Connectome-Based Neurosurgery in Primary Intra-Axial Neoplasms: Beyond the Traditional Modular Conception of Brain Architecture for the Preservation of Major Neurological Domains and Higher-Order Cognitive Functions
Abstract
:1. Introduction
2. The Connectomal Architecture of Neural Anatomy: Beyond the Concept of “Eloquent” and “Non-Eloquent” Brain
2.1. The Development of a Novel Model of Brain Architecture
2.2. Implications in Epilepsy, Schizophrenia and Depression
2.3. Implications in Neuro-Oncology
3. Application in Cancer Neuroscience: Connectomics for a Gentler and Safer Neurosurgical Act
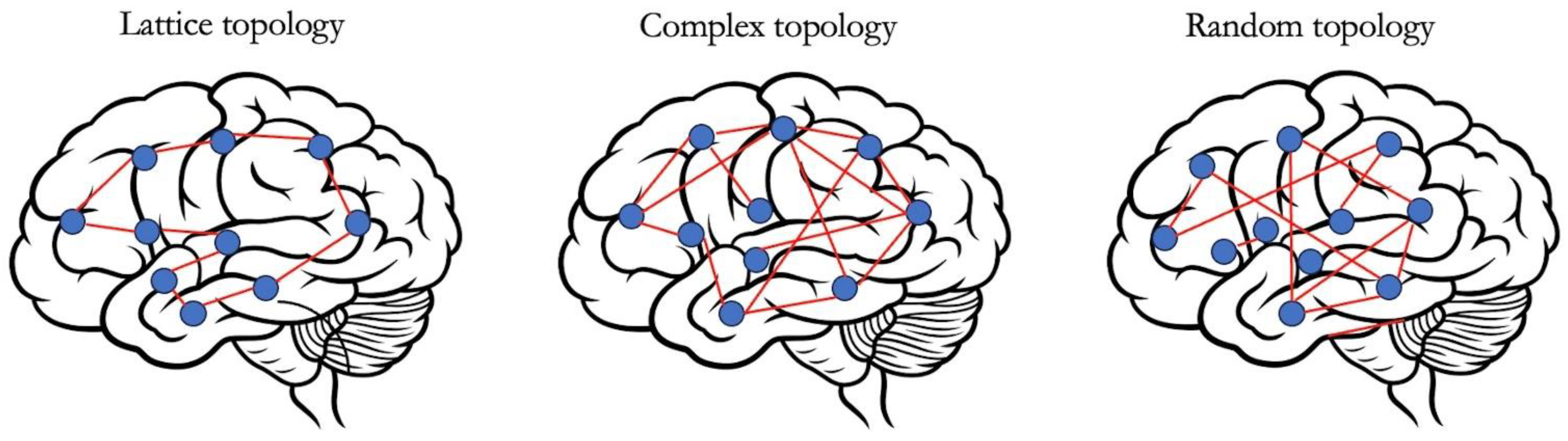
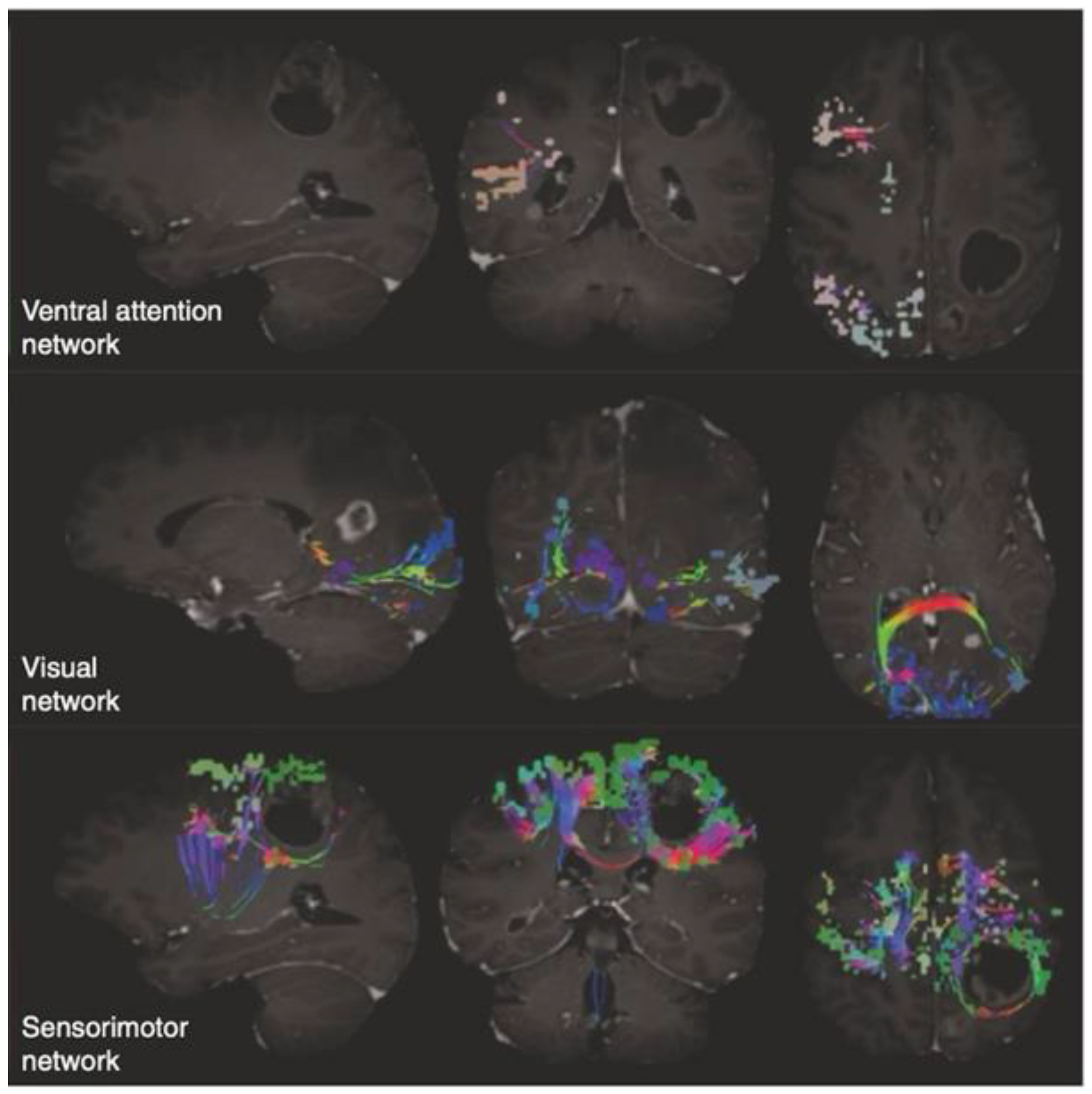
3.1. Connectome-Based Brain Mapping and Graph-Theoretical Analysis
- -
- Node degree: the number of connections of each parcel.
- -
- Clustering coefficient: a measure of network segregation or local specialization and it quantifies the number of connections that exist between the nearest neighbours of a node as a proportion of the maximum number of possible connections.
- -
- Path length: a measure of network integration or global information flow and it represents the minimum number of edges (i.e., connections) that must be traversed to go from one node to another.
- -
- Efficiency: the reciprocal of path length and it can be local or global. For higher values of path length, there is a reduction in the efficiency of the information flux.
- -
- Hubs: nodes with high degree or high centrality.
- -
- Centrality: it describes the importance of a node in the context of the overall network by quantifying the number of shortest paths between all the other node pairs in the network that pass through the given node.
- -
- Robustness: it reflects the changes in structural and functional integrity of a network after the removal of individual node(s) or edge(s).
- -
- Information centrality: it represents the percentage change in global efficiency due to the removal of a single node.
- -
- Random error: removal of casually selected nodes with subsequent measurement of the changes in network properties.
- -
3.2. An Insight into the Application of QuicktomeTM for the Identification of Brain Networks in Patients Affected by Primary Intra-Axial Neoplasms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sanai, N.; Berger, M.S. Surgical Oncology for Gliomas: The State of the Art. Nat. Rev. Clin. Oncol. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and Therapeutic Prognostic Factors in Adult Hemispheric World Health Organization Grade II Gliomas: A Series of 1097 Cases: Clinical Article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef]
- Duffau, H. Brain Connectomics Applied to Oncological Neuroscience: From a Traditional Surgical Strategy Focusing on Glioma Topography to a Meta-Network Approach. Acta Neurochir. 2021, 163, 905–917. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Talozzi, L.; Martinoni, M.; Manners, D.N.; Badaloni, F.; Testa, C.; Asioli, S.; Mitolo, M.; Bartiromo, F.; Rochat, M.J.; et al. From Neurosurgical Planning to Histopathological Brain Tumor Characterization: Potentialities of Arcuate Fasciculus Along-Tract Diffusion Tensor Imaging Tractography Measures. Front. Neurol. 2021, 12, 633209. [Google Scholar] [CrossRef] [PubMed]
- Raffa, G.; Scibilia, A.; Conti, A.; Cardali, S.M.; Rizzo, V.; Terranova, C.; Quattropani, M.C.; Marzano, G.; Ricciardo, G.; Vinci, S.L.; et al. Multimodal Surgical Treatment of High-Grade Gliomas in the Motor Area: The Impact of the Combination of Navigated Transcranial Magnetic Stimulation and Fluorescein-Guided Resection. World Neurosurg. 2019, 128, e378–e390. [Google Scholar] [CrossRef] [PubMed]
- Drewes, C.; Sagberg, L.M.; Jakola, A.S.; Solheim, O. Perioperative and Postoperative Quality of Life in Patients with Glioma–A Longitudinal Cohort Study. World Neurosurg. 2018, 117, e465–e474. [Google Scholar] [CrossRef]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The Economy of Brain Network Organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Broca, P. About the articulation language site. Bull. Soc. Anth. 1861, 6, 330–357, 398–407. [Google Scholar]
- Broca, P. About the cranial-cerebral topography. Rev. Anthrop. 1876, 5, 193–248. [Google Scholar]
- Jackson, J. Convulsive Spasms of the Right Hand and Arm Preceding Epileptic Seizures1. Med. Times Gaz. 1863, 1, 110–111. [Google Scholar]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.M.; WiIdrick, D.M. Neurosurgical Outcomes in a Modern Series of 400 Craniotomies for Treatment of Parenchymal Tumors. Neurosurgery 1998, 42, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr.; Thompson, P.M.; Stern, J.M.; Staba, R.J.; Bragin, A.; Mody, I. Connectomics and Epilepsy. Curr. Opin. Neurol. 2013, 26, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, B.C.; Hong, S.; Bernasconi, A.; Bernasconi, N. Imaging Structural and Functional Brain Networks in Temporal Lobe Epilepsy. Front. Hum. Neurosci. 2013, 7, 624. [Google Scholar] [CrossRef]
- Tavakol, S.; Royer, J.; Lowe, A.J.; Bonilha, L.; Tracy, J.I.; Jackson, G.D.; Duncan, J.S.; Bernasconi, A.; Bernasconi, N.; Bernhardt, B.C. Neuroimaging and Connectomics of Drug-resistant Epilepsy at Multiple Scales: From Focal Lesions to Macroscale Networks. Epilepsia 2019, 60, 593–604. [Google Scholar] [CrossRef]
- Bonilha, L.; Jensen, J.H.; Baker, N.; Breedlove, J.; Nesland, T.; Lin, J.J.; Drane, D.L.; Saindane, A.M.; Binder, J.R.; Kuzniecky, R.I. The Brain Connectome as a Personalized Biomarker of Seizure Outcomes after Temporal Lobectomy. Neurology 2015, 84, 1846–1853. [Google Scholar] [CrossRef]
- Bernhardt, B.C.; Fadaie, F.; Liu, M.; Caldairou, B.; Gu, S.; Jefferies, E.; Smallwood, J.; Bassett, D.S.; Bernasconi, A.; Bernasconi, N. Temporal Lobe Epilepsy: Hippocampal Pathology Modulates Connectome Topology and Controllability. Neurology 2019, 92, e2209–e2220. [Google Scholar] [CrossRef]
- Narr, K.L.; Leaver, A.M. Connectome and Schizophrenia. Curr. Opin. Psychiatry 2015, 28, 229–235. [Google Scholar] [CrossRef]
- Yun, J.-Y.; Kim, Y.-K. Graph Theory Approach for the Structural-Functional Brain Connectome of Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 111, 110401. [Google Scholar] [CrossRef]
- Polin, R.S.; Marko, N.F.; Ammerman, M.D.; Shaffrey, M.E.; Huang, W.; Anderson, F.A.; Caputy, A.J.; Laws, E.R. Functional Outcomes and Survival in Patients with High-Grade Gliomas in Dominant and Nondominant Hemispheres. J. Neurosurg. 2005, 102, 276–283. [Google Scholar] [CrossRef]
- Wang, J.; Zuo, X.; He, Y. Graph-Based Network Analysis of Resting-State Functional MRI. Front. Syst. Neurosci. 2010, 4, 1419. [Google Scholar] [CrossRef]
- Poologaindran, A.; Lowe, S.R.; Sughrue, M.E. The Cortical Organization of Language: Distilling Human Connectome Insights for Supratentorial Neurosurgery. J. Neurosurg. 2021, 134, 1959–1966. [Google Scholar] [CrossRef]
- Ren, H.; Zhu, J.; Su, X.; Chen, S.; Zeng, S.; Lan, X.; Zou, L.-Y.; Sughrue, M.E.; Guo, Y. Application of Structural and Functional Connectome Mismatch for Classification and Individualized Therapy in Alzheimer Disease. Front. Public Health 2020, 8, 584430. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum—Chapter 1: Introduction, Methods, and Significance. Oper. Neurosurg. 2018, 15, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Dadario, N.B.; Brahimaj, B.; Yeung, J.; Sughrue, M.E. Reducing the Cognitive Footprint of Brain Tumor Surgery. Front. Neurol. 2021, 12, 711646. [Google Scholar] [CrossRef]
- Hart, M.G.; Romero-Garcia, R.; Price, S.J.; Santarius, T.; Suckling, J. Connections, Tracts, Fractals, and the Rest: A Working Guide to Network and Connectivity Studies in Neurosurgery. World Neurosurg. 2020, 140, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.G.; Ypma, R.J.F.; Romero-Garcia, R.; Price, S.J.; Suckling, J. Graph Theory Analysis of Complex Brain Networks: New Concepts in Brain Mapping Applied to Neurosurgery. J. Neurosurg. 2016, 124, 1665–1678. [Google Scholar] [CrossRef]
- Fornito, A.; Zalesky, A.; Breakspear, M. The Connectomics of Brain Disorders. Nat. Rev. Neurosci. 2015, 16, 159–172. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex Brain Networks: Graph Theoretical Analysis of Structural and Functional Systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Hart, M.G.; Price, S.J.; Suckling, J. Connectome Analysis for Pre-Operative Brain Mapping in Neurosurgery. Br. J. Neurosurg. 2016, 30, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Girvan, M.; Newman, M.E.J. Community Structure in Social and Biological Networks. Proc. Natl. Acad. Sci. USA 2002, 99, 7821–7826. [Google Scholar] [CrossRef]
- Sporns, O.; Honey, C.J.; Kötter, R. Identification and Classification of Hubs in Brain Networks. PLoS ONE 2007, 2, e1049. [Google Scholar] [CrossRef]
- Bartolomei, F.; Bosma, I.; Klein, M.; Baayen, J.C.; Reijneveld, J.C.; Postma, T.J.; Heimans, J.J.; van Dijk, B.W.; de Munck, J.C.; de Jongh, A.; et al. Disturbed Functional Connectivity in Brain Tumour Patients: Evaluation by Graph Analysis of Synchronization Matrices. Clin. Neurophysiol. 2006, 117, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Ding, S.; Hu, X.; Yang, K.; Xiao, C.; Zou, Y.; Chen, Y.; Tao, L.; Liu, H.; Qian, Z. Reduced Efficiency of Functional Brain Network Underlying Intellectual Decline in Patients with Low-Grade Glioma. Neurosci. Lett. 2013, 543, 27–31. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, R.; Hu, X.; Ding, S.; Qian, J.; Lei, T.; Cao, X.; Tao, L.; Qian, Z.; Liu, H. Disturbed Small-World Networks and Neurocognitive Function in Frontal Lobe Low-Grade Glioma Patients. PLoS ONE 2014, 9, e94095. [Google Scholar] [CrossRef] [PubMed]
- Douw, L.; Baayen, H.; Bosma, I.; Klein, M.; Vandertop, P.; Heimans, J.; Stam, K.; de Munck, J.; Reijneveld, J. Treatment-Related Changes in Functional Connectivity in Brain Tumor Patients: A Magnetoencephalography Study. Exp. Neurol. 2008, 212, 285–290. [Google Scholar] [CrossRef]
- Mitolo, M.; Zoli, M.; Testa, C.; Morandi, L.; Rochat, M.J.; Zaccagna, F.; Martinoni, M.; Santoro, F.; Asioli, S.; Badaloni, F.; et al. Neuroplasticity Mechanisms in Frontal Brain Gliomas: A Preliminary Study. Front. Neurol. 2022, 13, 867048. [Google Scholar] [CrossRef]
- Conti, A.; Raffa, G.; Granata, F.; Rizzo, V.; Germanò, A.; Tomasello, F. Navigated Transcranial Magnetic Stimulation for “Somatotopic” Tractography of the Corticospinal Tract. Oper. Neurosurg. 2014, 10, 542–554. [Google Scholar] [CrossRef]
- Wu, Z.; Hu, G.; Cao, B.; Liu, X.; Zhang, Z.; Dadario, N.B.; Shi, Q.; Fan, X.; Tang, Y.; Cheng, Z.; et al. Non-Traditional Cognitive Brain Network Involvement in Insulo-Sylvian Gliomas: A Case Series Study and Clinical Experience Using Quicktome. Chin. Neurosurg. J. 2023, 9, 16. [Google Scholar] [CrossRef]
- Yeung, J.T.; Taylor, H.M.; Nicholas, P.J.; Young, I.M.; Jiang, I.; Doyen, S.; Sughrue, M.E.; Teo, C. Using Quicktome for Intracerebral Surgery: Early Retrospective Study and Proof of Concept. World Neurosurg. 2021, 154, e734–e742. [Google Scholar] [CrossRef] [PubMed]
- Morell, A.A.; Eichberg, D.G.; Shah, A.H.; Luther, E.; Lu, V.M.; Kader, M.; Higgins, D.M.O.; Merenzon, M.; Patel, N.V.; Komotar, R.J.; et al. Using Machine Learning to Evaluate Large-Scale Brain Networks in Patients with Brain Tumors: Traditional and Non-Traditional Eloquent Areas. Neuro-Oncol. Adv. 2022, 4, vdac142. [Google Scholar] [CrossRef] [PubMed]
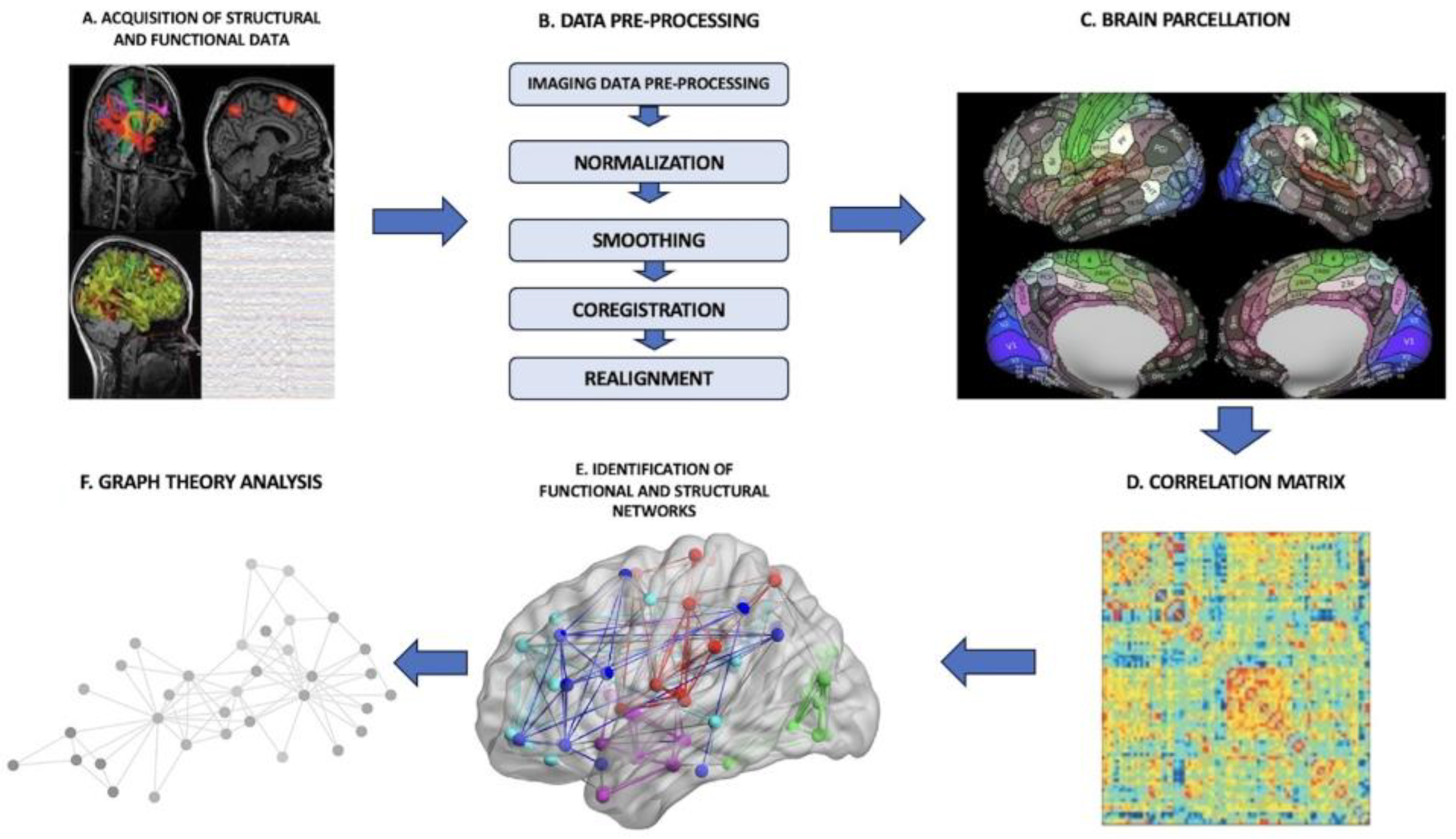
| Measure | Definition |
|---|---|
| Node degree | The number of connections/edges of a specific node |
| Clustering coefficient | The number of connections that exist between the nearest neighbours of a node as a proportion of the maximum number of possible connections |
| Path length | It is a measure of network integration or global information flow and represents the minimum number of edges (i.e., connections) that must be traversed to go from one node to another |
| Efficiency | It is the reciprocal of path length and can be local or global |
| Hubs | Nodes with high degree or high centrality |
| Centrality | It quantifies the number of shortest paths between all the other node pairs in the network that pass through a specific node. Therefore, it is a measure that describes the importance of that specific node in the overall network |
| Robustness | It reflects the changes in structural and functional integrity of a network after the removal of individual node(s) or edge(s) |
| Information centrality | It is a measure that describes the percentage change in global efficiency due to the removal of a single node |
| Random error | It consists in removing casually selected nodes and then measuring the change in network properties |
| Targeted attack | It consists in the suppression of specific nodes based on individual features such as their degree, centrality or clustering |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnani, M.; Rustici, A.; Zoli, M.; Tuleasca, C.; Chaurasia, B.; Franceschi, E.; Tonon, C.; Lodi, R.; Conti, A. Connectome-Based Neurosurgery in Primary Intra-Axial Neoplasms: Beyond the Traditional Modular Conception of Brain Architecture for the Preservation of Major Neurological Domains and Higher-Order Cognitive Functions. Life 2024, 14, 136. https://doi.org/10.3390/life14010136
Magnani M, Rustici A, Zoli M, Tuleasca C, Chaurasia B, Franceschi E, Tonon C, Lodi R, Conti A. Connectome-Based Neurosurgery in Primary Intra-Axial Neoplasms: Beyond the Traditional Modular Conception of Brain Architecture for the Preservation of Major Neurological Domains and Higher-Order Cognitive Functions. Life. 2024; 14(1):136. https://doi.org/10.3390/life14010136
Chicago/Turabian StyleMagnani, Marcello, Arianna Rustici, Matteo Zoli, Constantin Tuleasca, Bipin Chaurasia, Enrico Franceschi, Caterina Tonon, Raffaele Lodi, and Alfredo Conti. 2024. "Connectome-Based Neurosurgery in Primary Intra-Axial Neoplasms: Beyond the Traditional Modular Conception of Brain Architecture for the Preservation of Major Neurological Domains and Higher-Order Cognitive Functions" Life 14, no. 1: 136. https://doi.org/10.3390/life14010136







