No Object–Location Memory Improvement through Focal Transcranial Direct Current Stimulation over the Right Temporoparietal Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Overview
2.2. Baseline Neuropsychological Assessment
2.3. Training Task
2.4. Focal tDCS
2.5. MRI Assessment and Electric Field Modeling
2.6. Statistical Analyses
3. Results
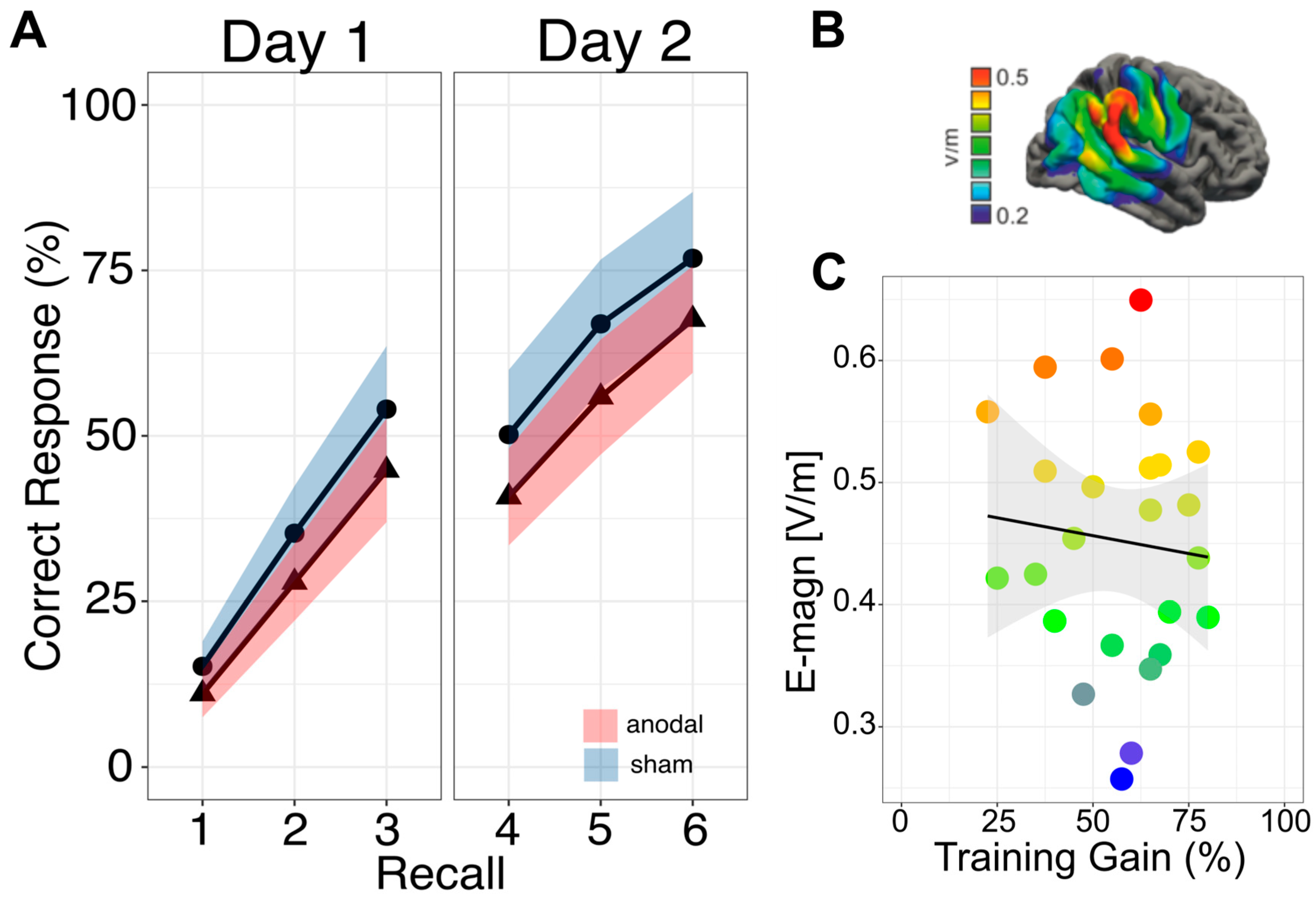
3.1. Primary Outcome: Immediate Effects
3.2. Secondary Outcome: Sustained Effects
3.3. Secondary Outcome: Electric Field Strength
3.4. Secondary Outcome: Link to General Cognitive Ability
3.5. Adverse Events and Blinding
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Postma, A.; Kessels, R.; Vanasselen, M. How the Brain Remembers and Forgets where Things Are: The Neurocognition of Object–Location Memory. Neurosci. Biobehav. Rev. 2008, 32, 1339–1345. [Google Scholar] [CrossRef]
- De Sousa, A.V.C.; Grittner, U.; Rujescu, D.; Külzow, N.; Flöel, A. Impact of 3-Day Combined Anodal Transcranial Direct Current Stimulation-Visuospatial Training on Object-Location Memory in Healthy Older Adults and Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2020, 75, 223–244. [Google Scholar] [CrossRef]
- Hedden, T.; Gabrieli, J.D.E. Insights into the Ageing Mind: A View from Cognitive Neuroscience. Nat. Rev. Neurosci. 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; Hobbel, D.; Postma, A. Aging, context memory and binding: A comparison of “what, where and when” in young and older adults. Int. J. Neurosci. 2007, 117, 795–810. [Google Scholar] [CrossRef]
- Liu, Y.; Paajanen, T.; Zhang, Y.; Westman, E.; Wahlund, L.-O.; Simmons, A.; Tunnard, C.; Sobow, T.; Mecocci, P.; Tsolaki, M.; et al. Combination Analysis of Neuropsychological Tests and Structural MRI Measures in Differentiating AD, MCI and Control Groups—The AddNeuroMed Study. Neurobiol. Aging 2011, 32, 1198–1206. [Google Scholar] [CrossRef]
- Shin, M.-S.; Park, S.-Y.; Park, S.-R.; Seol, S.-H.; Kwon, J.S. Clinical and Empirical Applications of the Rey–Osterrieth Complex Figure Test. Nat. Protoc. 2006, 1, 892–899. [Google Scholar] [CrossRef]
- Zimmermann, K.; Eschen, A. Brain Regions Involved in Subprocesses of Small-Space Episodic Object-Location Memory: A Systematic Review of Lesion and Functional Neuroimaging Studies. Memory 2017, 25, 487–519. [Google Scholar] [CrossRef]
- Cona, G.; Scarpazza, C. Where Is the “Where” in the Brain? A Meta-analysis of Neuroimaging Studies on Spatial Cognition. Hum. Brain Mapp. 2019, 40, 1867–1886. [Google Scholar] [CrossRef]
- Gillis, M.M.; Garcia, S.; Hampstead, B.M. Working Memory Contributes to the Encoding of Object Location Associations: Support for a 3-Part Model of Object Location Memory. Behav. Brain Res. 2016, 311, 192–200. [Google Scholar] [CrossRef]
- Frankland, P.W.; Bontempi, B. The Organization of Recent and Remote Memories. Nat. Rev. Neurosci. 2005, 6, 119–130. [Google Scholar] [CrossRef]
- Manelis, A.; Reder, L.M.; Hanson, S.J. Dynamic Changes in the Medial Temporal Lobe during Incidental Learning of Object–Location Associations. Cereb. Cortex 2012, 22, 828–837. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neural Correlates and Molecular Mechanisms of Memory and Learning. Int. J. Mol. Sci. 2024, 25, 2724. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Mazzà, M.; Tamietto, M.; Avenanti, A. Targeting Human Glucocorticoid Receptors in Fear Learning: A Multiscale Integrated Approach to Study Functional Connectivity. Int. J. Mol. Sci. 2024, 25, 864. [Google Scholar] [CrossRef]
- Thams, F.; Külzow, N.; Flöel, A.; Antonenko, D. Modulation of Network Centrality and Gray Matter Microstructure Using Multi-session Brain Stimulation and Memory Training. Hum. Brain Mapp. 2022, 43, 3416–3426. [Google Scholar] [CrossRef]
- Seghier, M.L. The Angular Gyrus: Multiple Functions and Multiple Subdivisions. Neuroscientist 2013, 19, 43–61. [Google Scholar] [CrossRef]
- Lou, W.; Shi, L.; Wong, A.; Chu, W.C.W.; Mok, V.C.T.; Wang, D. Changes of Cerebral Perfusion and Functional Brain Network Organization in Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 54, 397–409. [Google Scholar] [CrossRef]
- Valenzuela, M.J.; Turner, A.J.F.; Kochan, N.A.; Wen, W.; Suo, C.; Hallock, H.; McIntosh, R.A.; Sachdev, P.; Breakspear, M. Posterior Compensatory Network in Cognitively Intact Elders with Hippocampal Atrophy. Hippocampus 2015, 25, 581–593. [Google Scholar] [CrossRef]
- Brodt, S.; Gais, S.; Beck, J.; Erb, M.; Scheffler, K.; Schönauer, M. Fast Track to the Neocortex: A Memory Engram in the Posterior Parietal Cortex. Science 2018, 362, 1045–1048. [Google Scholar] [CrossRef]
- Buckner, R.L.; DiNicola, L.M. The Brain’s Default Network: Updated Anatomy, Physiology and Evolving Insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- Smallwood, J.; Bernhardt, B.C.; Leech, R.; Bzdok, D.; Jefferies, E.; Margulies, D.S. The Default Mode Network in Cognition: A Topographical Perspective. Nat. Rev. Neurosci. 2021, 22, 503–513. [Google Scholar] [CrossRef]
- Antonenko, D.; Hayek, D.; Netzband, J.; Grittner, U.; Flöel, A. tDCS-Induced Episodic Memory Enhancement and Its Association with Functional Network Coupling in Older Adults. Sci. Rep. 2019, 9, 2273. [Google Scholar] [CrossRef]
- Koch, G.; Bonnì, S.; Pellicciari, M.C.; Casula, E.P.; Mancini, M.; Esposito, R.; Ponzo, V.; Picazio, S.; Di Lorenzo, F.; Serra, L.; et al. Transcranial Magnetic Stimulation of the Precuneus Enhances Memory and Neural Activity in Prodromal Alzheimer’s Disease. NeuroImage 2018, 169, 302–311. [Google Scholar] [CrossRef]
- Momi, D.; Ozdemir, R.A.; Tadayon, E.; Boucher, P.; Shafi, M.M.; Pascual-Leone, A.; Santarnecchi, E. Network-Level Macroscale Structural Connectivity Predicts Propagation of Transcranial Magnetic Stimulation. NeuroImage 2021, 229, 117698. [Google Scholar] [CrossRef]
- Ozdemir, R.A.; Tadayon, E.; Boucher, P.; Momi, D.; Karakhanyan, K.A.; Fox, M.D.; Halko, M.A.; Pascual-Leone, A.; Shafi, M.M.; Santarnecchi, E. Individualized Perturbation of the Human Connectome Reveals Reproducible Biomarkers of Network Dynamics Relevant to Cognition. Proc. Natl. Acad. Sci. USA 2020, 117, 8115–8125. [Google Scholar] [CrossRef]
- Wang, J.X.; Rogers, L.M.; Gross, E.Z.; Ryals, A.J.; Dokucu, M.E.; Brandstatt, K.L.; Hermiller, M.S.; Voss, J.L. Targeted Enhancement of Cortical-Hippocampal Brain Networks and Associative Memory. Science 2014, 345, 1054–1057. [Google Scholar] [CrossRef]
- Nilakantan, A.S.; Bridge, D.J.; Gagnon, E.P.; VanHaerents, S.A.; Voss, J.L. Stimulation of the Posterior Cortical-Hippocampal Network Enhances Precision of Memory Recollection. Curr. Biol. 2017, 27, 465–470. [Google Scholar] [CrossRef]
- Tang, Y.; Xu, L.; Zhu, T.; Cui, H.; Qian, Z.; Kong, G.; Tang, X.; Wei, Y.; Zhang, T.; Hu, Y.; et al. Visuospatial Learning Selectively Enhanced by Personalized Transcranial Magnetic Stimulation over Parieto-Hippocampal Network among Patients at Clinical High-Risk for Psychosis. Schizophr. Bull. 2023, 49, 923–932. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability Changes Induced in the Human Motor Cortex by Weak Transcranial Direct Current Stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef]
- Bjekić, J.; Čolić, M.V.; Živanović, M.; Milanović, S.D.; Filipović, S.R. Transcranial Direct Current Stimulation (tDCS) over Parietal Cortex Improves Associative Memory. Neurobiol. Learn. Mem. 2019, 157, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Flöel, A.; Suttorp, W.; Kohl, O.; Kürten, J.; Lohmann, H.; Breitenstein, C.; Knecht, S. Non-Invasive Brain Stimulation Improves Object-Location Learning in the Elderly. Neurobiol. Aging 2012, 33, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Truong, D.Q.; Khadka, N.; Bikson, M. Spatial and Polarity Precision of Concentric High-Definition Transcranial Direct Current Stimulation (HD-tDCS). Phys. Med. Biol. 2016, 61, 4506–4521. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Bansal, V.; Diaz, J.; Patel, J.; Reato, D.; Bikson, M. Gyri-Precise Head Model of Transcranial Direct Current Stimulation: Improved Spatial Focality Using a Ring Electrode versus Conventional Rectangular Pad. Brain Stimul. 2009, 2, 201–207.e1. [Google Scholar] [CrossRef] [PubMed]
- Dmochowski, J.P.; Datta, A.; Bikson, M.; Su, Y.; Parra, L.C. Optimized Multi-Electrode Stimulation Increases Focality and Intensity at Target. J. Neural Eng. 2011, 8, 046011. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Külzow, N.; Sousa, A.; Prehn, K.; Grittner, U.; Flöel, A. Neuronal and Behavioral Effects of Multi-Day Brain Stimulation and Memory Training. Neurobiol. Aging 2018, 61, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Prehn, K.; Stengl, H.; Grittner, U.; Kosiolek, R.; Ölschläger, A.; Weidemann, A.; Flöel, A. Effects of Anodal Transcranial Direct Current Stimulation and Serotonergic Enhancement on Memory Performance in Young and Older Adults. Neuropsychopharmacol 2017, 42, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Külzow, N.; Cavalcanti De Sousa, A.V.; Cesarz, M.; Hanke, J.-M.; Günsberg, A.; Harder, S.; Koblitz, S.; Grittner, U.; Flöel, A. No Effects of Non-Invasive Brain Stimulation on Multiple Sessions of Object-Location-Memory Training in Healthy Older Adults. Front. Neurosci. 2018, 11, 746. [Google Scholar] [CrossRef]
- Evans, C.; Bachmann, C.; Lee, J.S.A.; Gregoriou, E.; Ward, N.; Bestmann, S. Dose-Controlled tDCS Reduces Electric Field Intensity Variability at a Cortical Target Site. Brain Stimul. 2020, 13, 125–136. [Google Scholar] [CrossRef]
- Gözenman, F.; Berryhill, M.E. Working Memory Capacity Differentially Influences Responses to tDCS and HD-tDCS in a Retro-Cue Task. Neurosci. Lett. 2016, 629, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Heusser, A.C.; Poeppel, D.; Ezzyat, Y.; Davachi, L. Episodic Sequence Memory Is Supported by a Theta–Gamma Phase Code. Nat. Neurosci. 2016, 19, 1374–1380. [Google Scholar] [CrossRef]
- Leshikar, E.D.; Duarte, A.; Hertzog, C. Task-Selective Memory Effects for Successfully Implemented Encoding Strategies. PLoS ONE 2012, 7, e38160. [Google Scholar] [CrossRef]
- Perceval, G.; Martin, A.K.; Copland, D.A.; Laine, M.; Meinzer, M. Multisession Transcranial Direct Current Stimulation Facilitates Verbal Learning and Memory Consolidation in Young and Older Adults. Brain Lang. 2020, 205, 104788. [Google Scholar] [CrossRef] [PubMed]
- Helmstaedter, C.; Durwen, H. VLMT: Verbaler Lern-Und Merkfähigkeitstest: Ein Praktikables Und Differenziertes Instrumentarium Zur Prüfung Der Verbalen Gedächtnisleistungen. Schweiz. Arch. Für Neurol. Neurochir. Und Psychiatr. 1990, 141, 21–30. [Google Scholar]
- Müller, H.; Hasse-Sander, I.; Horn, R.; Helmstaedter, C.; Elger, C.E. Rey Auditory-Verbal Learning Test: Structure of a Modified German Version. J. Clin. Psychol. 1997, 53, 663–671. [Google Scholar] [CrossRef]
- Rey, A.; Osterrieth, P.A.A. Rey-Osterrieth Complex Figure Copying Test. In APA PsycTests; American Psychological Association: Washington, DC, USA, 2011. [Google Scholar]
- Wechsler, D. Wechsler Adult Intelligence Scale, 3rd ed.; Pearson: Frankfurt am Main, Germany, 2019. [Google Scholar]
- Tombaugh, T. Trail Making Test A and B: Normative Data Stratified by Age and Education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Van Der Elst, W.; Van Boxtel, M.P.J.; Van Breukelen, G.J.P.; Jolles, J. The Stroop Color-Word Test: Influence of Age, Sex, and Education; and Normative Data for a Large Sample Across the Adult Age Range. Assessment 2006, 13, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Aschenbrenner, S.; Tucha, O.; Lange, K. RWT: Regensburger Wortflüssigkeits-Test; Hogrefe: Göttingen, Germany, 2000. [Google Scholar]
- Mack, W.J.; Freed, D.M.; Williams, B.W.; Henderson, V.W. Boston Naming Test: Shortened Versions for Use in Alzheimer’s Disease. J. Gerontol. 1992, 47, P154–P158. [Google Scholar] [CrossRef]
- Antal, A.; Alekseichuk, I.; Bikson, M.; Brockmöller, J.; Brunoni, A.R.; Chen, R.; Cohen, L.G.; Dowthwaite, G.; Ellrich, J.; Flöel, A.; et al. Low Intensity Transcranial Electric Stimulation: Safety, Ethical, Legal Regulatory and Application Guidelines. Clin. Neurophysiol. 2017, 128, 1774–1809. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Fernández, R.; Avendaño-Coy, J.; Velasco-Velasco, R.; Palomo-Carrión, R.; Bravo-Esteban, E.; Ferri-Morales, A. A New Approach to Assess Blinding for Transcranial Direct Current Stimulation Treatment in Patients with Fibromyalgia. A Randomized Clinical Trial. Brain Sci. 2021, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.; Ni, L.; Davis, C.E. Assessment of Blinding in Clinical Trials. Control. Clin. Trials 2004, 25, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Thielscher, A.; Antunes, A.; Saturnino, G.B. Field Modeling for Transcranial Magnetic Stimulation: A Useful Tool to Understand the Physiological Effects of TMS? In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 222–225. [Google Scholar]
- Saturnino, G.B.; Antunes, A.; Thielscher, A. On the Importance of Electrode Parameters for Shaping Electric Field Patterns Generated by tDCS. NeuroImage 2015, 120, 25–35. [Google Scholar] [CrossRef]
- Truong, D.Q.; Adair, D.; Bikson, M. Computer-Based Models of tDCS and tACS. In Transcranial Direct Current Stimulation in Neuropsychiatric Disorders; Brunoni, A., Nitsche, M., Loo, C., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 47–66. ISBN 978-3-319-33965-8. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67, 48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. lmerTest Package: Tests in Linear Mixed Effects Models. J. Stat. Soft. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Hsu, W.-Y.; Ku, Y.; Zanto, T.P.; Gazzaley, A. Effects of Noninvasive Brain Stimulation on Cognitive Function in Healthy Aging and Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurobiol. Aging 2015, 36, 2348–2359. [Google Scholar] [CrossRef] [PubMed]
- Strube, W.; Bunse, T.; Nitsche, M.A.; Nikolaeva, A.; Palm, U.; Padberg, F.; Falkai, P.; Hasan, A. Bidirectional Variability in Motor Cortex Excitability Modulation Following 1 mA Transcranial Direct Current Stimulation in Healthy Participants. Physiol. Rep. 2016, 4, e12884. [Google Scholar] [CrossRef]
- Wiethoff, S.; Hamada, M.; Rothwell, J.C. Variability in Response to Transcranial Direct Current Stimulation of the Motor Cortex. Brain Stimul. 2014, 7, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Thielscher, A.; Saturnino, G.B.; Aydin, S.; Ittermann, B.; Grittner, U.; Flöel, A. Towards Precise Brain Stimulation: Is Electric Field Simulation Related to Neuromodulation? Brain Stimul. 2019, 12, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Zich, C.; Lee, J.S.A.; Ward, N.; Bestmann, S. Inter-Individual Variability in Current Direction for Common tDCS Montages. NeuroImage 2022, 260, 119501. [Google Scholar] [CrossRef]
- Hill, A.T.; Rogasch, N.C.; Fitzgerald, P.B.; Hoy, K.E. Effects of Single versus Dual-Site High-Definition Transcranial Direct Current Stimulation (HD-tDCS) on Cortical Reactivity and Working Memory Performance in Healthy Subjects. Brain Stimul. 2018, 11, 1033–1043. [Google Scholar] [CrossRef]
- Müller, D.; Habel, U.; Brodkin, E.S.; Weidler, C. High-Definition Transcranial Direct Current Stimulation (HD-tDCS) for the Enhancement of Working Memory—A Systematic Review and Meta-Analysis of Healthy Adults. Brain Stimul. 2022, 15, 1475–1485. [Google Scholar] [CrossRef]
- Nikolin, S.; Lauf, S.; Loo, C.K.; Martin, D. Effects of High-Definition Transcranial Direct Current Stimulation (HD-tDCS) of the Intraparietal Sulcus and Dorsolateral Prefrontal Cortex on Working Memory and Divided Attention. Front. Integr. Neurosci. 2019, 12, 64. [Google Scholar] [CrossRef]
- Nissim, N.R.; O’Shea, A.; Indahlastari, A.; Telles, R.; Richards, L.; Porges, E.; Cohen, R.; Woods, A.J. Effects of In-Scanner Bilateral Frontal tDCS on Functional Connectivity of the Working Memory Network in Older Adults. Front. Aging Neurosci. 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed]
- Scheldrup, M.; Greenwood, P.M.; McKendrick, R.; Strohl, J.; Bikson, M.; Alam, M.; McKinley, R.A.; Parasuraman, R. Transcranial Direct Current Stimulation Facilitates Cognitive Multi-Task Performance Differentially Depending on Anode Location and Subtask. Front. Hum. Neurosci. 2014, 8, 665. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.A.; Bestmann, S.; Evans, C. A Future of Current Flow Modelling for Transcranial Electrical Stimulation? Curr. Behav. Neurosci. Rep. 2021, 8, 150–159. [Google Scholar] [CrossRef]
- Opitz, A.; Yeagle, E.; Thielscher, A.; Schroeder, C.; Mehta, A.D.; Milham, M.P. On the Importance of Precise Electrode Placement for Targeted Transcranial Electric Stimulation. NeuroImage 2018, 181, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Menardi, A.; Momi, D.; Vallesi, A.; Barabási, A.-L.; Towlson, E.K.; Santarnecchi, E. Maximizing Brain Networks Engagement via Individualized Connectome-Wide Target Search. Brain Stimul. 2022, 15, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Mikkonen, M.; Laakso, I.; Tanaka, S.; Hirata, A. Cost of Focality in TDCS: Interindividual Variability in Electric Fields. Brain Stimul. 2020, 13, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Jamil, A.; Batsikadze, G.; Kuo, H.; Meesen, R.L.J.; Dechent, P.; Paulus, W.; Nitsche, M.A. Current Intensity- and Polarity-specific Online and Aftereffects of Transcranial Direct Current Stimulation: An fMRI Study. Hum. Brain Mapp. 2020, 41, 1644–1666. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, D.-W.; Chang, W.H.; Kim, Y.-H.; Kim, K.; Im, C.-H. Inconsistent Outcomes of Transcranial Direct Current Stimulation May Originate from Anatomical Differences among Individuals: Electric Field Simulation Using Individual MRI Data. Neurosci. Lett. 2014, 564, 6–10. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Liu, N.; Wang, P.; Sun, K.; Xiao, W. High-Definition Transcranial Direct Current Stimulation of the Left Middle Temporal Complex Does Not Affect Visual Motion Perception Learning. Front. Neurosci. 2022, 16, 988590. [Google Scholar] [CrossRef]
- Antonenko, D.; Schubert, F.; Bohm, F.; Ittermann, B.; Aydin, S.; Hayek, D.; Grittner, U.; Flöel, A. tDCS-Induced Modulation of GABA Levels and Resting-State Functional Connectivity in Older Adults. J. Neurosci. 2017, 37, 4065–4073. [Google Scholar] [CrossRef]
- He, Q.; Beveridge, E.H.; Starnes, J.; Goodroe, S.C.; Brown, T.I. Environmental Overlap and Individual Encoding Strategy Modulate Memory Interference in Spatial Navigation. Cognition 2021, 207, 104508. [Google Scholar] [CrossRef] [PubMed]
- Riby, L.M.; Orme, E. A Familiar Pattern? Semantic Memory Contributes to the Enhancement of Visuo-Spatial Memories. Brain Cogn. 2013, 81, 215–222. [Google Scholar] [CrossRef] [PubMed]
- McBride, D.M.; Anne Dosher, B. A Comparison of Conscious and Automatic Memory Processes for Picture and Word Stimuli: A Process Dissociation Analysis. Conscious. Cogn. 2002, 11, 423–460. [Google Scholar] [CrossRef] [PubMed]
- Brandimonte, M.A.; Hitch, G.J.; Bishop, D.V.M. Verbal Recoding of Visual Stimuli Impairs Mentalimagetransformations. Mem. Cogn. 1992, 20, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Baadte, C.; Meinhardt-Injac, B. The Picture Superiority Effect in Associative Memory: A Developmental Study. Br. J. Dev. Psychol. 2019, 37, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Kosslyn, S.M.; Koenig, O.; Barrett, A.; Cave, C.B.; Tang, J.; Gabrieli, J.D.E. Evidence for Two Types of Spatial Representations: Hemispheric Specialization for Categorical and Coordinate Relations. J. Exp. Psychol. Hum. Percept. Perform. 1989, 15, 723–735. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, D.; Fromm, A.E.; Thams, F.; Grittner, U.; Meinzer, M.; Flöel, A. Microstructural and Functional Plasticity Following Repeated Brain Stimulation during Cognitive Training in Older Adults. Nat. Commun. 2023, 14, 3184. [Google Scholar] [CrossRef] [PubMed]
- Cimadevilla, J.M.; Piccardi, L. Spatial Skills. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 175, pp. 65–79. ISBN 978-0-444-64123-6. [Google Scholar]
- Park, J.; Shin, G.I.; Park, Y.M.; Kim, I.Y.; Jang, D.P. Sex Differences of Cognitive Load Effects on Object-Location Binding Memory. Biomed. Eng. Lett. 2017, 7, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Perceval, G.; Flöel, A.; Meinzer, M. Can Transcranial Direct Current Stimulation Counteract Age-Associated Functional Impairment? Neurosci. Biobehav. Rev. 2016, 65, 157–172. [Google Scholar] [CrossRef]


| Total | Anodal | Sham | ||
|---|---|---|---|---|
| N (females) | 52 (40) | 26 (20) | 26 (20) | |
| Age [years] | 22.8 (2.6) | 22.5 (2.7) | 23.2 (2.5) | |
| Verbal Learning [%] | 90.1 (5.8) | 89.8 (6.8) | 91.3 (4.7) | |
| Spatial Memory [%] | 86.9 (7.6) | 86.7 (8.3) | 87.1 (7.0) | |
| Digit Span [n] | forward | 8.2 (1.7) | 8.2 (1.4) | 8.2 (1.9) |
| backward | 7.6 (1.7) | 7.4 (1.5) | 7.9 (1.8) | |
| Stroop [s] | words | 26.4 (3.2) | 26.1 (2.6) | 26.7 (3.8) |
| color | 42.8 (8.3) | 42.4 (7.6) | 43.2 (9.1) | |
| interference | 63.9 (14.9) | 62.5 (11.8) | 65.3 (15.9) | |
| TMT [s] | A | 19.9 (6.7) | 21.1 (7.3) | 18.7 (6.0) |
| B | 40.7 (11.5) | 42.0 (13.5) | 39.5 (9.2) | |
| Verbal Fluency [n] | phonematic | 18.5 (4.3) | 18.5 (4.0) | 18.6 (4.7) |
| semantic | 32.9 (6.4) | 33.9 (6.7) | 31.8 (6.0) | |
| switch | 19.3 (3.0) | 19.3 (3.8) | 19.3 (2.1) | |
| Baseline OLM [%] | 31.5 (16.9) | 33.7 (18.6) | 29.5 (15.1) |
| Total n = 52 | Anodal Group n = 26 | Sham Group n = 26 | Incidence Rate Ratio for Group Differences (95% CI) | |
|---|---|---|---|---|
| Total number of adverse events | 76 | 41 | 35 | 1.3 (0.9–2.0) |
| Itching | 18/1.6 (0.6) | 11/1.8 (0.6) | 7/1.1 (0.4) | 2.5 (1.1–6.0) |
| Pain | 5/1.4 (0.5) | 1/1 | 4/1.5 (0.6) | 0.2 (0.0–1.0) |
| Burning | 18/1.3 (0.6) | 13/1.4 (0.7) | 5/1 (0) | 3.6 (1.4–10.9) |
| Warmth/heat | 17/1.4 (0.6) | 9/1.6 (0.7) | 8/1.3 (0.5) | 1.4 (0.6–3.2) |
| Metallic taste | 1/1 | 0 | 1/1 | NA |
| Fatigue/decreased alertness | 5/1 (0) | 3/1 (0) | 2/1 (0) | 1.5 (0.2–11.4) |
| Other | 12/1–3 (0.7) | 4/1 (0) | 8/1.5 (0.8) | 0.3 (0.1–1.0) |
| Assignment | Response | ||||
|---|---|---|---|---|---|
| Anodal | Sham | DK | Total | ||
| Anodal | 9 | 12 | 5 | 26 | |
| Sham | 8 | 11 | 7 | 26 | |
| Total | 17 | 23 | 12 | 52 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fromm, A.E.; Grittner, U.; Brodt, S.; Flöel, A.; Antonenko, D. No Object–Location Memory Improvement through Focal Transcranial Direct Current Stimulation over the Right Temporoparietal Cortex. Life 2024, 14, 539. https://doi.org/10.3390/life14050539
Fromm AE, Grittner U, Brodt S, Flöel A, Antonenko D. No Object–Location Memory Improvement through Focal Transcranial Direct Current Stimulation over the Right Temporoparietal Cortex. Life. 2024; 14(5):539. https://doi.org/10.3390/life14050539
Chicago/Turabian StyleFromm, Anna Elisabeth, Ulrike Grittner, Svenja Brodt, Agnes Flöel, and Daria Antonenko. 2024. "No Object–Location Memory Improvement through Focal Transcranial Direct Current Stimulation over the Right Temporoparietal Cortex" Life 14, no. 5: 539. https://doi.org/10.3390/life14050539





