Recurrent Somatic Copy Number Alterations and Their Association with Oncogene Expression Levels in High-Grade Ovarian Serous Carcinoma
Abstract
:1. Introduction
2. Material and Methods
2.1. TCGA, PanCancer Atlas High-Grade Ovarian Serous Carcinoma Profiled Samples
2.2. Identification of Genes with Recurrent Somatic Copy Number Alterations
2.3. Correlation between Genes with Recurrent SCNA Correlation and mRNA Expression
2.4. Identification of Cancer-Related Genes with Recurrent SCNA
2.5. Association of Cancer Related Genes and Clinicopathological Parameters
3. Results
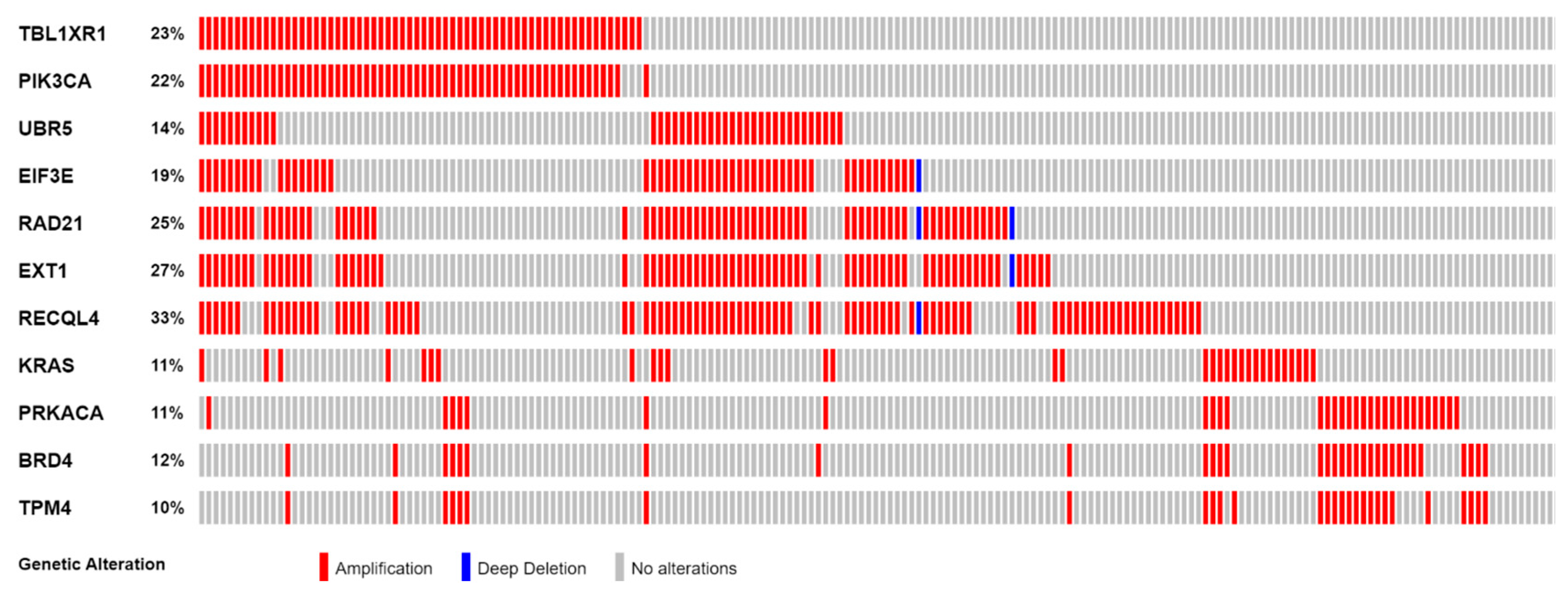
3.1. Genes with Recurrent SCNA
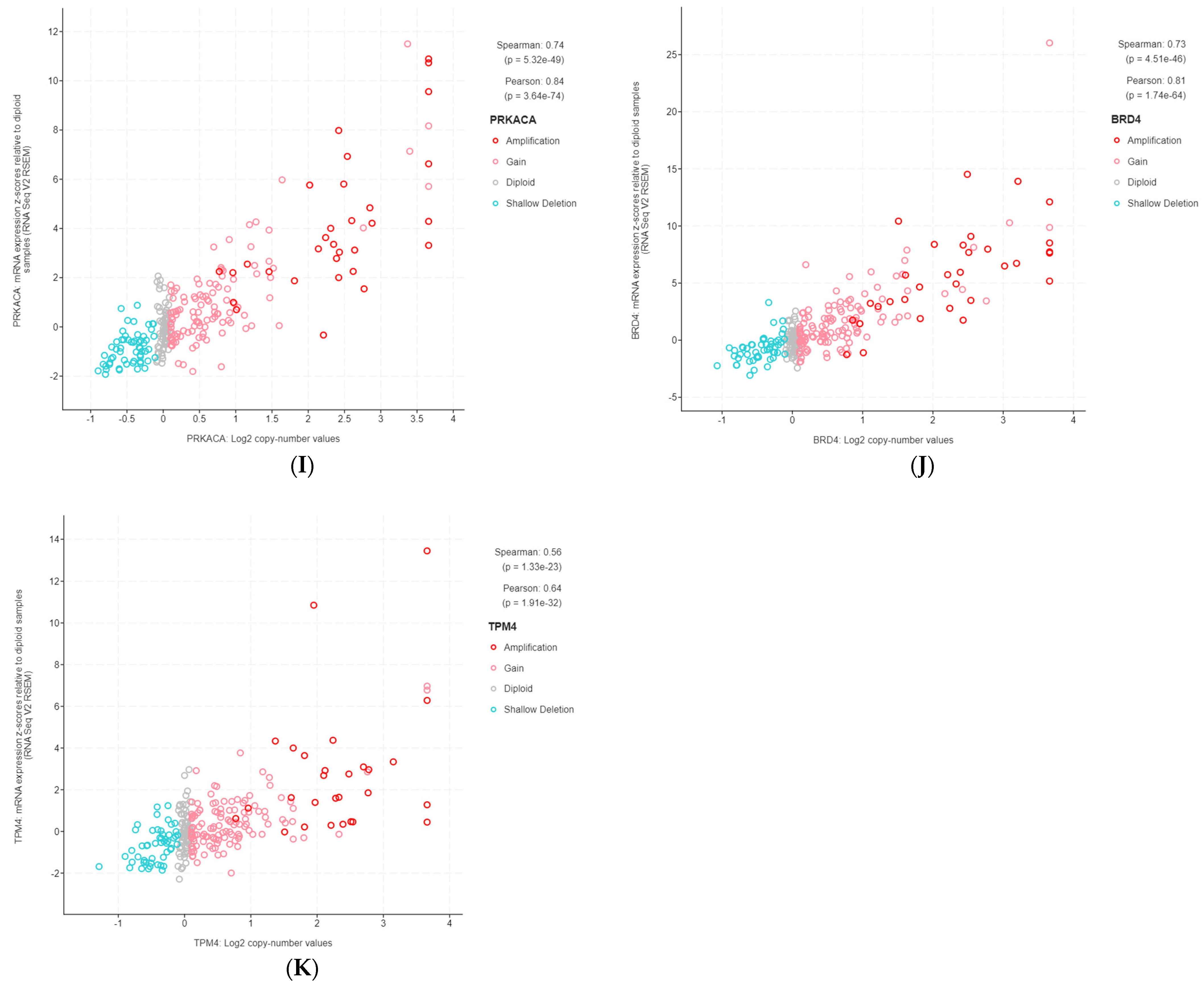
3.2. Highly Correlated, Tier 1 CGC-COSMIC Genes with Recurrent SCNA
3.3. Association of Highly Correlated, Cancer-Related Genes with Clinicopathological Parameters
3.3.1. Association with Age at Diagnosis
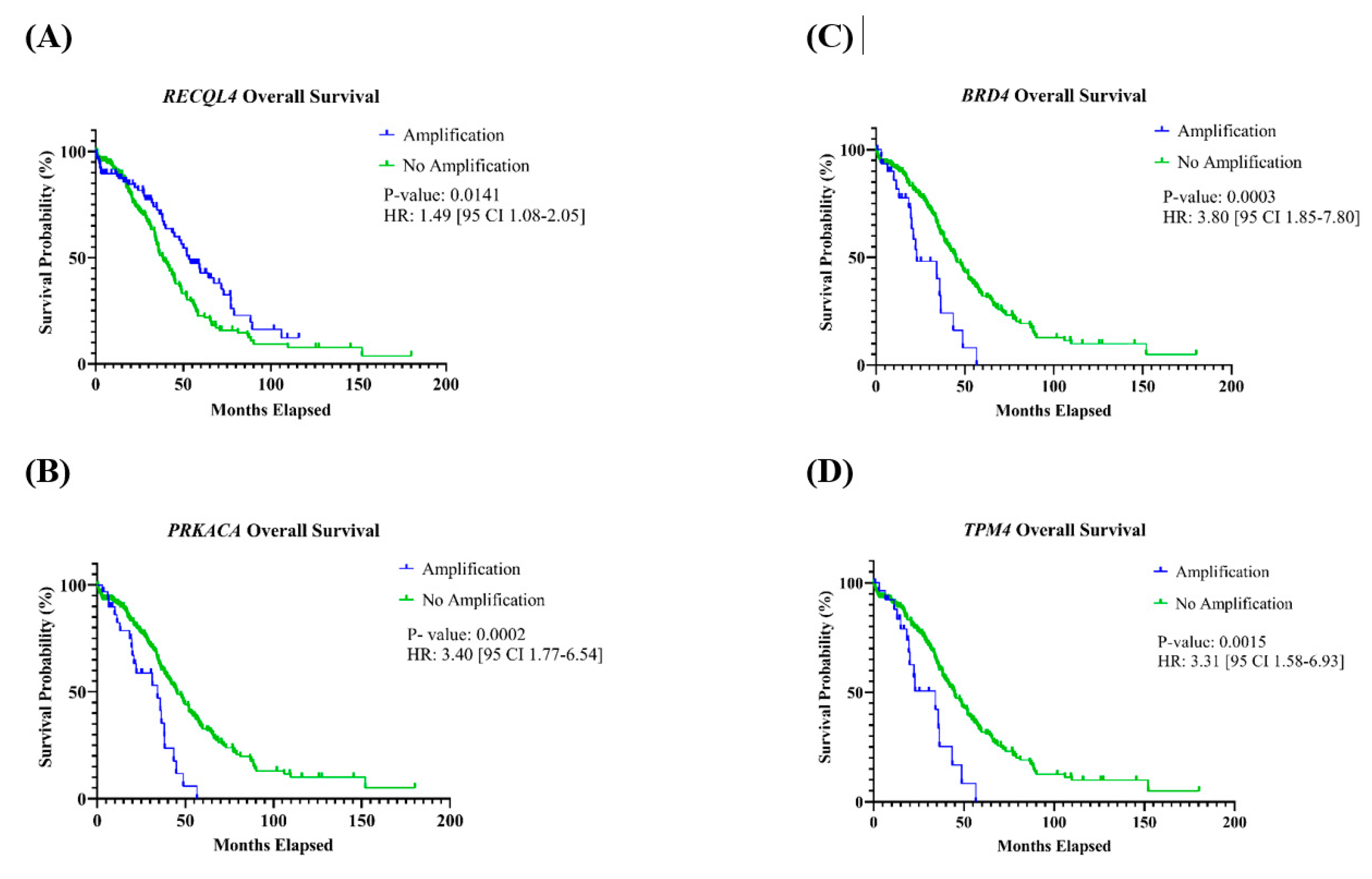
3.3.2. Association with Overall and Progression-Free Survival
3.3.3. Association with Disease Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Hirst, J.; Crow, J.; Godwin, A. Ovarian Cancer Genetics: Subtypes and Risk Factors; IntechOpen: London, UK, 2018. [Google Scholar]
- Nitecki, R.; Cobb, L.P.; Jazaeri, A.A.; Rauh-Hain, J.A. Ovarian Cancer. In The MD Anderson Manual of Medical Oncology, 4th ed.; Kantarjian, H.M., Wolff, R.A., Rieber, A.G., Eds.; McGraw Hill Education: New York, NY, USA, 2022. [Google Scholar]
- WHO. Classification of Tumours Editorial Board. Female Genital Tumours; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- Hunter, S.M.; Anglesio, M.S.; Ryland, G.L.; Sharma, R.; Chiew, Y.E.; Rowley, S.M.; Doyle, M.A.; Li, J.; Gilks, C.B.; Moss, P.; et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget 2015, 6, 37663–37677. [Google Scholar] [CrossRef] [PubMed]
- Kotsopoulos, J.; Zamani, N.; Rosen, B.; McLaughlin, J.R.; Risch, H.A.; Kim, S.J.; Sun, P.; Akbari, M.R.; Narod, S.A. Impact of germline mutations in cancer-predisposing genes on long-term survival in patients with epithelial ovarian cancer. Br. J. Cancer 2022, 127, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.T.; Guan, B.; Feng, Y.; Mao, T.L.; Chen, X.; Jinawath, N.; Wang, Y.; Kurman, R.J.; Shih, I.-M.; Wang, T.L. Analysis of DNA copy number alterations in ovarian serous tumors identifies new molecular genetic changes in low-grade and high-grade carcinomas. Cancer Res. 2009, 69, 4036–4042. [Google Scholar] [CrossRef]
- Petersen, S.; Wilson, A.J.; Hirst, J.; Roby, K.F.; Fadare, O.; Crispens, M.A.; Beeghly-Fadiel, A.; Khabele, D. CCNE1 and BRD4 co-amplification in high-grade serous ovarian cancer is associated with poor clinical outcomes. Gynecol. Oncol. 2020, 157, 405–410. [Google Scholar] [CrossRef]
- Rhyasen, G.W.; Yao, Y.; Zhang, J.; Dulak, A.; Castriotta, L.; Jacques, K.; Zhao, W.; Gharahdaghi, F.; Hattersley, M.M.; Lyne, P.D.; et al. BRD4 amplification facilitates an oncogenic gene expression program in high-grade serous ovarian cancer and confers sensitivity to BET inhibitors. PLoS ONE 2018, 13, e0200826. [Google Scholar] [CrossRef]
- Sung, C.O.; Song, I.H.; Sohn, I. A distinctive ovarian cancer molecular subgroup characterized by poor prognosis and somatic focal copy number amplifications at chromosome 19. Gynecol. Oncol. 2014, 132, 343–350. [Google Scholar] [CrossRef]
- Drumond-Bock, A.L.; Bieniasz, M. The role of distinct BRD4 isoforms and their contribution to high-grade serous ovarian carcinoma pathogenesis. Mol. Cancer 2021, 20, 145. [Google Scholar] [CrossRef]
- Quintela, M.; James, D.W.; Pociute, A.; Powell, L.; Edwards, K.; Coombes, Z.; Garcia, J.; Garton, N.; Das, N.; Lutchman-Singh, K.; et al. Bromodomain inhibitor i-BET858 triggers a unique transcriptional response coupled to enhanced DNA damage, cell cycle arrest and apoptosis in high-grade ovarian carcinoma cells. Clin. Epigenetics 2023, 15, 63. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Mirza, H.; Ennis, D.P.; Smith, P.; Morrill Gavarro, L.; Sokota, C.; Giannone, G.; Goranova, T.; Bradley, T.; Piskorz, A.; et al. The Genomic Landscape of Early-Stage Ovarian High-Grade Serous Carcinoma. Clin. Cancer Res. 2022, 28, 2911–2922. [Google Scholar] [CrossRef] [PubMed]
- Engler, D.A.; Gupta, S.; Growdon, W.B.; Drapkin, R.I.; Nitta, M.; Sergent, P.A.; Allred, S.F.; Gross, J.; Deavers, M.T.; Kuo, W.L.; et al. Genome wide DNA copy number analysis of serous type ovarian carcinomas identifies genetic markers predictive of clinical outcome. PLoS ONE 2012, 7, e30996. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network; Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Ben-David, U.; Amon, A. Context is everything: Aneuploidy in cancer. Nat. Rev. Genet. 2020, 21, 44–62. [Google Scholar] [CrossRef] [PubMed]
- Knouse, K.A.; Davoli, T.; Elledge, S.J.; Amon, A. Aneuploidy in Cancer: Seq-ing Answers to Old Questions. Annu. Rev. Cancer Biol. 2017, 1, 335–354. [Google Scholar] [CrossRef]
- Hoadley, K.A.; Yau, C.; Hinoue, T.; Wolf, D.M.; Lazar, A.J.; Drill, E.; Shen, R.; Taylor, A.M.; Cherniack, A.D.; Thorsson, V.; et al. Cell-of-Origin Patterns Dominate the Molecular Classification of 10,000 Tumors from 33 Types of Cancer. Cell 2018, 173, 291–304.E6. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Guo, L.; Li, Y.; Zhao, C.; Peng, J.; Song, K.; Chen, L.; Zhang, P.; Ma, H.; Yuan, C.; Yan, S.; et al. RECQL4, Negatively Regulated by miR-10a-5p, Facilitates Cell Proliferation and Invasion via MAFB in Ovarian Cancer. Front. Oncol. 2020, 10, 524128. [Google Scholar] [CrossRef]
- McCarroll, S.A.; Kuruvilla, F.G.; Korn, J.M.; Cawley, S.; Nemesh, J.; Wysoker, A.; Shapero, M.H.; de Bakker, P.I.; Maller, J.B.; Kirby, A.; et al. Integrated detection and population-genetic analysis of SNPs and copy number variation. Nat. Genet. 2008, 40, 1166–1174. [Google Scholar] [CrossRef]
- Beroukhim, R.; Getz, G.; Nghiemphu, L.; Barretina, J.; Hsueh, T.; Linhart, D.; Vivanco, I.; Lee, J.C.; Huang, J.H.; Alexander, S.; et al. Assessing the significance of chromosomal aberrations in cancer: Methodology and application to glioma. Proc. Natl. Acad. Sci. USA 2007, 104, 20007–20012. [Google Scholar] [CrossRef] [PubMed]
- Mermel, C.H.; Schumacher, S.E.; Hill, B.; Meyerson, M.L.; Beroukhim, R.; Getz, G. GISTIC2.0 facilitates sensitive and confident localization of the targets of focal somatic copy-number alteration in human cancers. Genome Biol. 2011, 12, R41. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal 2013, 6, pl1. [Google Scholar] [CrossRef] [PubMed]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Gruning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef]
- Sondka, Z.; Bamford, S.; Cole, C.G.; Ward, S.A.; Dunham, I.; Forbes, S.A. The COSMIC Cancer Gene Census: Describing genetic dysfunction across all human cancers. Nat. Rev. Cancer 2018, 18, 696–705. [Google Scholar] [CrossRef]
- Deng, P.; Wang, Z.; Chen, J.; Liu, S.; Yao, X.; Liu, S.; Liu, L.; Yu, Z.; Huang, Y.; Xiong, Z.; et al. RAD21 amplification epigenetically suppresses interferon signaling to promote immune evasion in ovarian cancer. J. Clin. Investig. 2022, 132, e159628. [Google Scholar] [CrossRef]
- Ucar, D.; Lin, D.I. Amplification of the bromodomain-containing protein 4 gene in ovarian high-grade serous carcinoma is associated with worse prognosis and survival. Mol. Clin. Oncol. 2015, 3, 1291–1294. [Google Scholar] [CrossRef]
- Vias, M.; Morrill Gavarro, L.; Sauer, C.M.; Sanders, D.A.; Piskorz, A.M.; Couturier, D.L.; Ballereau, S.; Hernando, B.; Schneider, M.P.; Hall, J.; et al. High-grade serous ovarian carcinoma organoids as models of chromosomal instability. Elife 2023, 12, e83867. [Google Scholar] [CrossRef]
- Birkeland, E.; Wik, E.; Mjos, S.; Hoivik, E.A.; Trovik, J.; Werner, H.M.; Kusonmano, K.; Petersen, K.; Raeder, M.B.; Holst, F.; et al. KRAS gene amplification and overexpression but not mutation associates with aggressive and metastatic endometrial cancer. Br. J. Cancer 2012, 107, 1997–2004. [Google Scholar] [CrossRef]
- Saglam, O.; Tang, Z.; Tang, G.; Medeiros, L.J.; Toruner, G.A. KAT6A amplifications are associated with shorter progression-free survival and overall survival in patients with endometrial serous carcinoma. PLoS ONE 2020, 15, e0238477. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. 3q26 Amplifications in Cervical Squamous Carcinomas. Curr. Oncol. 2021, 28, 2868–2880. [Google Scholar] [CrossRef] [PubMed]
- Silvera, D.; Formenti, S.C.; Schneider, R.J. Translational control in cancer. Nat. Rev. Cancer 2010, 10, 254–266. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; Jiang, T.; Wang, J.; Lu, H.; Tang, H.; Teng, M.; Fan, J. Overexpression of eIF3e is correlated with colon tumor development and poor prognosis. Int. J. Clin. Exp. Pathol. 2014, 7, 6462–6474. [Google Scholar]
- Yong, Z.W.; Zaini, Z.M.; Kallarakkal, T.G.; Karen-Ng, L.P.; Rahman, Z.A.; Ismail, S.M.; Sharifah, N.A.; Mustafa, W.M.; Abraham, M.T.; Tay, K.K.; et al. Genetic alterations of chromosome 8 genes in oral cancer. Sci. Rep. 2014, 4, 6073. [Google Scholar] [CrossRef]
- Cheng, H.; Zhang, N.; Pati, D. Cohesin subunit RAD21: From biology to disease. Gene 2020, 758, 144966. [Google Scholar] [CrossRef]
- Dong, S.; Wu, Y.; Yu, S.; Yang, Y.; Lu, L.; Fan, S. Increased EXT1 gene copy number correlates with increased mRNA level predicts short disease-free survival in hepatocellular carcinoma without vascular invasion. Medicine 2018, 97, e12625. [Google Scholar] [CrossRef]
- Luong, T.T.; Bernstein, K.A. Role and Regulation of the RECQL4 Family during Genomic Integrity Maintenance. Genes 2021, 12, 1919. [Google Scholar] [CrossRef]
- Turnham, R.E.; Scott, J.D. Protein kinase A catalytic subunit isoform PRKACA; History, function and physiology. Gene 2016, 577, 101–108. [Google Scholar] [CrossRef]
- Graham, R.P.; Jin, L.; Knutson, D.L.; Kloft-Nelson, S.M.; Greipp, P.T.; Waldburger, N.; Roessler, S.; Longerich, T.; Roberts, L.R.; Oliveira, A.M.; et al. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod. Pathol. 2015, 28, 822–829. [Google Scholar] [CrossRef]
- Lin, J.J.; Eppinga, R.D.; Warren, K.S.; McCrae, K.R. Human tropomyosin isoforms in the regulation of cytoskeleton functions. Adv. Exp. Med. Biol. 2008, 644, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ye, T.; Zhang, Q.; Li, X.; Ma, L.; Yan, J. The expression and clinical significance of TPM4 in hepatocellular carcinoma. Int. J. Med. Sci. 2021, 18, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Wu, Z.; Sun, P. Overexpression of TPM4 is associated with worse prognosis and immune infiltration in patients with glioma. BMC Neurol. 2023, 23, 17. [Google Scholar] [CrossRef] [PubMed]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol. 2017, 2017, PO.17.00011. [Google Scholar] [CrossRef] [PubMed]
- Kusnoor, S.V.; Koonce, T.Y.; Levy, M.A.; Lovly, C.M.; Naylor, H.M.; Anderson, I.A.; Micheel, C.M.; Chen, S.C.; Ye, F.; Giuse, N.B. My Cancer Genome: Evaluating an Educational Model to Introduce Patients and Caregivers to Precision Medicine Information. AMIA Jt. Summits Transl. Sci. Proc. 2016, 2016, 112–121. [Google Scholar]



| Cytoband | GRCh38 Coordinates | Size (Mb) | CNV | Tier 1 CGC-COSMIC Genes |
|---|---|---|---|---|
| 1p34.3-p34.2 | chr1:38838197–41242306 | 2.40 Mb | AMP | |
| 3q35.2-q29 | chr3:155290226–198222513 | 42.93 Mb | AMP | MECOM, TBL1XR1 *, PIK3CA *, SOX2, MAP3K13, ETV5, EIF4A2, BCL6, LPP, TP63 |
| 5q11.2-q12.1 | chr5:58969037–60488065 | 1.52 Mb | DEL | |
| 8p23.3-p23.2 | chr8:166085–4994914 | 4.83 Mb | DEL | |
| 8q22.2-q23.2 | chr8:98426957–109975771 | 11.55 Mb | AMP | UBR5 *, RSPO2, EIF3E * |
| 8q23.3-q24.23 | chr8:112222927–135647610 | 23.42 Mb | AMP | RAD21 *, EXT1 *, MYC, NDRG1 |
| 8q24.23-q24.3 | chr8:138130022–145056030 | 6.93 Mb | AMP | RECQL4 * |
| 11q14.1-q14.1 | chr11:77589952–79441030 | 1.85 Mb | AMP | |
| 12p12.1-p11.23 | chr12:23529503–26833194 | 3.30 Mb | AMP | KRAS * |
| 19p13.13-p13.11 | chr19:13731751–16471970 | 2.74 Mb | AMP | PRKACA *, DNAJB1, BRD4 *, TPM4 * |
| 19p12-p12 | chr19:23914885–24163447 | 0.25 Mb | AMP | |
| 19q12-q13.11 | chr19:28965130–34677159 | 5.71 Mb | AMP | CCNE1 |
| 19q13.2-q13.2 | chr19:38303557–39934634 | 1.63 Mb | AMP | |
| 22q13.32-q13.33 | chr22:48489552–50799637 | 2.31 Mb | DEL |
| Tier 1 CGC-COSMIC Gene | Association with Stage | Association with Age | Association with PFS | Association with OS |
|---|---|---|---|---|
| TBL1XR1 | p = 1.000 | p = 0.2251 | p = 0.1131 | p = 0.1011 |
| PIK3CA | p = 1.000 | p = 0.0896 | p = 0.3638 | p = 0.2521 |
| UBR5 | p = 0.4816 | p = 0.0665 | p = 0.1242 | p = 0.3576 |
| EIF3E | p = 0.2104 | p = 7.077 × 10−5 * | p = 0.7110 | p = 0.7091 |
| RAD21 | p = 0.7707 | p = 0.0003 * | p = 0.3300 | p = 0.1463 |
| EXT1 | p = 0.5722 | p = 0.0001 * | p = 0.8829 | p = 0.3558 |
| RECQL4 | p = 0.5947 | p = 0.0002 * | p = 0.2713 | p = 0.0141 * |
| KRAS | p = 1.000 | p = 0.5480 | p = 0.4719 | p = 0.1313 |
| PRKACA | p = 0.7026 | p = 0.1137 | p = 0.4412 | p = 0.0002 * |
| BRD4 | p = 0.2362 | p = 0.4320 | p = 0.7229 | p = 0.0003 * |
| TPM4 | p = 1.000 | p = 0.5322 | p = 0.7904 | p = 0.0015 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esplen, H.P.; Yang, R.K.; Kalia, A.; Tang, Z.; Tang, G.; Medeiros, L.J.; Toruner, G.A. Recurrent Somatic Copy Number Alterations and Their Association with Oncogene Expression Levels in High-Grade Ovarian Serous Carcinoma. Life 2023, 13, 2192. https://doi.org/10.3390/life13112192
Esplen HP, Yang RK, Kalia A, Tang Z, Tang G, Medeiros LJ, Toruner GA. Recurrent Somatic Copy Number Alterations and Their Association with Oncogene Expression Levels in High-Grade Ovarian Serous Carcinoma. Life. 2023; 13(11):2192. https://doi.org/10.3390/life13112192
Chicago/Turabian StyleEsplen, Hillary P., Richard K. Yang, Awdhesh Kalia, Zhenya Tang, Guilin Tang, L. Jeffrey Medeiros, and Gokce A. Toruner. 2023. "Recurrent Somatic Copy Number Alterations and Their Association with Oncogene Expression Levels in High-Grade Ovarian Serous Carcinoma" Life 13, no. 11: 2192. https://doi.org/10.3390/life13112192





