Biological Activities of Molecules Derived from Olea europaea L. Tested In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extracts Tested In Vitro
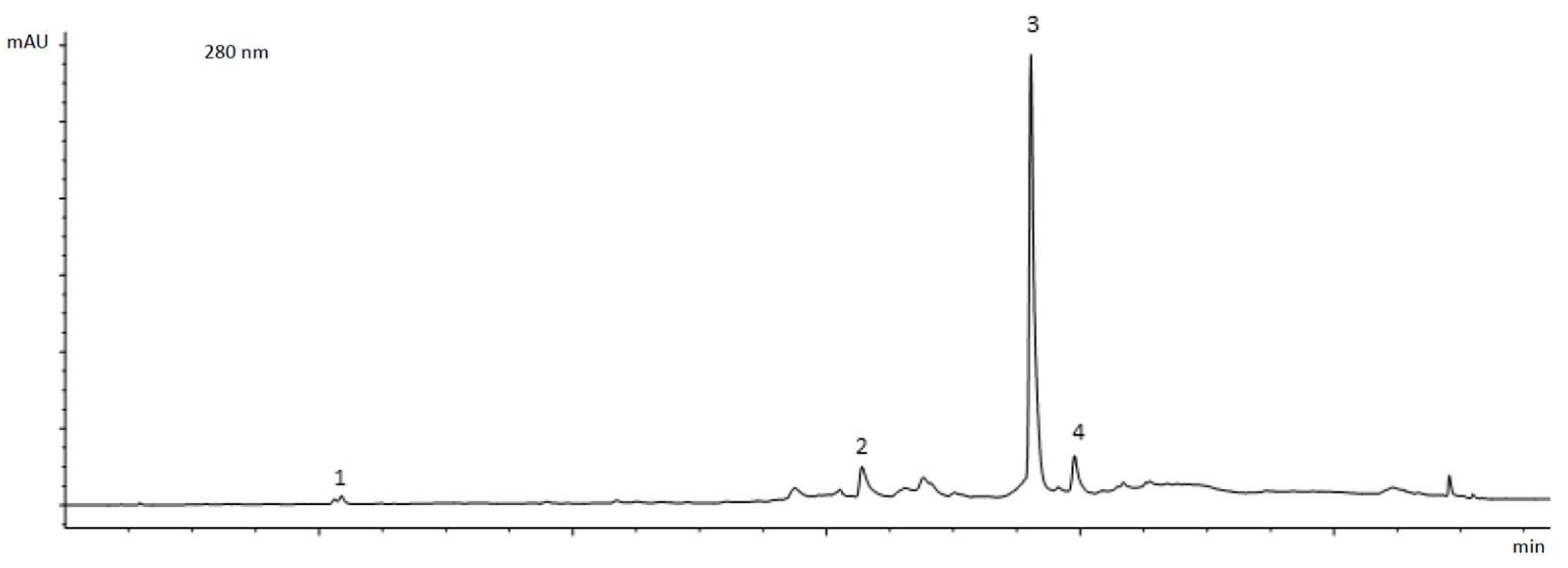
2.2. In Vitro Study
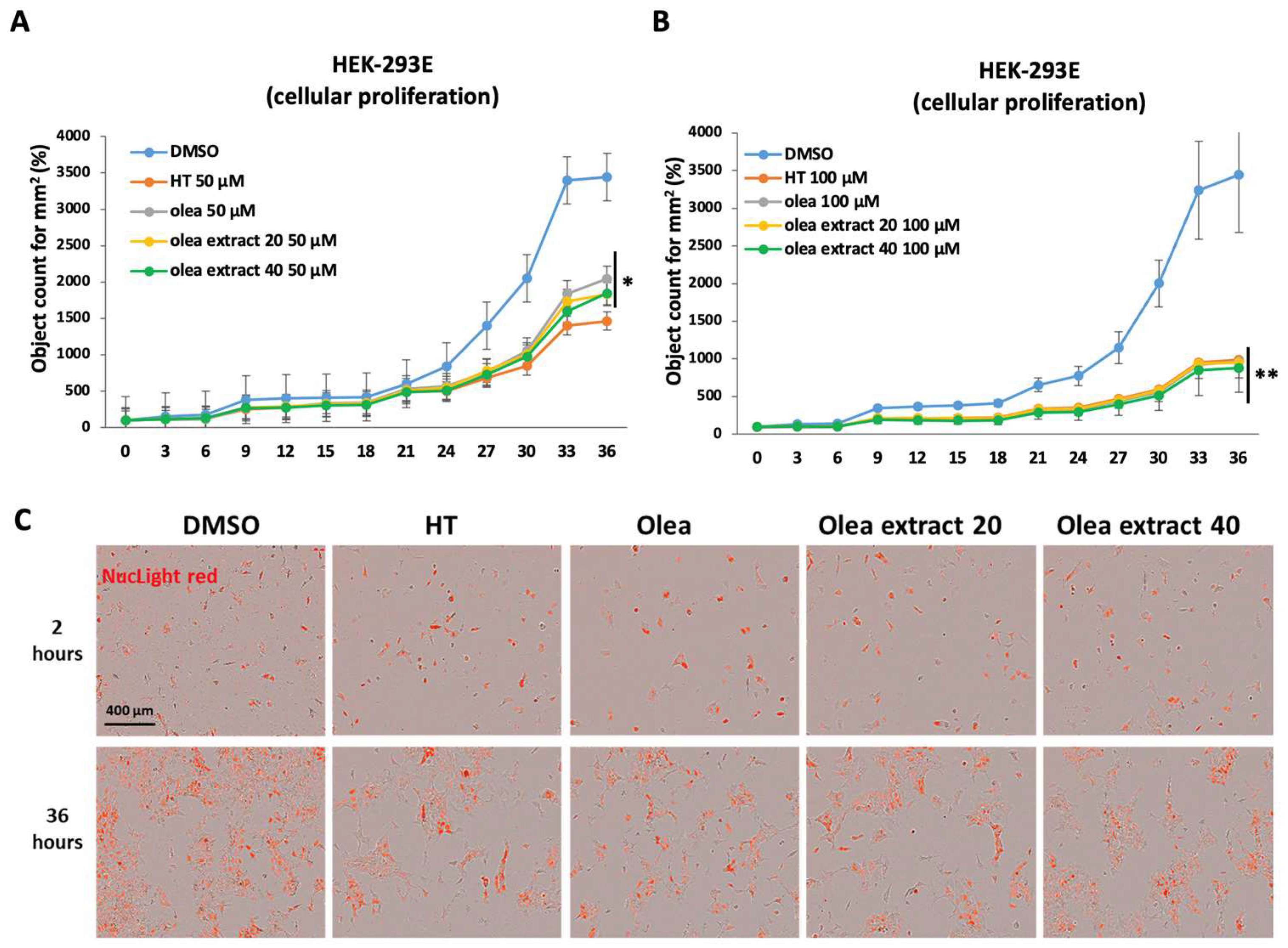
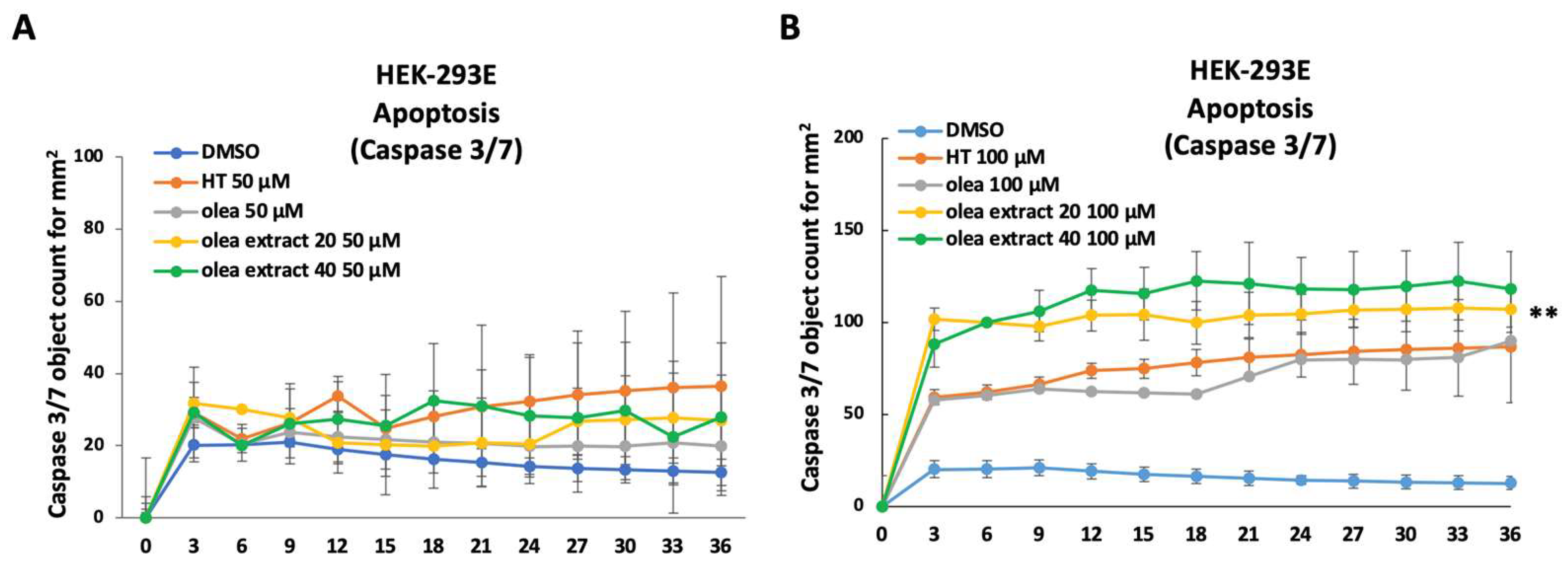
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caprara, G. Mediterranean-Type Dietary Pattern and Physical Activity: The Winning Combination to Counteract the Rising Burden of Non-Communicable Diseases (NCDs). Nutrients 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Mayr, H.L.; Thomas, C.J. The anti-inflammatory effects of a Mediterranean diet: A review. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Corella, D.; Castaner, O.; Lamuela-Raventos, R.M.; Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Ros, E.; Estruch, R. Long-Term Immunomodulatory Effects of a Mediterranean Diet in Adults at High Risk of Cardiovascular Disease in the PREvencion con DIeta MEDiterranea (PREDIMED) Randomized Controlled Trial. J. Nutr. 2016, 146, 1684–1693. [Google Scholar] [CrossRef] [PubMed]
- Tafesse, T.B.; Hymete, A.; Mekonnen, Y.; Tadesse, M. Antidiabetic activity and phytochemical screening of extracts of the leaves of Ajuga remota Benth on alloxan-induced diabetic mice. BMC Complement. Altern. Med. 2017, 17, 243. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Carpena, M.; Lourenco-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance of normal blood HDL-cholesterol concentrations (ID 1639), maintenance of normal blood pressure (ID 3781), “anti-inflammatory properties” (ID 1882), “contributes to the upper respiratory tract health” (ID 3468), “can help to maintain a normal function of gastrointestinal tract” (3779), and “contributes to body defences against external agents” (ID 3467) pursuant to Article 13(1) of Regulation (EC) No 1924/20061. EFSA J. 2011, 9, 2033. [Google Scholar]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondiere, U.R.; Hemon, B.; Casagrande, C.; Vignat, J.; et al. European Prospective Investigation into Cancer and Nutrition (EPIC): Study populations and data collection. Public Health Nutr. 2002, 5, 1113–1124. [Google Scholar] [CrossRef]
- Gaforio, J.J.; Visioli, F.; Alarcon-de-la-Lastra, C.; Castaner, O.; Delgado-Rodriguez, M.; Fito, M.; Hernandez, A.F.; Huertas, J.R.; Martinez-Gonzalez, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Masala, G.; Ceroti, M.; Pala, V.; Krogh, V.; Vineis, P.; Sacerdote, C.; Saieva, C.; Salvini, S.; Sieri, S.; Berrino, F.; et al. A dietary pattern rich in olive oil and raw vegetables is associated with lower mortality in Italian elderly subjects. Br. J. Nutr. 2007, 98, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Nadeem, M.; Gilani, S.A.; Khan, S.; Sajid, M.W.; Amir, R.M. Antitumor Perspectives of Oleuropein and Its Metabolite Hydroxytyrosol: Recent Updates. J. Food Sci. 2018, 83, 1781–1791. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols From Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef] [PubMed]
- Clodoveo, M.L.; Crupi, P.; Annunziato, A.; Corbo, F. Innovative Extraction Technologies for Development of Functional Ingredients Based on Polyphenols from Olive Leaves. Foods 2021, 11, 103. [Google Scholar] [CrossRef] [PubMed]
- Voss, L.; Guttek, K.; Reddig, A.; Reinhold, A.; Voss, M.; Schraven, B.; Reinhold, D. Screening of FDA-Approved Drug Library Identifies Adefovir Dipivoxil as Highly Potent Inhibitor of T Cell Proliferation. Front. Immunol. 2020, 11, 616570. [Google Scholar] [CrossRef]
- Feitelson, M.A.; Arzumanyan, A.; Kulathinal, R.J.; Blain, S.W.; Holcombe, R.F.; Mahajna, J.; Marino, M.; Martinez-Chantar, M.L.; Nawroth, R.; Sanchez-Garcia, I.; et al. Sustained proliferation in cancer: Mechanisms and novel therapeutic targets. Semin. Cancer Biol. 2015, 35, S25–S54. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Gaglia, G.; Kabraji, S.; Rammos, D.; Dai, Y.; Verma, A.; Wang, S.; Mills, C.E.; Chung, M.; Bergholz, J.S.; Coy, S.; et al. Temporal and spatial topography of cell proliferation in cancer. Nat. Cell Biol. 2022, 24, 316–326. [Google Scholar] [CrossRef]
- Fezai, M.; Senovilla, L.; Jemaa, M.; Ben-Attia, M. Analgesic, anti-inflammatory and anticancer activities of extra virgin olive oil. J. Lipids 2013, 2013, 129736. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Nocella, C.; Cammisotto, V.; Bartimoccia, S.; Monticolo, R.; D’Amico, A.; Stefanini, L.; Pagano, F.; Pastori, D.; Cangemi, R.; et al. Antioxidant activity from extra virgin olive oil via inhibition of hydrogen peroxide-mediated NADPH-oxidase 2 activation. Nutrition 2018, 55–56, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Bernini, R.; Noce, A.; Urciuoli, S.; Di Lauro, M.; Pietroboni Zaitseva, A.; Marrone, G.; Di Daniele, N. Potential Beneficial Effects of Extra Virgin Olive Oils Characterized by High Content in Minor Polar Compounds in Nephropathic Patients: A Pilot Study. Molecules 2020, 25, 4757. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Urciuoli, S.; Di Daniele, F.; Di Lauro, M.; Pietroboni Zaitseva, A.; Di Daniele, N.; Romani, A. Usefulness of Extra Virgin Olive Oil Minor Polar Compounds in the Management of Chronic Kidney Disease Patients. Nutrients 2021, 13, 581. [Google Scholar] [CrossRef] [PubMed]
- Franconi, F.; Campesi, I.; Romani, A. Is Extra Virgin Olive Oil an Ally for Women’s and Men’s Cardiovascular Health? Cardiovasc. Ther. 2020, 2020, 6719301. [Google Scholar] [CrossRef] [PubMed]
- Vanni, G.; Materazzo, M.; Pellicciaro, M.; Morando, L.; Portarena, I.; Anemona, L.; D’Angelillo, M.R.; Barbarino, R.; Chiaravalloti, A.; Buonomo, O.C.; et al. Does Age Matter? Estimating Risks of Locoregional Recurrence After Breast-conservative Surgery. In Vivo 2020, 34, 1125–1132. [Google Scholar] [CrossRef]
- Vanni, G.; Pellicciaro, M.; Materazzo, M.; Pedini, D.; Portarena, I.; Buonomo, C.; Perretta, T.; Rizza, S.; Pistolese, C.A.; Buonomo, O.C.; et al. Advanced Stages and Increased Need for Adjuvant Treatments in Breast Cancer Patients: The Effect of the One-year COVID-19 Pandemic. Anticancer Res. 2021, 41, 2689–2696. [Google Scholar] [CrossRef]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal exerts antitumor effects on human liver and colon cancer cells through ROS generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef]
- Cuyas, E.; Gumuzio, J.; Lozano-Sanchez, J.; Segura-Carretero, A.; Verdura, S.; Bosch-Barrera, J.; Martin-Castillo, B.; Nonell-Canals, A.; Llebaria, A.; Cabello, S.; et al. Mimetics of extra virgin olive oil phenols with anti-cancer stem cell activity. Aging 2020, 12, 21057–21075. [Google Scholar] [CrossRef]
- Carpi, S.; Polini, B.; Manera, C.; Digiacomo, M.; Salsano, J.E.; Macchia, M.; Scoditti, E.; Nieri, P. miRNA Modulation and Antitumor Activity by the Extra-Virgin Olive Oil Polyphenol Oleacein in Human Melanoma Cells. Front. Pharmacol. 2020, 11, 574317. [Google Scholar] [CrossRef]
- Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef] [PubMed]
- Benedetto, N.; Calabrone, L.; Gutmanska, K.; Macri, N.; Cerrito, M.G.; Ricotta, R.; Pelosi, G.; Bruno, A.; Noonan, D.M.; Albini, A. An Olive Oil Mill Wastewater Extract Improves Chemotherapeutic Activity Against Breast Cancer Cells While Protecting From Cardiotoxicity. Front. Cardiovasc. Med. 2022, 9, 867867. [Google Scholar] [CrossRef] [PubMed]
- Rishmawi, S.; Haddad, F.; Dokmak, G.; Karaman, R. A Comprehensive Review on the Anti-Cancer Effects of Oleuropein. Life 2022, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Shamshoum, H.; Vlavcheski, F.; Tsiani, E. Anticancer effects of oleuropein. Biofactors 2017, 43, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Stoneham, M.; Goldacre, M.; Seagroatt, V.; Gill, L. Olive oil, diet and colorectal cancer: An ecological study and a hypothesis. J. Epidemiol. Community Health 2000, 54, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, R.; Fernandez-Banares, F.; Navarro, E.; Castella, E.; Mane, J.; Alvarez, M.; Pastor, C.; Cabre, E.; Gassull, M.A. Effect of olive oil on early and late events of colon carcinogenesis in rats: Modulation of arachidonic acid metabolism and local prostaglandin E(2) synthesis. Gut 2000, 46, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Giner, E.; Recio, M.C.; Rios, J.L.; Cerda-Nicolas, J.M.; Giner, R.M. Chemopreventive effect of oleuropein in colitis-associated colorectal cancer in c57bl/6 mice. Mol. Nutr. Food Res. 2016, 60, 242–255. [Google Scholar] [CrossRef]
- Nunez, F.; Bravo, S.; Cruzat, F.; Montecino, M.; De Ferrari, G.V. Wnt/beta-catenin signaling enhances cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer cells. PLoS ONE 2011, 6, e18562. [Google Scholar] [CrossRef]
- Deng, L.; Liang, H.; Han, Y. Cyclooxygenase-2 and beta-Catenin as Potential Diagnostic and Prognostic Markers in Endometrial Cancer. Front. Oncol. 2020, 10, 56. [Google Scholar] [CrossRef]
- Morana, J.M.; Leal-Hernande, O.; Canal-Macias, M.L.; Roncero-Martin, R.; Guerrero-Bonmatty, R.; Aliaga, I.; Zamorano, J.D. Antiproliferative Properties of Oleuropein in Human Osteosarcoma Cells. Nat. Prod. Commun. 2016, 11, 491–492. [Google Scholar] [PubMed]
- Bossio, S.; Perri, A.; Malivindi, R.; Giordano, F.; Rago, V.; Mirabelli, M.; Salatino, A.; Brunetti, A.; Greco, E.A.; Aversa, A. Oleuropein Counteracts Both the Proliferation and Migration of Intra- and Extragonadal Seminoma Cells. Nutrients 2022, 14, 2323. [Google Scholar] [CrossRef] [PubMed]
- Ci, Y.; Qiao, J.; Han, M. Molecular Mechanisms and Metabolomics of Natural Polyphenols Interfering with Breast Cancer Metastasis. Molecules 2016, 21, 1634. [Google Scholar] [CrossRef] [PubMed]
- Papachristodoulou, A.; Tsoukala, M.; Benaki, D.; Kostidis, S.; Gioti, K.; Aligiannis, N.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Mikros, E.; et al. Oleuropein is a Powerful Sensitizer of Doxorubicin-mediated Killing of Prostate Cancer Cells and Exerts Its Action via Induction of Autophagy. J. Cancer Res. Treat. 2016, 4, 61–68. [Google Scholar] [CrossRef]
- Andreadou, I.; Mikros, E.; Ioannidis, K.; Sigala, F.; Naka, K.; Kostidis, S.; Farmakis, D.; Tenta, R.; Kavantzas, N.; Bibli, S.I.; et al. Oleuropein prevents doxorubicin-induced cardiomyopathy interfering with signaling molecules and cardiomyocyte metabolism. J. Mol. Cell. Cardiol. 2014, 69, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Noce, A.; Marrone, G.; Di Lauro, M.; Mitterhofer, A.P.; Ceravolo, M.J.; Di Daniele, N.; Manenti, G.; De Lorenzo, A. The Onco-Nephrology Field: The Role of Personalized Chemotherapy to Prevent Kidney Damage. Cancers 2023, 15, 2254. [Google Scholar] [CrossRef] [PubMed]
- Ruzzolini, J.; Peppicelli, S.; Andreucci, E.; Bianchini, F.; Scardigli, A.; Romani, A.; la Marca, G.; Nediani, C.; Calorini, L. Oleuropein, the Main Polyphenol of Olea europaea Leaf Extract, Has an Anti-Cancer Effect on Human BRAF Melanoma Cells and Potentiates the Cytotoxicity of Current Chemotherapies. Nutrients 2018, 10, 1950. [Google Scholar] [CrossRef] [PubMed]
- de la Puerta, R.; Ruiz Gutierrez, V.; Hoult, J.R. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- de la Puerta, R.; Martinez Dominguez, M.E.; Ruiz-Gutierrez, V.; Flavill, J.A.; Hoult, J.R. Effects of virgin olive oil phenolics on scavenging of reactive nitrogen species and upon nitrergic neurotransmission. Life Sci. 2001, 69, 1213–1222. [Google Scholar] [CrossRef]
- Andrikopoulos, N.K.; Kaliora, A.C.; Assimopoulou, A.N.; Papageorgiou, V.P. Inhibitory activity of minor polyphenolic and nonpolyphenolic constituents of olive oil against in vitro low-density lipoprotein oxidation. J. Med. Food 2002, 5, 1–7. [Google Scholar] [CrossRef]
- Hormozi, M.; Salehi Marzijerani, A.; Baharvand, P. Effects of Hydroxytyrosol on Expression of Apoptotic Genes and Activity of Antioxidant Enzymes in LS180 Cells. Cancer Manag. Res. 2020, 12, 7913–7919. [Google Scholar] [CrossRef] [PubMed]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Qabaha, K.; Al-Rimawi, F.; Qasem, A.; Naser, S.A. Oleuropein Is Responsible for the Major Anti-Inflammatory Effects of Olive Leaf Extract. J. Med. Food 2018, 21, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Mirlekar, B.; Pylayeva-Gupta, Y. IL-12 Family Cytokines in Cancer and Immunotherapy. Cancers 2021, 13, 167. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Kamohara, H.; Matsuyama, W.; Shimozato, O.; Abe, K.; Galligan, C.; Hashimoto, S.; Matsushima, K.; Yoshimura, T. Regulation of tumour necrosis factor-related apoptosis-inducing ligand (TRAIL) and TRAIL receptor expression in human neutrophils. Immunology 2004, 111, 186–194. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- Carmona, I.; Aguirre, I.; Griffith, D.M.; Garcia-Borrego, A. Towards a circular economy in virgin olive oil production: Valorization of the olive mill waste (OMW) “alpeorujo” through polyphenol recovery with natural deep eutectic solvents (NADESs) and vermicomposting. Sci. Total Environ. 2023, 872, 162198. [Google Scholar] [CrossRef] [PubMed]
- Centrone, M.; D’Agostino, M.; Difonzo, G.; De Bruno, A.; Di Mise, A.; Ranieri, M.; Montemurro, C.; Valenti, G.; Poiana, M.; Caponio, F.; et al. Antioxidant Efficacy of Olive By-Product Extracts in Human Colon HCT8 Cells. Foods 2020, 10, 11. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrone, G.; Urciuoli, S.; Candi, E.; Bernini, R.; Vanni, G.; Masci, C.; Guerriero, C.; Mancini, M.; De Lorenzo, A.; Vignolini, P.; et al. Biological Activities of Molecules Derived from Olea europaea L. Tested In Vitro. Life 2024, 14, 49. https://doi.org/10.3390/life14010049
Marrone G, Urciuoli S, Candi E, Bernini R, Vanni G, Masci C, Guerriero C, Mancini M, De Lorenzo A, Vignolini P, et al. Biological Activities of Molecules Derived from Olea europaea L. Tested In Vitro. Life. 2024; 14(1):49. https://doi.org/10.3390/life14010049
Chicago/Turabian StyleMarrone, Giulia, Silvia Urciuoli, Eleonora Candi, Roberta Bernini, Gianluca Vanni, Claudia Masci, Cristina Guerriero, Mara Mancini, Antonino De Lorenzo, Pamela Vignolini, and et al. 2024. "Biological Activities of Molecules Derived from Olea europaea L. Tested In Vitro" Life 14, no. 1: 49. https://doi.org/10.3390/life14010049




