Decellularized Wharton Jelly Implants Do Not Trigger Collagen and Cartilaginous Tissue Production in Tracheal Injury in Rabbits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Umbilical Cord
2.2. WJ Decellularization
2.3. Surgical Procedure
2.4. Euthanasia
2.5. Histopathology
2.6. Statistical Analysis
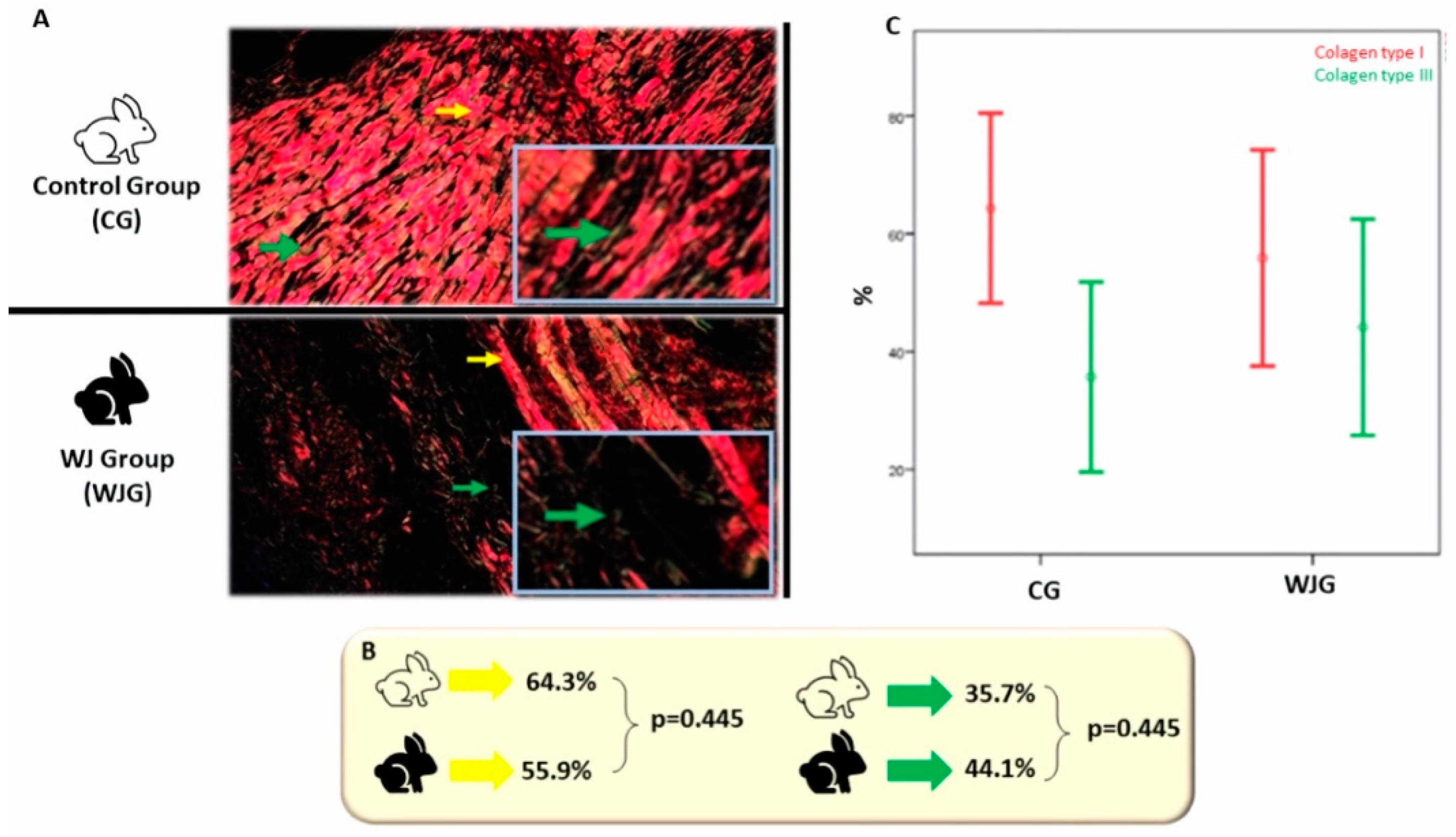
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhasmana, A.; Singh, A.; Rawal, S. Biomedical grafts for tracheal tissue repairing and regeneration Tracheal tissue engineering: An overview. J. Tissue Eng. Regen. Med. 2020, 14, 653–672. [Google Scholar] [CrossRef] [PubMed]
- Vachiéry, J.L.; Delcroix, M.; Al-Hiti, H.; Efficace, M.; Hutyra, M.; Lack, G.; Papadakis, K.; Rubin, L.J. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur. Respir. J. 2018, 51, 1701886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turakhia, A.; Little, B.P.; Henry, T.S. Tracheal Narrowing and Tracheomalacia. In Chest Imaging; American Thoracic Society: New York, NY, USA, 2019. [Google Scholar]
- Jeong, H.J.; Nam, H.; Jang, J.; Lee, S.J. 3D bioprinting strategies for the regeneration of functional tubular tissues and organs. Bioengineering 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorge, L.F.; Francisco, J.C.; Bergonse, N.; Baena, C.; Carvalho, K.A.T.; Abdelwahid, E.; Neto, J.R.F.; Moreira, L.F.P.; Guarita–Souza, L.C. Tracheal repair with acellular human amniotic membrane in a rabbit model. J. Tissue Eng. Regen. Med. 2018, 12, e1525–e1530. [Google Scholar] [CrossRef]
- Park, J.H.; Jang, J.; Lee, J.S.; Cho, D.W. Current advances in three-dimensional tissue/organ printing. Tissue Eng. Regen. Med. 2016, 13, 612–621. [Google Scholar] [CrossRef]
- Laurenti, M.; Cauda, V. ZnO nanostructures for tissue engineering applications. Nanomaterials 2017, 7, 374. [Google Scholar] [CrossRef] [Green Version]
- Wurtz, A.; Hysi, I.; Kipnis, E.; Zawadzki, C.; Hubert, T.; Jashari, R.; Copin, M.C.; Jude, B. Tracheal reconstruction with a composite graft: Fascial flap-wrapped allogenic aorta with external cartilage-ring support. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Neto, N.B.; Jorge, L.F.; Francisco, J.C.; Erbano, B.O.; Barboza, B.E.G.; Da Silva, L.L.G.; Olandoski, M.; De Carvalho, K.A.T.; Moreira, L.F.P.; Neto, J.R.F.; et al. Regeneration of tracheal tissue in partial defects using porcine small intestinal submucosa. Stem Cells Int. 2018, 2018, 5102630. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Jadalannagari, S.; Converse, G.; McFall, C.; Buse, E.; Filla, M.; Villar, M.T.; Artigues, A.; Mellot, A.J.; Wang, J.; Detamore, M.S.; et al. Decellularized Wharton’s Jelly from human umbilical cord as a novel 3D scaffolding material for tissue engineering applications. PLoS ONE 2017, 12, e0172098. [Google Scholar] [CrossRef]
- Arutyunyan, I.; Fatkhudinov, T.; Sukhikh, G. Umbilical cord tissue cryopreservation: A short review. Stem Cell Res. Ther. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, V.L.; Dodson, R.B. Bioengineering aspects of the umbilical cord. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 144, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for in situ tissue regeneration: A review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, L.; Castaldi, M.A.; Rosamilio, R.; Ragni, E.; Vitolo, R.; Fulgione, C.; Castaldi, S.G.; Serio, B.; Bianco, R.; Guida, M.; et al. Mesenchymal stem cells from the Wharton’s jelly of the human umbilical cord: Biological properties and therapeutic potential. Int. J. Stem Cells 2019, 12, 218–226. [Google Scholar] [CrossRef]
- Hopper, R.A.; Woodhouse, K.; Semple, J.L. Acellularization of Human Placenta with Preservation of the Basement Membrane: A Potential Matrix for Tissue Engineering. Ann. Plast. Surg. 2003, 51, 598–602. [Google Scholar] [CrossRef]
- Francisco, J.C.; Correa Cunha, R.; Cardoso, M.A.; Baggio Simeoni, R.; Mogharbel, B.F.; Picharski, G.L.; Silva Moreira Dziedzic, D.; Guarita-Souza, L.C.; Carvalho, K.A.T. Decellularized Amniotic Membrane Scaffold as a Pericardial Substitute: An In Vivo Study. Transplant. Proc. 2016, 48, 2845–2849. [Google Scholar] [CrossRef]
- Orth, P.; Eldracher, M.; Cucchiarini, M.; Madry, H. Small-diameter subchondral drilling improves dna and proteoglycan content of the cartilaginous repair tissue in a large animal model of a full-thickness chondral defect. J. Clin. Med. 2020, 9, 1903. [Google Scholar] [CrossRef]
- Wurtz, A.; Hysi, I.; Zawadzki, C.; Soenen, V.; Hubert, T.; Banfi, C.; Jashari, R.; Copin, M.C. Construction of a tube-shaped tracheal substitute using fascial flap-wrapped revascularized allogenic aorta. Eur. J. Cardiothorac. Surg. 2012, 41, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Ott, L.M.; Zabel, T.A.; Walker, N.K.; Farris, A.L.; Chakroff, J.T.; Ohst, D.G.; Johnson, J.K.; Gehrke, S.H.; Weatherly, R.A.; Detamore, M.S. Mechanical evaluation of gradient electrospun scaffolds with 3D printed ring reinforcements for tracheal defect repair. Biomed. Mater. 2016, 11, 25020. [Google Scholar] [CrossRef] [Green Version]
- Schopf, L.F.; Fraga, J.C.; Porto, R.; Santos, L.A.; Marques, D.R.; Sanchez, P.R.; Meyer, F.S.; Ulbrich, J.M. Experimental use of new absorbable tracheal stent. J. Pediatr. Surg. 2018, 53, 1305–1309. [Google Scholar] [CrossRef]
- Choi, J.S.; Kim, J.M.; Kim, J.W.; Kim, Y.M.; Park, I.S.; Yang, S.G. Prevention of tracheal inflammation and fibrosis using nitinol stent coated with doxycycline. Laryngoscope 2018, 128, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fang, Q.; Lin, H.; Yu, X.; Zheng, H.; Wan, Y. Alginate-poloxamer/silk fibroin hydrogels with covalently and physically cross-linked networks for cartilage tissue engineering. Carbohydr. Polym. 2020, 247, 116593. [Google Scholar] [CrossRef] [PubMed]
- Main, B.J.; Maffulli, N.; Valk, J.A.; Rodriguez, H.C.; Gupta, M.; El-Amin, S.F.; Gupta, A. Umbilical cord-derived wharton’s jelly for regenerative medicine applications: A systematic review. Pharmaceuticals 2021, 14, 1090. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Caramella, C.; Catenacci, L.; Conti, B.; Dorati, R.; Ferrari, F.; Genta, I.; Modena, T.; Perteghella, S.; Rossi, S.; et al. Biomaterials for soft tissue repair and regeneration: A focus on italian research in the field. Pharmaceutics 2021, 13, 1341. [Google Scholar] [CrossRef]
- Chiang, T.; Pepper, V.; Best, C.; Onwuka, E.; Breuer, C.K. Clinical Translation of Tissue Engineered Trachea Grafts. Ann. Otol. Rhinol. Laryngol. 2016, 125, 873–885. [Google Scholar] [CrossRef] [Green Version]
- Schenke-Layland, K.; Opitz, F.; Gross, M.; Döring, C.; Halbhuber, K.J.; Schirrmeister, F.; Wahlers, T.; Stock, U.A. Complete dynamic repopulation of decellularized heart valves by application of defined physical signals—An in vitro study. Cardiovasc. Res. 2003, 60, 497–509. [Google Scholar] [CrossRef]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A Comprehensive Review on Metallic Implant Biomaterials and Their Subtractive Manufacturing; Springer: Berlin/Heidelberg, Germany, 2022; ISBN 0123456789. [Google Scholar]
- Xu, Y.; Duan, L.; Li, Y.; She, Y.; Zhu, J.; Zhou, G.; Jiang, G.; Yang, Y. Nanofibrillar Decellularized Wharton’s Jelly Matrix for Segmental Tracheal Repair. Adv. Funct. Mater. 2020, 30, 1910067. [Google Scholar] [CrossRef]
- Baggio Simeoni, P.R.; Simeoni, R.B.; Bispo Machado Júnior, P.A.; de Almeida, M.B.; Dziedzic, D.S.M.; da Rosa, N.N.; Stricker, P.E.F.; Dos Santos Miggiolaro, A.F.R.; Naves, G.; Neto, N.B.; et al. Tracheal repair with human umbilical cord mesenchymal stem cells differentiated in chondrocytes grown on an acellular amniotic membrane: A pre-clinical approach. Life 2021, 11, 879. [Google Scholar] [CrossRef]



| Control Group (n = 10) | WJ Group (n = 10) | p | |
|---|---|---|---|
| Weight (kg) * | 2.91 ± 0.30 | 2.77 ± 0.21 | 0.261 |
| Tracheal area (µm²) * | 9,654,271.1 ± 4,687,386.9 | 10,546,349.7 ± 3,724,181.3 | 0.643 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foltz, K.M.; Neto, A.E.; Francisco, J.C.; Simeoni, R.B.; Miggiolaro, A.F.R.d.S.; do Nascimento, T.G.; Mogharbel, B.F.; de Carvalho, K.A.T.; Faria-Neto, J.R.; de Noronha, L.; et al. Decellularized Wharton Jelly Implants Do Not Trigger Collagen and Cartilaginous Tissue Production in Tracheal Injury in Rabbits. Life 2022, 12, 942. https://doi.org/10.3390/life12070942
Foltz KM, Neto AE, Francisco JC, Simeoni RB, Miggiolaro AFRdS, do Nascimento TG, Mogharbel BF, de Carvalho KAT, Faria-Neto JR, de Noronha L, et al. Decellularized Wharton Jelly Implants Do Not Trigger Collagen and Cartilaginous Tissue Production in Tracheal Injury in Rabbits. Life. 2022; 12(7):942. https://doi.org/10.3390/life12070942
Chicago/Turabian StyleFoltz, Katia Martins, Aloysio Enck Neto, Júlio César Francisco, Rossana Baggio Simeoni, Anna Flávia Ribeiro dos Santos Miggiolaro, Thatyanne Gradowski do Nascimento, Bassam Felipe Mogharbel, Katherine Athayde Teixeira de Carvalho, José Rocha Faria-Neto, Lúcia de Noronha, and et al. 2022. "Decellularized Wharton Jelly Implants Do Not Trigger Collagen and Cartilaginous Tissue Production in Tracheal Injury in Rabbits" Life 12, no. 7: 942. https://doi.org/10.3390/life12070942






