Changes in the Bacterial Communities of Biocomposites with Different Flame Retardants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biocomposite Board Formation
2.2. Genomic DNA Extraction from Biobased Composite Boards
2.3. Next Generation Sequencing
2.4. Bioinformatic Data Analysis
2.5. Assessing the Impact of the EG and Flovan Solutions on Bacterial Growth
3. Results
3.1. Bacterial Composition of BcBs and Hemp Shives
3.2. The Viability of Pseudomonas aeruguinosa and Bacilus subtilis in the Growth Media Containing EG or Flovan
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, M.; Chen, L.; Wang, J.; Msigwa, G.; Osman, A.I.; Fawzy, S.; Rooney, D.W.; Yap, P.S. Circular Economy Strategies for Combating Climate Change and Other Environmental Issues. Environ. Chem. Lett. 2023, 21, 55–80. [Google Scholar] [CrossRef]
- Velenturf, A.P.M.; Purnell, P. Principles for a Sustainable Circular Economy. Sustain. Prod. Consum. 2021, 27, 1437–1457. [Google Scholar] [CrossRef]
- Li, X.; Tabil, L.G.; Panigrahi, S. Chemical Treatments of Natural Fiber for Use in Natural Fiber-Reinforced Composites: A Review. J. Polym. Environ. 2007, 15, 5–33. [Google Scholar] [CrossRef]
- Madyaratri, E.W.; Ridho, M.R.; Aristri, M.A.; Lubis, M.A.R.; Iswanto, A.H.; Nawawi, D.S.; Antov, P.; Kristak, L.; Majlingová, A.; Fatriasari, W. Recent Advances in the Development of Fire-Resistant Biocomposites—A Review. Polymers 2022, 14, 362. [Google Scholar] [CrossRef] [PubMed]
- Przybylak, M.; Maciejewski, H.; Dutkiewicz, A.; Wesołek, D.; Władyka-Przybylak, M. Multifunctional, Strongly Hydrophobic and Flame-Retarded Cotton Fabrics Modified with Flame Retardant Agents and Silicon Compounds. Polym. Degrad. Stab. 2016, 128, 55–64. [Google Scholar] [CrossRef]
- Sun, L.; Fugetsu, B. Mass Production of Graphene Oxide from Expanded Graphite. Mater. Lett. 2013, 109, 207–210. [Google Scholar] [CrossRef]
- Acuña, P.; Li, Z.; Santiago-Calvo, M.; Villafañe, F.; Rodríguez-Perez, M.Á.; Wang, D.Y. Influence of the Characteristics of Expandable Graphite on the Morphology, Thermal Properties, Fire Behaviour and Compression Performance of a Rigid Polyurethane Foam. Polymers 2019, 11, 168. [Google Scholar] [CrossRef] [PubMed]
- Laachachi, A.; Burger, N.; Apaydin, K.; Sonnier, R.; Ferriol, M. Is Expanded Graphite Acting as Flame Retardant in Epoxy Resin? Polym. Degrad. Stab. 2015, 117, 22–29. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Maksimova, N.V.; Manylov, M.S.; Kirichenko, A.N.; Kalachev, I.L.; Malakho, A.P.; Avdeev, V.V. Gas Permeability of Graphite Foil Prepared from Exfoliated Graphite with Different Microstructures Chemical Routes to Materials. J. Mater. Sci. 2021, 56, 4197–4211. [Google Scholar] [CrossRef]
- Bykkam, S.; Narsingam, S.; Ahmadipour, M.; Dayakar, T.; Venkateswara Rao, K.; Shilpa Chakra, C.; Kalakotla, S. Few Layered Graphene Sheet Decorated by ZnO Nanoparticles for Anti-Bacterial Application. Superlattices Microstruct. 2015, 83, 776–784. [Google Scholar] [CrossRef]
- Maas, M. Carbon Nanomaterials as Antibacterial Colloids. Materials 2016, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.C.; Wu, K.H.; Lyu, D.Y.; Cheng, K.F.; Huang, W.C. Preparation and Characterization of Expanded Graphite/Metal Oxides for Antimicrobial Application. Mater. Sci. Eng. C 2017, 75, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Tremblay, P.L. Graphene: An Antibacterial Agent or a Promoter of Bacterial Proliferation? iScience 2020, 23, 101787. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.; Simon, L.; Ioannidis, N.; Patel, S.; Ophir, Z.; Gogos, C.; Jaffe, M.; Tirmizi, S.; Bonnett, P.; Abbate, P. Nitration Kinetics of Cellulose Fibers Derived from Wood Pulp in Mixed Acids. Ind. Eng. Chem. Res. 2018, 57, 1883–1893. [Google Scholar] [CrossRef]
- Sally, R.; Day, J. Flame Retardant Compositions Containing a Non-Salt Ammonia Neutralisate of a Nitrilotris (Alkylenephosphonic Acid). U.K. Patent GB 2306477, 7 May 1997. [Google Scholar]
- Shaik, N.R.; Kerru, N.; Chintha, V.; Khatana, K.; Sirigiri, D.N.R.; Chinnam, S.; Chamarthi, N.R. Synthesis of Novel Alkylphosphonates as Promising Antimicrobial Drugs: Computational Molecular Docking Studies. Phosphorus Sulfur Silicon Relat. Elem. 2021, 196, 722–730. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, G.; Wang, P.; Dai, F. A Novel ε-Polylysine-Derived Durable Phosphorus-nitrogen-based Flame Retardant for Cotton Fabrics. Cellulose 2021, 28, 3807–3822. [Google Scholar] [CrossRef]
- Wan, C.; Tian, P.; Liu, M.; Zhang, G.; Zhang, F. Synthesis of a Phosphorus-nitrogen Flame Retardant Endowing Cotton with High Whiteness and Washability. Ind. Crop. Prod. 2019, 141, 111738. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Zhang, J.; Wang, G.; Liu, Y.; Gao, C. Preparation and Properties of Flame Retardant Biobased Polyamide Elastomer with Shape Memory. Polym. Adv. Technol. 2023, 34, 1381–1392. [Google Scholar] [CrossRef]
- McLain, V.C. Final Report on the Safety Assessment of Triethanolamine, Diethanolamine and Monoethanolamine. J. Am. Coll. Toxicol. 1983, 2, 183–235. [Google Scholar]
- Zardini, H.Z.; Davarpanah, M.; Shanbedi, M.; Amiri, A.; Maghrebi, M.; Ebrahimi, L. Microbial Toxicity of Ethanolamines—Multiwalled Carbon Nanotubes. J. Biomed. Mater. Res. A 2014, 102, 1774–1781. [Google Scholar] [CrossRef]
- Auer, L.; Lazuka, A.; Sillam-Dussès, D.; Miambi, E.; O’Donohue, M.; Hernandez-Raquet, G. Uncovering the Potential of Termite Gut Microbiome for Lignocellulose Bioconversion in Anaerobic Batch Bioreactors. Front. Microbiol. 2017, 8, 2623. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Dong, Y.; Nguyen, T.T.; Chen, X.; Guo, M. Environment-Friendly Wood Fibre Composite with High Bonding Strength and Water Resistance. R. Soc. Open Sci. 2018, 5, 172002. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, D.; Sas-Paszt, L.; Derkowska, E.; Lisek, A.; Russel, S. Occurrence of Arbuscular Mycorrhizal Fungi in Hemp (Cannabis sativa) Plants and Soil Fertilized with Sewage Sludge and Phosphogypsum. J. Nat. Fibers 2021, 18, 250–260. [Google Scholar] [CrossRef]
- Taghinasab, M.; Jabaji, S. Cannabis Microbiome and the Role of Endophytes in Modulating the Production of Secondary Metabolites: An Overview. Microorganisms 2020, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Ji, A.; Jia, L.; Kumar, D.; Yoo, C.G. Recent Advancements in Biological Conversion of Industrial Hemp for Biofuel and Value-Added Products. Fermentation 2021, 7, 6. [Google Scholar] [CrossRef]
- Hidayat, B.J.; Felby, C.; Johansen, K.S.; Thygesen, L.G. Cellulose Is Not Just Cellulose: A Review of Dislocations as Reactive Sites in the Enzymatic Hydrolysis of Cellulose Microfibrils. Cellulose 2012, 19, 1481–1493. [Google Scholar] [CrossRef]
- Vasiliauskienė, D.; Balčiūnas, G.; Boris, R.; Kairytė, A.; Kremensas, A.; Urbonavičius, J. The Effect of Different Plant Oil Impregnation and Hardening Temperatures on Physical-Mechanical Properties of Modified Biocomposite Boards Made of Hemp Shives and Corn Starch. Materials 2020, 13, 5275. [Google Scholar] [CrossRef]
- Guerrini, C.J.; Botkin, J.R.; McGuire, A.L. Clarify the HIPAA Right of Access to Individuals’ Research Data. Nat. Biotechnol. 2019, 37, 850–852. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Maidak, B.L.; Cole, J.R.; Lilburn, T.G.; Parker, C.T.; Saxman, P.R.; Farris, R.J.; Garrity, G.M.; Olsen, G.J.; Schmidt, T.M.; Tiedje, J.M. The RDP-II (Ribosomal Database Project). Nucleic Acids Res. 2001, 29, 173–174. [Google Scholar] [CrossRef]
- Vasiliauskienė, D.; Boris, R.; Balčiūnas, G.; Kairytė, A.; Urbonavičius, J. Impact of Cellulolytic Fungi on Biodegradation of Hemp Shives and Corn Starch-Based Composites with Different Flame-Retardants. Microorganisms 2022, 10, 1830. [Google Scholar] [CrossRef]
- Vasiliauskienė, D.; Balčiūnas, G.; Boris, R.; Kairytė, A.; Urbonavičius, J. The Impact of Microorganisms on the Performance of Linseed Oil and Tung Tree Oil Impregnated Composites Made of Hemp Shives and Corn Starch. Microorganisms 2023, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bourdot, A.; Moussa, T.; Gacoin, A.; Maalouf, C.; Vazquez, P.; Thomachot-Schneider, C.; Bliard, C.; Merabtine, A.; Lachi, M.; Douzane, O.; et al. Characterization of a Hemp-Based Agro-Material: Influence of Starch Ratio and Hemp Shive Size on Physical, Mechanical, and Hygrothermal Properties. Energy Build. 2017, 153, 501–512. [Google Scholar] [CrossRef]
- Pundiene, I.; Vitola, L.; Pranckeviciene, J.; Bajare, D. Hemp Shive-Based Bio-Composites Bounded by Potato Starch Binder: The Roles of Aggregate Particle Size and Aspect Ratio. J. Ecol. Eng. 2022, 23, 220–234. [Google Scholar] [CrossRef]
- Khan, B.A.; Warner, P.; Wang, H. Antibacterial Properties of Hemp and Other Natural Fibre Plants: A Review. Bioresources 2014, 9, 3642–3659. [Google Scholar] [CrossRef]
- Peng, X.; Zheng, Q.; Liu, L.; He, Y.; Li, T.; Jia, X. Efficient Biodegradation of Tetrabromobisphenol A by the Novel Strain Enterobacter Sp. T2 with Good Environmental Adaptation: Kinetics, Pathways and Genomic Characteristics. J. Hazard. Mater. 2022, 429, 128335. [Google Scholar] [CrossRef]
- Green, S.K.; Schroth, M.N.; Cho, J.J.; Kominos, S.D.; Vitanza-Jack, V.B. Agricultural plants and soil as a reservoir for Pseudomonas aeruginosa. Appl. Microbiol. 1974, 28, 987–991. [Google Scholar] [CrossRef]
- Earl, A.M.; Losick, R.; Kolter, R. Ecology and genomics of Bacillus subtilis. Trends Microbiol. 2008, 16, 269–275. [Google Scholar] [CrossRef]
- Su, Y.; Liu, C.; Fang, H.; Zhang, D. Bacillus subtilis: A universal cell factory for industry, agriculture, biomaterials and medicine. Microb. Cell Fact. 2020, 19, 173. [Google Scholar] [CrossRef]
- Mulcahy, L.R.; Isabella, V.M.; Lewis, K. Pseudomonas aeruginosa biofilms in disease. Microb. Ecol. 2014, 68, 1–12. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Mu, C.; Meng, L.; Fei, Z.; Dyson, P.J. Recent Advances in Graphite Carbon Nitride-Based Nanocomposites: Structure, Antibacterial Properties and Synergies. Nanoscale Adv. 2021, 3, 3708–3729. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial Properties of Graphene-Based Nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, Y.; Wang, G.; Lu, H.; Ma, J.; Zhang, M.; Chen, X.; Yin, C.; Mao, Z. Controlled-Release Diammonium Phosphate Alleviates Apple Replant Disease: An Integrated Analysis of Soil Properties, Plant Growth, and the Soil Microbiome. J. Agric Food Chem. 2022, 70, 8942–8954. [Google Scholar] [CrossRef]

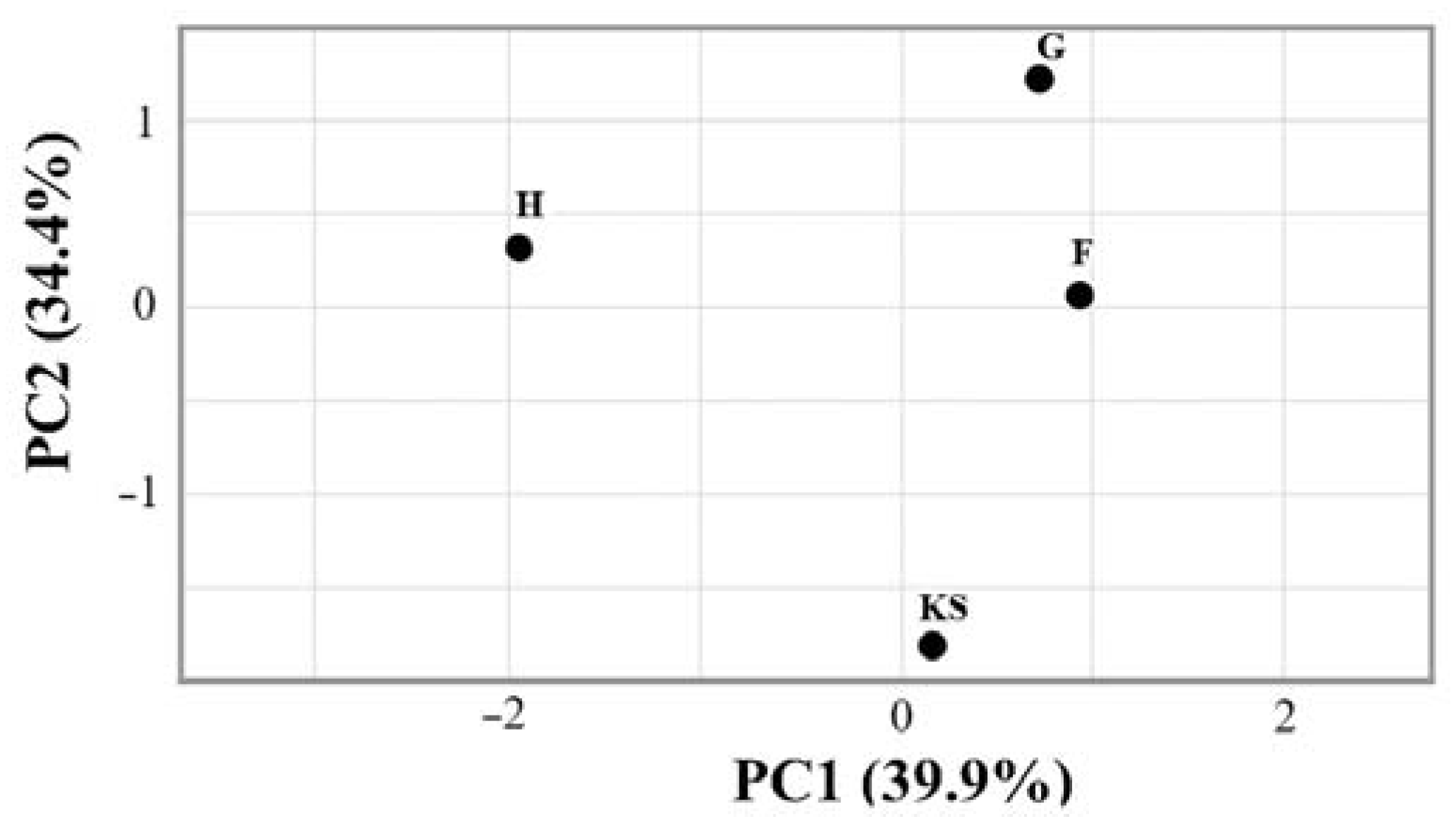
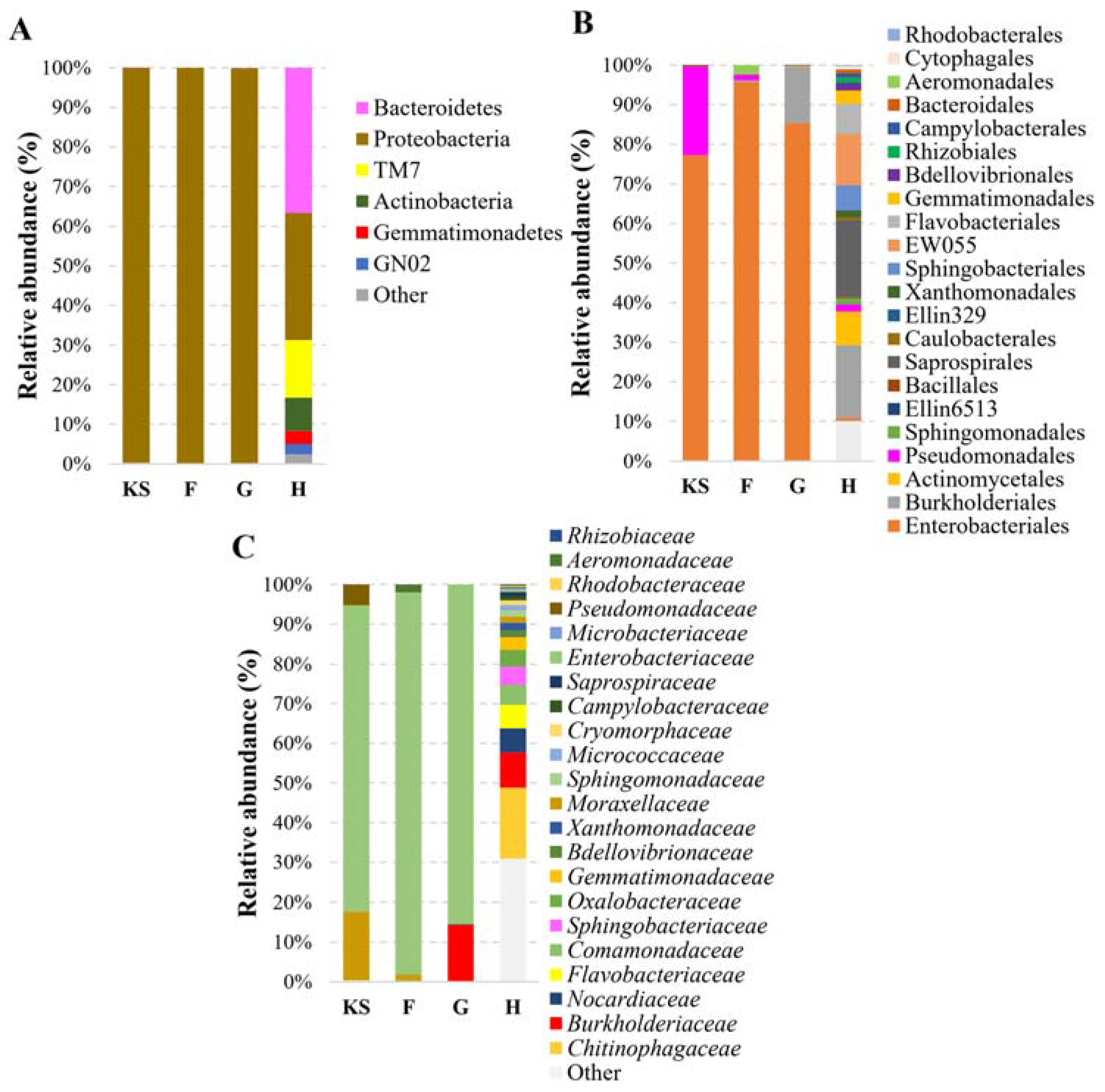
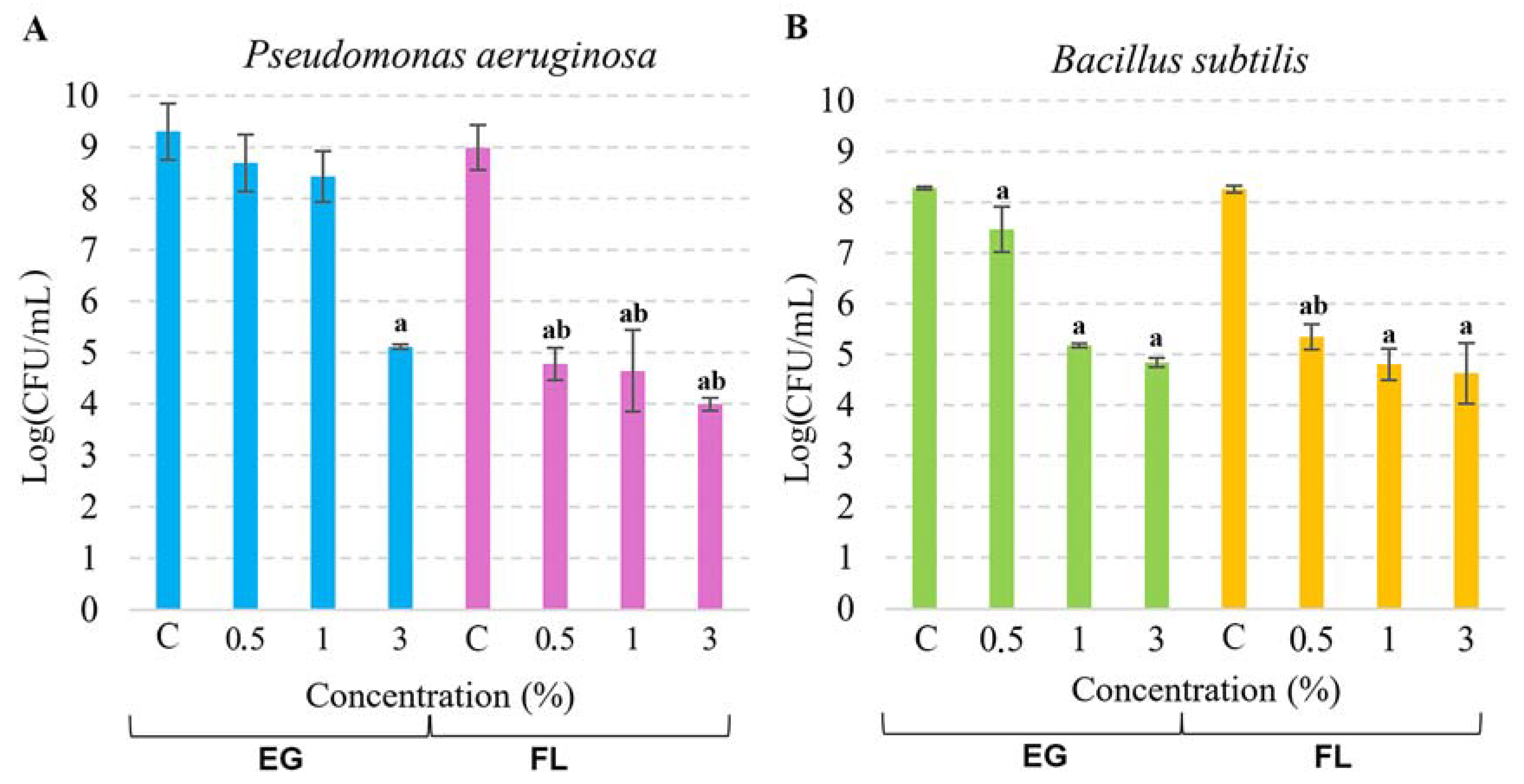
| Sample ID | Total Reads | HQ Reads | Shannon | ASVs | Faith_pd |
|---|---|---|---|---|---|
| KS | 113,788 | 44,218 | 4.2 | 52 | 2.59 |
| G | 110,043 | 59,534 | 2.1 | 15 | 3.97 |
| F | 97,329 | 48,342 | 3.3 | 37 | 1.45 |
| H | 94,245 | 42,038 | 6.3 | 390 | 40.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasiliauskienė, D.; Lukša, J.; Servienė, E.; Urbonavičius, J. Changes in the Bacterial Communities of Biocomposites with Different Flame Retardants. Life 2023, 13, 2306. https://doi.org/10.3390/life13122306
Vasiliauskienė D, Lukša J, Servienė E, Urbonavičius J. Changes in the Bacterial Communities of Biocomposites with Different Flame Retardants. Life. 2023; 13(12):2306. https://doi.org/10.3390/life13122306
Chicago/Turabian StyleVasiliauskienė, Dovilė, Juliana Lukša, Elena Servienė, and Jaunius Urbonavičius. 2023. "Changes in the Bacterial Communities of Biocomposites with Different Flame Retardants" Life 13, no. 12: 2306. https://doi.org/10.3390/life13122306






