6):780. https://doi.org/10.3390/life12060780
Chicago/Turabian StyleKimuro, Keiji, Kazuya Hosokawa, Kohtaro Abe, Kohei Masaki, Satomi Imakiire, Takafumi Sakamoto, and Hiroyuki Tsutsui. 2022. "Beneficial Effects of Pulmonary Vasodilators on Pre-Capillary Pulmonary Hypertension in Patients with Chronic Kidney Disease on Hemodialysis" Life 12, no. 6: 780. https://doi.org/10.3390/life12060780
APA StyleKimuro, K., Hosokawa, K., Abe, K., Masaki, K., Imakiire, S., Sakamoto, T., & Tsutsui, H. (2022). Beneficial Effects of Pulmonary Vasodilators on Pre-Capillary Pulmonary Hypertension in Patients with Chronic Kidney Disease on Hemodialysis. Life, 12(6), 780. https://doi.org/10.3390/life12060780






