Gut Microbiota Is Not Essential for Survival and Development in Blattella germanica, but Affects Uric Acid Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Blattella germanica Rearing Conditions
2.2. Generation of Germ-Free Cockroaches and Quality Control
2.3. Experimental Design and Fitness Parameters’ Determination
2.4. Cockroach Dissection and In Vivo Visualization of Fat Body Morphology
2.5. Electron Microscopy
2.6. Fat Body DNA Extraction and Blattabacterium Quantification
2.7. Uric Acid Extraction and Quantification
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perreau, J.; Moran, N.A. Genetic innovations in animal–microbe symbioses. Nat. Rev. Genet. 2021, 23, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Baldo, L.; Werren, J.H. Evolutionary genetics of microbial symbiosis. Genes 2021, 12, 327. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Engel, P. Mechanisms underlying gut microbiota-host interactions in insects. J. Exp. Biol. 2021, 224, jeb207696. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, D.; Engel, M.S. Evolution of the Insects; Cambridge University Press: New York, NY, USA, 2015. [Google Scholar]
- Douglas, A.E. The molecular basis of bacterial–insect symbiosis. J. Mol. Biol. 2014, 426, 3830–3837. [Google Scholar] [CrossRef] [PubMed]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Siddiqui, R.; Elmashak, Y.; Khan, N.A. Cockroaches: A potential source of novel bioactive molecule(s) for the benefit of human health. Appl. Entomol. Zool. 2023, 58, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bandi, C.; Damiani, G.; Magrassi, L.; Grigolo, A.; Fani, R.; Sacchi, L. Flavobacteria as intracellular symbionts in cockroaches. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1994, 257, 43–48. [Google Scholar] [CrossRef]
- Engel, P.; Moran, N.A. The gut microbiota of insects—Diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Cruden, D.L.; Markovetz, A.J. Microbial ecology of the cockroach gut. Annu. Rev. Microbiol. 1987, 41, 617–643. [Google Scholar] [CrossRef]
- Sacchi, L.; Grigolo, A.; Laudani, U.; Ricevuti, G.; Dealessi, F. Behavior of symbionts during oogenesis and early stages of development in the German cockroach, Blattella germanica (Blattodea). J. Invertebr. Pathol. 1985, 46, 139–152. [Google Scholar] [CrossRef]
- Sacchi, L.; Grigolo, A.; Mazzinit, M.; Bigliardi:, E.; Baccetri, B.; Laudani, U. Symbionts in the occytes of Blattella germanica (L.) (Dictyoptera: Blattellidae): Their mode of transmission. Int. J. Lnsect Morphol. Embryol. 1988, 17, 437–446. [Google Scholar] [CrossRef]
- Sacchi, L.; Corona, S.; Grigolo, A.; Laudani, U.; Selmi, M.G.; Bigliardi, E. The fate of the endocytobionts of Blattella germanica (Blattaria: Blattellidae) and Periplaneta americana (Blattaria: Blattidae) during embryo development. Ital. J. Zool. 1996, 63, 1–11. [Google Scholar] [CrossRef]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 201504031. [Google Scholar] [CrossRef]
- Rosas, T.; García-Ferris, C.; Domínguez-Santos, R.; Llop, P.; Latorre, A.; Moya, A. Rifampicin treatment of Blattella germanica evidences a fecal transmission route of their gut microbiota. FEMS Microbiol. Ecol. 2018, 94, fiy002. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Santos, R.; Pérez-Cobas, A.E.; Artacho, A.; Castro, J.A.; Talón, I.; Moya, A.; García-Ferris, C.; Latorre, A. Unraveling assemblage, functions and stability of the gut microbiota of Blattella germanica by antibiotic treatment. Front. Microbiol. 2020, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, P.; Pérez-Cobas, A.E.; van de Pol, C.; Baixeras, J.; Moya, A.; Latorre, A. Succession of the gut microbiota in the cockroach Blattella germanica. Int. Microbiol. 2014, 17, 99–109. [Google Scholar] [CrossRef] [PubMed]
- López-Sánchez, M.J.; Neef, A.; Peretó, J.; Patiño-Navarrete, R.; Pignatelli, M.; Latorre, A.; Moya, A. Evolutionary convergence and nitrogen metabolism in Blattabacterium strain Bge, primary endosymbiont of the cockroach Blattella germanica. PLoS Genet. 2009, 5, e1000721. [Google Scholar] [CrossRef]
- Sabree, Z.L.; Kambhampati, S.; Moran, N.A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl. Acad. Sci. USA 2009, 106, 19521–19526. [Google Scholar] [CrossRef]
- Tokuda, G.; Elbourne, L.D.H.H.; Kinjo, Y.; Saitoh, S.; Sabree, Z.; Hojo, M.; Yamada, A.; Hayashi, Y.; Shigenobu, S.; Bandi, C.; et al. Maintenance of essential amino acid synthesis pathways in the Blattabacterium cuenoti symbiont of a wood-feeding cockroach. Biol. Lett. 2013, 9, 20121153. [Google Scholar] [CrossRef]
- Ayayee, P.A.; Larsen, T.; Sabree, Z. Symbiotic essential amino acids provisioning in the American cockroach, Periplaneta americana (Linnaeus) under various dietary conditions. PeerJ 2016, 4, e2046. [Google Scholar] [CrossRef]
- Patiño-Navarrete, R.; Piulachs, M.-D.; Belles, X.; Moya, A.; Latorre, A.; Peretó, J. The cockroach Blattella germanica obtains nitrogen from uric acid through a metabolic pathway shared with its bacterial endosymbiont. Biol. Lett. 2014, 10, 20140407. [Google Scholar] [CrossRef] [PubMed]
- Tegtmeier, D.; Thompson, C.L.; Schauer, C.; Brune, A. Oxygen affects gut bacterial colonization and metabolic activities in a gnotobiotic cockroach model. Appl. Environ. Microbiol. 2016, 82, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Mikaelyan, A.; Thompson, C.L.; Hofer, M.J.; Brunea, A. Deterministic assembly of complex bacterial communities in guts of germ-free cockroaches. Appl. Environ. Microbiol. 2016, 82, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Kakumanu, M.L.; Maritz, J.M.; Carlton, J.M.; Schal, C. Overlapping community compositions of gut and fecal microbiomes in lab-reared and field-collected German cockroaches. Appl. Environ. Microbiol. 2018, 84, e01037-18. [Google Scholar] [CrossRef] [PubMed]
- Pietri, J.E.; Tiffany, C.; Liang, D. Disruption of the microbiota affects physiological and evolutionary aspects of insecticide resistance in the German cockroach, an important urban pest. PLoS ONE 2018, 13, e0207985. [Google Scholar] [CrossRef]
- Jahnes, B.C.; Herrmann, M.; Sabree, Z.L. Conspecific coprophagy stimulates normal development in a germ-free model invertebrate. PeerJ 2019, 7, e6914. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, M.; Domínguez-Santos, R.; Marín-Miret, J.; Gil, R.; Latorre, A.; García-Ferris, C. Exploring gut microbial dynamics and symbiotic interaction in Blattella germanica using rifampicin. Biology 2023, 12, 955. [Google Scholar] [CrossRef]
- Dougherty, E.C. introduction to axenic culture of invertebrate metazoa: A goal. Ann. N. Y. Acad. Sci. 1959, 77, 27–54. [Google Scholar] [CrossRef]
- Doll, J.P.; Trexler, P.C.; Reynolds, L.I.; Bernard, G.R. The Use of peracetic acid to obtain germfree invertebrate eggs for gnotobiotic studies. Am. Midl. Nat. 1963, 69, 231. [Google Scholar] [CrossRef]
- Greenberg, B. Sterilizing procedures and agents, antibiotics and inhibitors in mass rearing of insects. Bull. Entomol. Soc. Am. 1970, 16, 31–36. [Google Scholar] [CrossRef]
- Benschoter, C.A.; Wrenn, R.T. Germfree techniques for establishment and maintenance of a colony of aseptic german cockroaches. Ann. Entomol. Soc. Am. 1972, 65, 641–644. [Google Scholar] [CrossRef]
- Brill, N.L.; Watson, D.W.; Abney, M.R. Surface disinfection technique for Plectris aliena grubs (Coleoptera: Scarabaeidae) using sodium hypochlorite. J. Agric. Urban Entomol. 2013, 29, 10–15. [Google Scholar] [CrossRef]
- Coon, K.L.; Vogel, K.J.; Brown, M.R.; Strand, M.R. Mosquitoes rely on their gut microbiota for development. Mol. Ecol. 2014, 23, 2727–2739. [Google Scholar] [CrossRef] [PubMed]
- Vera-Ponce de León, A.; Jahnes, B.C.; Otero-Bravo, A.; Sabree, Z.L. Microbiota perturbation or elimination can inhibit normal development and elicit a starvation-like response in an omnivorous model invertebrate. mSystems 2021, 6, e00802-21. [Google Scholar] [CrossRef] [PubMed]
- Clayton, R.B. A simplified method for the culture of Blatella germanica under aseptic conditions. Nature 1959, 184, 1166–1167. [Google Scholar] [CrossRef]
- Karnovsky, M.J. A formaldehyde-glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A. [Google Scholar]
- Millonig, G. Advantages of a phosphate buffer for OsO4 solutions in fixation. Proceedings of the 19th Annual Meeting of the Electron Microscope Society of America. J. Appl. Phys. 1961, 32, 1637. [Google Scholar]
- Wu, J.; Wang, Q.; Wang, D.; Wong, A.C.N.; Wang, G.H. Axenic and gnotobiotic insect technologies in research on host–microbiota interactions. Trends Microbiol. 2023, 31, 858–871. [Google Scholar] [CrossRef]
- Dukes, H.E.; Dyer, J.E.; Ottesen, E.A. Establishment and maintenance of gnotobiotic american cockroaches (Periplaneta americana). J. Vis. Exp. 2021, 2021, e61316. [Google Scholar] [CrossRef]
- Park, M.S.; Park, P.; Takeda, M. Roles of fat body trophocytes, mycetocytes and urocytes in the American cockroach, Periplaneta americana under starvation conditions: An ultrastructural study. Arthropod Struct. Dev. 2013, 42, 287–295. [Google Scholar] [CrossRef]
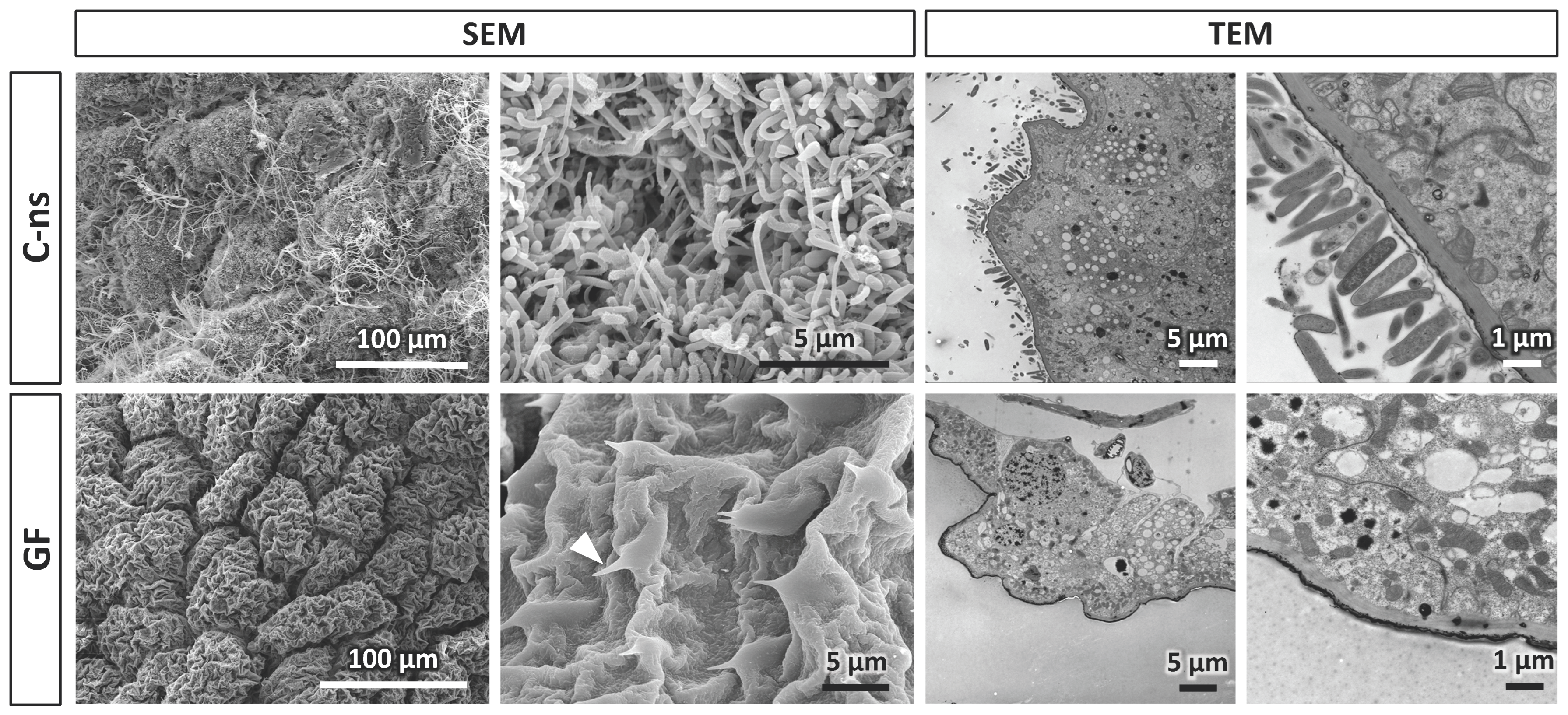
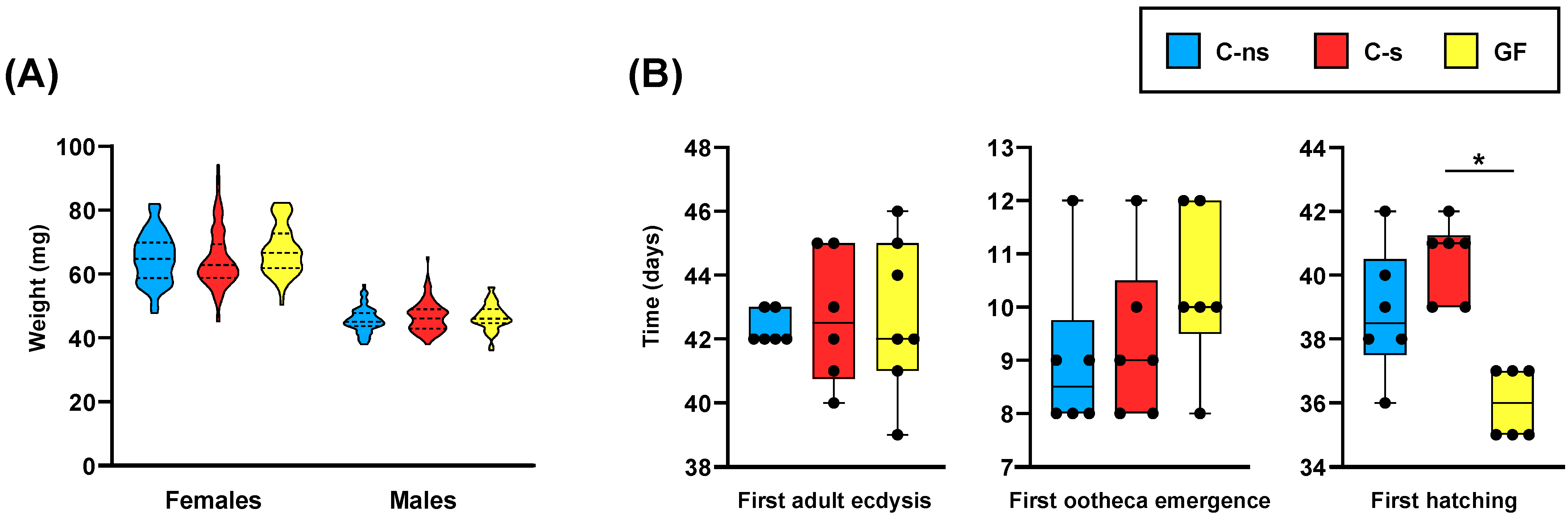


| Condition | Number of Oothecae | Number of Individuals Reaching Adult Ecdysis Each Day (d) | Total Individuals | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 d | 42 d | 44 d | 46 d | 48 d | 50 d | 52 d | 54 d | 56 d | 58 d | 60 d | 62 d | 64 d | 66 d | 68 d | |||
| C-ns | 6 | 0 | 10 | 35 | 29 | 12 | 16 | 14 | 8 | 3 | 3 | 0 | 0 | 0 | 0 | 0 | 130 |
| C-s | 6 | 0 | 22 | 37 | 28 | 15 | 28 | 29 | 16 | 9 | 2 | 1 | 2 | 0 | 0 | 1 | 190 |
| GF | 7 | 4 | 7 | 9 | 27 | 17 | 26 | 22 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Santos, R.; Baixeras, J.; Moya, A.; Latorre, A.; Gil, R.; García-Ferris, C. Gut Microbiota Is Not Essential for Survival and Development in Blattella germanica, but Affects Uric Acid Storage. Life 2024, 14, 153. https://doi.org/10.3390/life14010153
Domínguez-Santos R, Baixeras J, Moya A, Latorre A, Gil R, García-Ferris C. Gut Microbiota Is Not Essential for Survival and Development in Blattella germanica, but Affects Uric Acid Storage. Life. 2024; 14(1):153. https://doi.org/10.3390/life14010153
Chicago/Turabian StyleDomínguez-Santos, Rebeca, Joaquín Baixeras, Andrés Moya, Amparo Latorre, Rosario Gil, and Carlos García-Ferris. 2024. "Gut Microbiota Is Not Essential for Survival and Development in Blattella germanica, but Affects Uric Acid Storage" Life 14, no. 1: 153. https://doi.org/10.3390/life14010153







