Novel Compounds Derived from DFPM Induce Root Growth Arrest through the Specific VICTR Alleles of Arabidopsis Accessions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Materials and Root Growth Assay
2.3. Reporter Gene Expression Analysis
2.4. Quantitative Real-Time PCR
2.5. Comparative Sequence Analysis
2.6. Map-Based Cloning
3. Results
3.1. DFPM Derivatives Have Specific Effects on Root Growth
3.2. Selected Chemicals Affect Expression Patterns of the ABA-Responsive Genes and the Pathogen-Responsive Genes
3.3. Selected DFPM Derivatives May Interfere with Root Growth through the Same Signal Transduction Pathway Controlled by DFPM
3.4. Selected DFPM Derivatives Cause Accession-Specific Root Growth Arrest in Col-0, Nie1.2, and Leo1
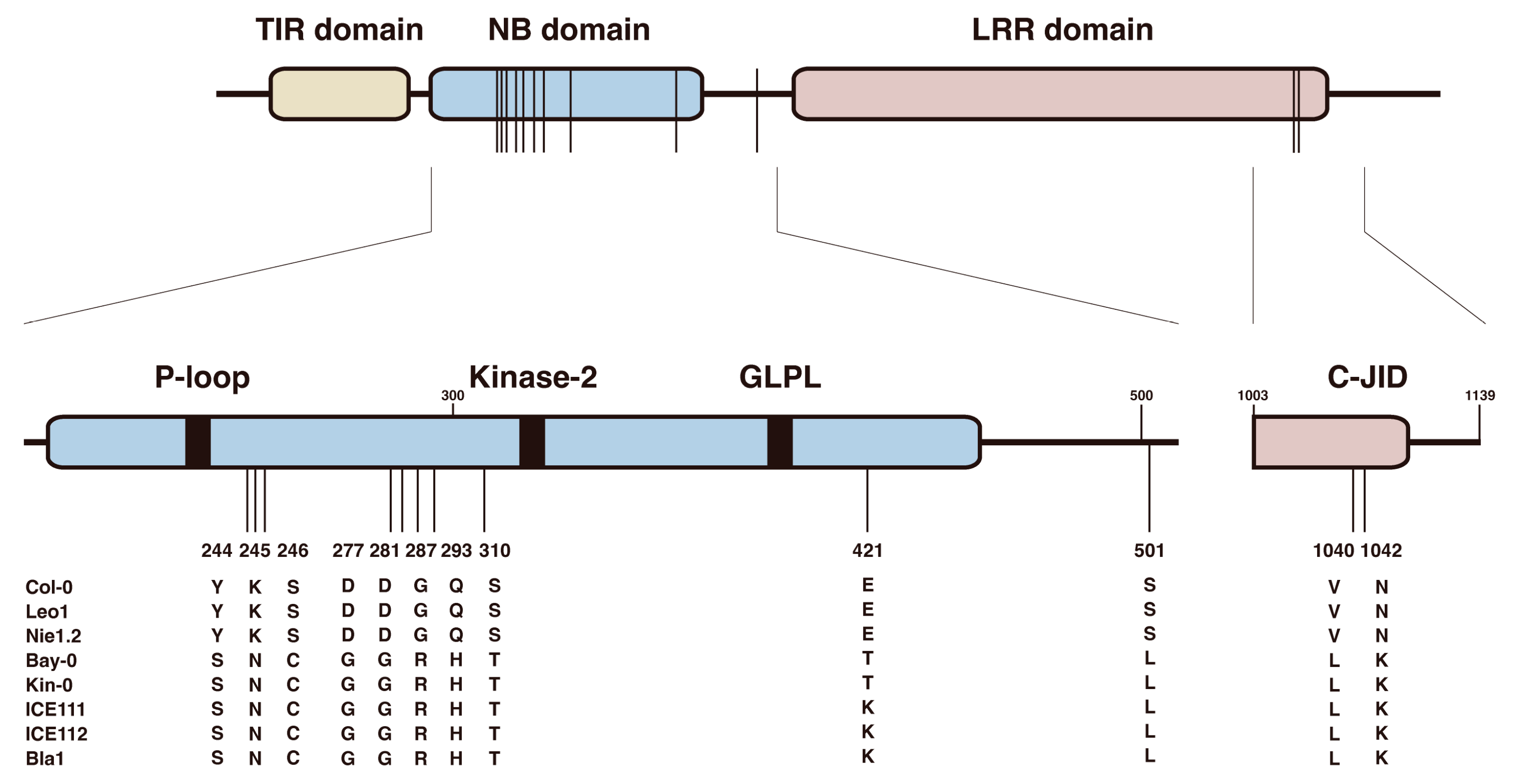
3.5. Twelve Natural Variation Sites in the TNL Receptor VICTR Are Required for Triggering the DFPM-Induced Root Growth Arrest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lepri, A.; Longo, C.; Messore, A.; Kazmi, H.; Madia, V.N.; Di Santo, R.; Costi, R.; Vittorioso, P. Plants and Small Molecules: An Up-and-Coming Synergy. Plants 2023, 12, 1729. [Google Scholar] [CrossRef] [PubMed]
- Pasquer, Q.T.L.; Tsakoumagkos, I.A.; Hoogendoorn, S. From Phenotypic Hit to Chemical Probe: Chemical Biology Approaches to Elucidate Small Molecule Action in Complex Biological Systems. Molecules 2020, 25, 5702. [Google Scholar] [CrossRef] [PubMed]
- Dejonghe, W.; Russinova, E. Plant Chemical Genetics: From Phenotype-Based Screens to Synthetic Biology. Plant Physiol. 2017, 174, 5–20. [Google Scholar] [CrossRef]
- Xuan, W.; Murphy, E.; Beeckman, T.; Audenaert, D.; De Smet, I. Synthetic molecules: Helping to unravel plant signal transduction. J. Chem. Biol. 2013, 6, 43–50. [Google Scholar] [CrossRef]
- Hewage, K.A.H.; Yang, J.F.; Wang, D.; Hao, G.F.; Yang, G.F.; Zhu, J.K. Chemical Manipulation of Abscisic Acid Signaling: A New Approach to Abiotic and Biotic Stress Management in Agriculture. Adv. Sci. 2020, 7, 2001265. [Google Scholar] [CrossRef]
- Hu, D.; Wei, L.; Liao, W. Brassinosteroids in Plants: Crosstalk with Small-Molecule Compounds. Biomolecules 2021, 11, 1800. [Google Scholar] [CrossRef]
- Chini, A.; Monte, I.; Fernandez-Barbero, G.; Boter, M.; Hicks, G.; Raikhel, N.; Solano, R. A small molecule antagonizes jasmonic acid perception and auxin responses in vascular and nonvascular plants. Plant Physiol. 2021, 187, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Asami, T. Chemical regulators of plant hormones and their applications in basic research and agriculture. Biosci. Biotechnol. Biochem. 2018, 82, 1265–1300. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef]
- Ng, L.M.; Melcher, K.; Teh, B.T.; Xu, H.E. Abscisic acid perception and signaling: Structural mechanisms and applications. Acta Pharmacol. Sin. 2014, 35, 567–584. [Google Scholar] [CrossRef] [PubMed]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef]
- Kim, T.H.; Hauser, F.; Ha, T.; Xue, S.; Bohmer, M.; Nishimura, N.; Munemasa, S.; Hubbard, K.; Peine, N.; Lee, B.H.; et al. Chemical genetics reveals negative regulation of abscisic acid signaling by a plant immune response pathway. Curr. Biol. 2011, 21, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tsuda, K.; Parker, J.E. Effector-triggered immunity: From pathogen perception to robust defense. Annu. Rev. Plant Biol. 2015, 66, 487–511. [Google Scholar] [CrossRef]
- Wu, L.; Chen, H.; Curtis, C.; Fu, Z.Q. Go in for the kill: How plants deploy effector-triggered immunity to combat pathogens. Virulence 2014, 5, 710–721. [Google Scholar] [CrossRef] [PubMed]
- van Wersch, S.; Tian, L.; Hoy, R.; Li, X. Plant NLRs: The Whistleblowers of Plant Immunity. Plant Commun. 2020, 1, 100016. [Google Scholar] [CrossRef] [PubMed]
- Eitas, T.K.; Dangl, J.L. NB-LRR proteins: Pairs, pieces, perception, partners, and pathways. Curr. Opin. Plant Biol. 2010, 13, 472–477. [Google Scholar] [CrossRef]
- Cesari, S. Multiple strategies for pathogen perception by plant immune receptors. New Phytol. 2018, 219, 17–24. [Google Scholar] [CrossRef]
- Shao, Z.Q.; Xue, J.Y.; Wu, P.; Zhang, Y.M.; Wu, Y.; Hang, Y.Y.; Wang, B.; Chen, J.Q. Large-Scale Analyses of Angiosperm Nucleotide-Binding Site-Leucine-Rich Repeat Genes Reveal Three Anciently Diverged Classes with Distinct Evolutionary Patterns. Plant Physiol. 2016, 170, 2095–2109. [Google Scholar] [CrossRef]
- Jia, A.; Huang, S.; Ma, S.; Chang, X.; Han, Z.; Chai, J. TIR-catalyzed nucleotide signaling molecules in plant defense. Curr. Opin. Plant Biol. 2023, 73, 102334. [Google Scholar] [CrossRef]
- Essuman, K.; Milbrandt, J.; Dangl, J.L.; Nishimura, M.T. Shared TIR enzymatic functions regulate cell death and immunity across the tree of life. Science 2022, 377, eabo0001. [Google Scholar] [CrossRef] [PubMed]
- Lapin, D.; Johanndrees, O.; Wu, Z.; Li, X.; Parker, J.E. Molecular innovations in plant TIR-based immunity signaling. Plant Cell 2022, 34, 1479–1496. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, W.; Zhang, T.; Gong, Z.; Zhao, H.; Han, G.Z. Out of Water: The Origin and Early Diversification of Plant R-Genes. Plant Physiol. 2018, 177, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.C.; Weigel, D. Plant NLR diversity: The known unknowns of pan-NLRomes. Plant Cell 2021, 33, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef]
- Van de Weyer, A.L.; Monteiro, F.; Furzer, O.J.; Nishimura, M.T.; Cevik, V.; Witek, K.; Jones, J.D.G.; Dangl, J.L.; Weigel, D.; Bemm, F. A Species-Wide Inventory of NLR Genes and Alleles in Arabidopsis thaliana. Cell 2019, 178, 1260–1272. [Google Scholar] [CrossRef]
- Gu, L.; Si, W.; Zhao, L.; Yang, S.; Zhang, X. Dynamic evolution of NBS-LRR genes in bread wheat and its progenitors. Mol. Genet. Genom. 2015, 290, 727–738. [Google Scholar] [CrossRef]
- van Wersch, S.; Li, X. Stronger When Together: Clustering of Plant NLR Disease resistance Genes. Trends Plant Sci. 2019, 24, 688–699. [Google Scholar] [CrossRef]
- Kim, T.H.; Kunz, H.H.; Bhattacharjee, S.; Hauser, F.; Park, J.; Engineer, C.; Liu, A.; Ha, T.; Parker, J.E.; Gassmann, W.; et al. Natural variation in small molecule-induced TIR-NB-LRR signaling induces root growth arrest via EDS1- and PAD4-complexed R protein VICTR in Arabidopsis. Plant Cell 2012, 24, 5177–5192. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.I. An Efficient Synthesis of N, N-Dialkyl-5-(chlorophenyl)-2-furancarbothioamides from 2-Furoic Acid. J. Korean Chem. Soc. 2016, 60, 457–461. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Mahanta, N.; Szantai-Kis, D.M.; Petersson, E.J.; Mitchell, D.A. Biosynthesis and Chemical Applications of Thioamides. ACS Chem. Biol. 2019, 14, 142–163. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, J.K.; Lobana, T.S.; Sood, H.; Arora, D.S.; Kaur, R.; Singh, J.; Garcia-Santos, I.; Kaur, M.; Jasinski, J.P. Silver derivatives of multi-donor heterocyclic thioamides as antimicrobial/anticancer agents: Unusual bio-activity against methicillin resistant S. aureus, S. epidermidis, and E. faecalis and human bone cancer MG63 cell line. RSC Adv. 2019, 9, 15470–15487. [Google Scholar] [CrossRef]
- Chen, X.; Mietlicki-Baase, E.G.; Barrett, T.M.; McGrath, L.E.; Koch-Laskowski, K.; Ferrie, J.J.; Hayes, M.R.; Petersson, E.J. Thioamide Substitution Selectively Modulates Proteolysis and Receptor Activity of Therapeutic Peptide Hormones. J. Am. Chem. Soc. 2017, 139, 16688–16695. [Google Scholar] [CrossRef] [PubMed]
- Mikhailovskii, A.G.; Yusov, A.S.; Makhmudov, R.R.; Starkova, A.V.; Rudakova, I.P. Synthesis and Analgesic, Anthelmintic, and Insecticidal Activity of 3,3-Dialkyl-1-(2-Phenylamino-2-Thioxoethyl)-3,4-Dihydroisoquinolinium Chlorides. Pharm. Chem. J. 2018, 52, 716–720. [Google Scholar] [CrossRef]
- Nishida, C.R.; Ortiz de Montellano, P.R. Bioactivation of antituberculosis thioamide and thiourea prodrugs by bacterial and mammalian flavin monooxygenases. Chem. Biol. Interact. 2011, 192, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Khatri, B.; Raghunathan, S.; Chakraborti, S.; Rahisuddin, R.; Kumaran, S.; Tadala, R.; Wagh, P.; Priyakumar, U.D.; Chatterjee, J. Desolvation of Peptide Bond by O to S Substitution Impacts Protein Stability. Angew. Chem. Int. Ed. Engl. 2021, 60, 24870–24874. [Google Scholar] [CrossRef]
- Dubey, N.; Singh, K. Role of NBS-LRR proteins in plant defense. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 115–138. [Google Scholar]
- Chen, J.; Zhang, X.; Rathjen, J.P.; Dodds, P.N. Direct recognition of pathogen effectors by plant NLR immune receptors and downstream signalling. Essays Biochem. 2022, 66, 471–483. [Google Scholar] [CrossRef]
- Maruta, N.; Burdett, H.; Lim, B.Y.J.; Hu, X.; Desa, S.; Manik, M.K.; Kobe, B. Structural basis of NLR activation and innate immune signalling in plants. Immunogenetics 2022, 74, 5–26. [Google Scholar] [CrossRef]
- Martin, R.; Qi, T.; Zhang, H.; Liu, F.; King, M.; Toth, C.; Nogales, E.; Staskawicz, B.J. Structure of the activated ROQ1 resistosome directly recognizing the pathogen effector XopQ. Science 2020, 370, 1185. [Google Scholar] [CrossRef]
- Saucet, S.B.; Esmenjaud, D.; Van Ghelder, C. Integrity of the post-LRR domain is required for TIR-NB-LRR function. Mol. Plant-Microbe Interact. 2021, 34, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Lapin, D.; Liu, L.; Sun, Y.; Song, W.; Zhang, X.; Logemann, E.; Yu, D.; Wang, J.; Jirschitzka, J.; et al. Direct pathogen-induced assembly of an NLR immune receptor complex to form a holoenzyme. Science 2020, 370, 1184. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Cho, M.; Kim, T.-H. Novel Compounds Derived from DFPM Induce Root Growth Arrest through the Specific VICTR Alleles of Arabidopsis Accessions. Life 2023, 13, 1797. https://doi.org/10.3390/life13091797
Kim S, Cho M, Kim T-H. Novel Compounds Derived from DFPM Induce Root Growth Arrest through the Specific VICTR Alleles of Arabidopsis Accessions. Life. 2023; 13(9):1797. https://doi.org/10.3390/life13091797
Chicago/Turabian StyleKim, Seojung, Miri Cho, and Tae-Houn Kim. 2023. "Novel Compounds Derived from DFPM Induce Root Growth Arrest through the Specific VICTR Alleles of Arabidopsis Accessions" Life 13, no. 9: 1797. https://doi.org/10.3390/life13091797



