Navigating Post-Traumatic Osteoporosis: A Comprehensive Review of Epidemiology, Pathophysiology, Diagnosis, Treatment, and Future Directions
Abstract
:1. Introduction
2. Epidemiology
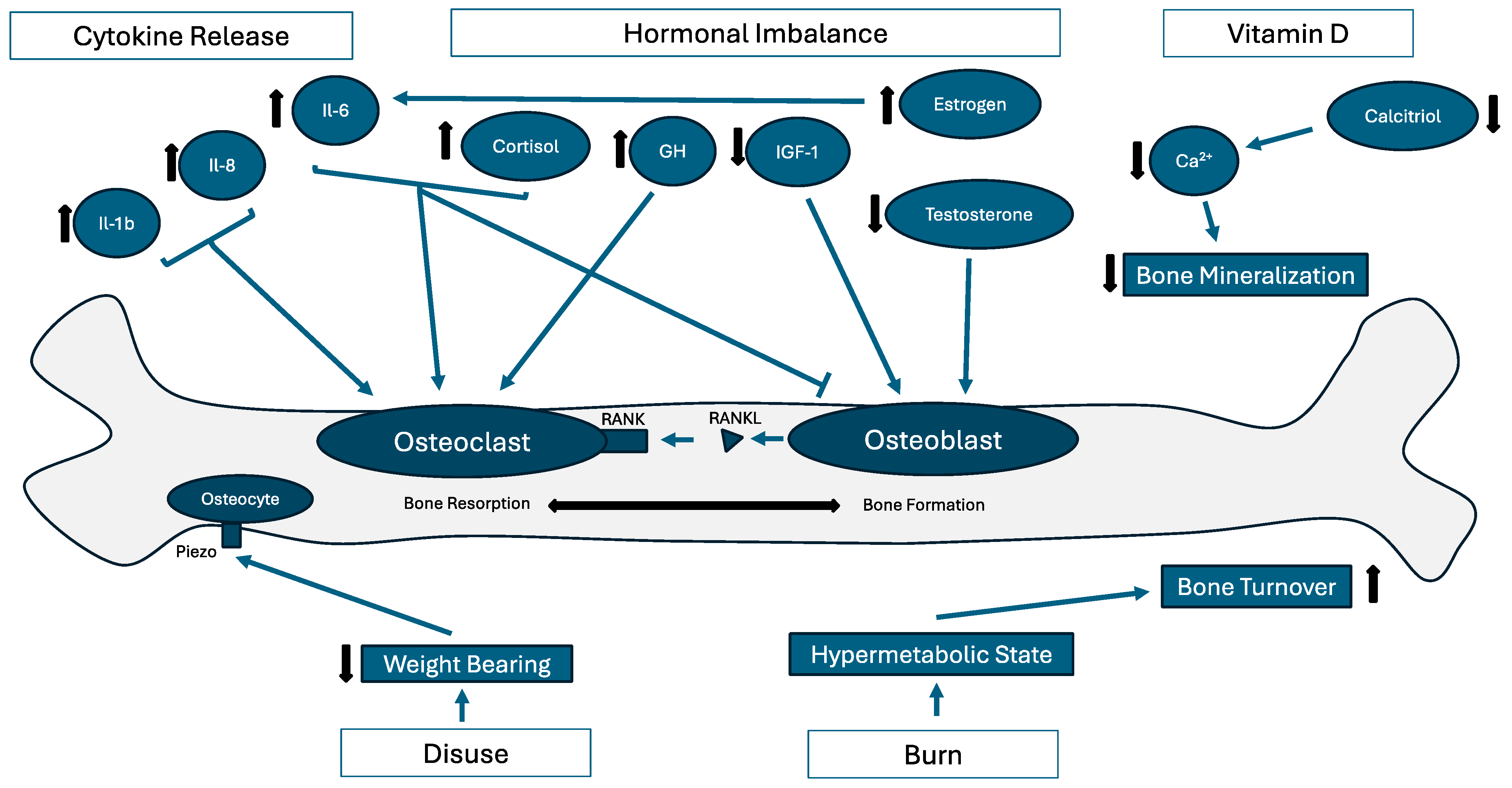
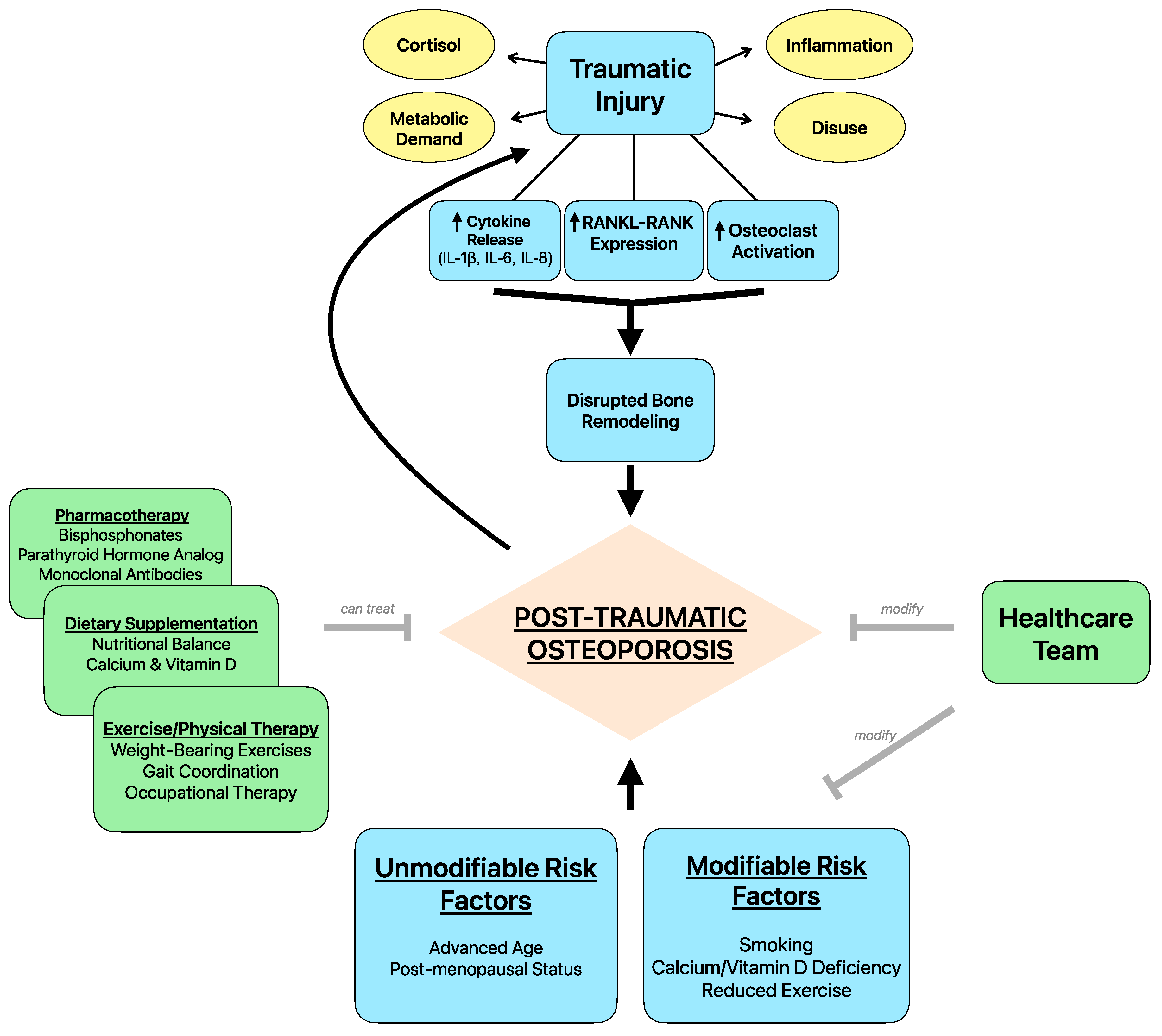
3. Pathophysiology
4. Risk Factors
5. Role of Healthcare Providers in Reducing Risk
6. Symptoms and Diagnosis
7. Treatment and Management
8. Prognosis and Follow-Up
9. Future Directions
10. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Fontaine, R.; Herrmann, L.G. Post-Traumatic Painful Osteoporosis. Ann. Surg. 1933, 97, 26–61. [Google Scholar] [CrossRef] [PubMed]
- Kaewboonchoo, O.; Sung, F.C.; Lin, C.L.; Hsu, H.C.; Kuo, C.T. Risk of osteoporosis and fracture in victims with burn injury. Osteoporos. Int. 2019, 30, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Moore-Lotridge, S.N.; Ihejirika, R.; Gibson, B.H.Y.; Posey, S.L.; Mignemi, N.A.; Cole, H.A.; Hawley, G.D.; Uppuganti, S.; Nyman, J.S.; Schoenecker, J.G. Severe injury-induced osteoporosis and skeletal muscle mineralization: Are these related complications? Bone Rep. 2021, 14, 100743. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.; Janjić, Z.; Marić, D. Principles of management of high-energy injuries of the leg. Med. Pregl. 2002, 55, 437–442. [Google Scholar] [PubMed]
- Saito, M.; Moore-Lotridge, S.N.; Uppuganti, S.; Egawa, S.; Yoshii, T.; Robinette, J.P.; Posey, S.L.; Gibson, B.H.; Cole, H.A.; Hawley, G.D.; et al. Determining the pharmacologic window of bisphosphonates that mitigates severe injury-induced osteoporosis and muscle calcification, while preserving fracture repair. Osteoporos. Int. 2022, 33, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.B.; Konopka, J.A.; Azam, M.T.; Ubillus, H.A.; Mercer, N.P.; Kennedy, J.G. Calcaneal reconstruction using a femoral head allograft and biologic adjuncts: A case report. SAGE Open Med. Case Rep. 2022, 10, 2050313x221129782. [Google Scholar] [CrossRef] [PubMed]
- Armas, L.A.; Recker, R.R. Pathophysiology of osteoporosis: New mechanistic insights. Endocrinol. Metab. Clin. N. Am. 2012, 41, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Gold, T.; Williams, S.A.; Weiss, R.J.; Wang, Y.; Watkins, C.; Carroll, J.; Middleton, C.; Silverman, S. Impact of fractures on quality of life in patients with osteoporosis: A US cross-sectional survey. J. Drug Assess. 2019, 8, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Willers, C.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Borgström, F.; Kanis, J.A. Osteoporosis in Europe: A compendium of country-specific reports. Arch. Osteoporos. 2022, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Burden, A.M.; Tanaka, Y.; Xu, L.; Ha, Y.C.; McCloskey, E.; Cummings, S.R.; Glüer, C.C. Osteoporosis case ascertainment strategies in European and Asian countries: A comparative review. Osteoporos. Int. 2021, 32, 817–829. [Google Scholar] [CrossRef]
- Sato, M.; Vietri, J.; Flynn, J.A.; Fujiwara, S. Treatment for Osteoporosis among Women in Japan: Associations with Patient Characteristics and Patient-Reported Outcomes in the 2008–2011 Japan National Health and Wellness Surveys. J. Osteoporos. 2014, 2014, 909153. [Google Scholar] [CrossRef] [PubMed]
- Iki, M. Epidemiology of osteoporosis in Japan. Clin. Calcium 2012, 22, 797–803. [Google Scholar] [PubMed]
- Cheung, C.L.; Ang, S.B.; Chadha, M.; Chow, E.S.; Chung, Y.S.; Hew, F.L.; Jaisamrarn, U.; Ng, H.; Takeuchi, Y.; Wu, C.H. An updated hip fracture projection in Asia: The Asian Federation of Osteoporosis Societies study. Osteoporos. Sarcopenia 2018, 4, 16–21. [Google Scholar] [CrossRef]
- Office of the Surgeon General (US). Bone Health and Osteoporosis: A Report of the Surgeon General; Reports of the Surgeon General; Office of the Surgeon General (US): Rockville, MD, USA, 2004. [Google Scholar]
- Teng, G.G.; Curtis, J.R.; Saag, K.G. Mortality and osteoporotic fractures: Is the link causal, and is it modifiable? Clin. Exp. Rheumatol. 2008, 26 (Suppl. S51), S125–S137. [Google Scholar]
- Neuman, M.D.; Silber, J.H.; Magaziner, J.S.; Passarella, M.A.; Mehta, S.; Werner, R.M. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern. Med. 2014, 174, 1273–1280. [Google Scholar] [CrossRef]
- Comarr, A.E.; Hutchinson, R.H.; Bors, E. Extremity fractures of patients with spinal cord injuries. Am. J. Surg. 1962, 103, 732–739. [Google Scholar] [CrossRef]
- Ragnarsson, K.T.; Sell, G.H. Lower extremity fractures after spinal cord injury: A retrospective study. Arch. Phys. Med. Rehabil. 1981, 62, 418–423. [Google Scholar]
- Ingram, R.R.; Suman, R.K.; Freeman, P.A. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia 1989, 27, 133–139. [Google Scholar] [CrossRef]
- Vestergaard, P.; Krogh, K.; Rejnmark, L.; Mosekilde, L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998, 36, 790–796. [Google Scholar] [CrossRef]
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Mechanisms of osteoporosis in spinal cord injury. Clin. Endocrinol. 2006, 65, 555–565. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Center, J.R.; Eisman, J.A. Osteoporosis: Underrated, underdiagnosed and undertreated. Med. J. Aust. 2004, 180, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Emkey, G.R.; Epstein, S. Secondary osteoporosis: Pathophysiology & diagnosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 911–935. [Google Scholar] [PubMed]
- Yu, B.; Wang, C.Y. Osteoporosis: The Result of an ‘Aged’ Bone Microenvironment. Trends Mol. Med. 2016, 22, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Sapan, H.B.; Paturusi, I.; Islam, A.A.; Yusuf, I.; Patellongi, I.; Massi, M.N.; Pusponegoro, A.D.; Arief, S.K.; Labeda, I.; Rendy, L.; et al. Interleukin-6 and interleukin-10 plasma levels and mRNA expression in polytrauma patients. Chin. J. Traumatol. 2017, 20, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Khurana, S.; Kumar, S.; Sagar, S.; Gupta, A.; Mishra, B.; Soni, K.D.; Mathur, P. CD14+ Monocytic Cytokines: Impact on Outcome in Severely Injured Patients. Indian. J. Crit. Care Med. 2018, 22, 528–532. [Google Scholar] [PubMed]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Kopesky, P.; Tiedemann, K.; Alkekhia, D.; Zechner, C.; Millard, B.; Schoeberl, B.; Komarova, S.V. Autocrine signaling is a key regulatory element during osteoclastogenesis. Biol. Open 2014, 3, 767–776. [Google Scholar] [CrossRef]
- Liu, Y.; Krishnamurthy, A.; Hensvold, A.H.; Joshua, V.; Sun, M.; Engstrom, M.; Wähämaa, H.; Malmström, V.; Jopling, L.A.; Rethi, B.; et al. AB0078 Role of IL-8 and Its Receptor in Anti-Citrullinated Protein Antibody Mediated Osteoclastogenesis in RA. Ann. Rheum. Dis. 2016, 75 (Suppl. S2), 923. [Google Scholar] [CrossRef]
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Wang, T.; He, C. TNF-α and IL-6: The Link between Immune and Bone System. Curr. Drug Targets 2020, 21, 213–227. [Google Scholar] [PubMed]
- Ilias, I.; Milionis, C.; Zoumakis, E. An Overview of Glucocorticoid-Induced Osteoporosis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Alesci, S.; De Martino, M.U.; Ilias, I.; Gold, P.W.; Chrousos, G.P. Glucocorticoid-induced osteoporosis: From basic mechanisms to clinical aspects. Neuroimmunomodulation 2005, 12, 1–19. [Google Scholar] [CrossRef]
- Atwood, R.E.; Golden, D.M.; Kaba, S.A.; Bradley, M.J. Characterization of the cortisol response to traumatic hemorrhage and intra-abdominal contamination models in Cynomologus Macaques. Mol. Cell Endocrinol. 2020, 518, 111036. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Chai, Y.M.; Zhao, G.; Wen, G.; Han, P. Glucocorticoid receptorβ isoform exhibits a disproportionate increase over the α isoform in the lungs of a polytrauma rat model. Int. J. Clin. Exp. Pathol. 2018, 11, 3046–3051. [Google Scholar]
- Carroll, P.V. Protein metabolism and the use of growth hormone and insulin-like growth factor-I in the critically ill patient. Growth Horm. IGF Res. 1999, 9, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Teng Chung, T.; Hinds, C.J. Treatment with GH and IGF-1 in critical illness. Crit. Care Clin. 2006, 22, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, K.; Sugimoto, T.; Kaji, H.; Kanatani, M.; Kobayashi, T.; Chihara, K. Stimulatory effect of growth hormone on bone resorption and osteoclast differentiation. Endocrinology 1996, 137, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, H.F.; Moyer, R.F.; Yacoub, D.; Coughlin, D.; Birmingham, T.B. Effects of Recombinant Human Growth Hormone for Osteoporosis: Systematic Review and Meta-Analysis. Can. J. Aging 2017, 36, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.; Wu, X.; Pang, L.; Lou, M.; Rosen, C.J.; Qiu, T.; Crane, J.; Frassica, F.; Zhang, L.; Rodriguez, J.P.; et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat. Med. 2012, 18, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Z.; Duan, N.; Zhu, G.; Schwarz, E.M.; Xie, C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018, 59, 99–107. [Google Scholar] [CrossRef]
- Dolecek, R. Endocrine changes after burn trauma—A review. Keio J. Med. 1989, 38, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Schröder, J.; Kahlke, V.; Staubach, K.H.; Zabel, P.; Stüber, F. Gender differences in human sepsis. Arch. Surg. 1998, 133, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.J.; Faunce, D.E.; Messingham, K.A. Ethanol and burn injury: Estrogen modulation of immunity. Alcohol 2004, 33, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Gregory, M.S.; Duffner, L.A.; Faunce, D.E.; Kovacs, E.J. Estrogen mediates the sex difference in post-burn immunosuppression. J. Endocrinol. 2000, 164, 129–138. [Google Scholar] [CrossRef]
- Lephart, E.D.; Baxter, C.R.; Parker, C.R., Jr. Effect of burn trauma on adrenal and testicular steroid hormone production. J. Clin. Endocrinol. Metab. 1987, 64, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.; Munker, R.; Nagel, D.; Weber, M.; Engelhardt, D. Serum androgens in intensive-care patients: Correlations with clinical findings. Clin. Endocrinol. 1991, 34, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Fourrier, F.; Jallot, A.; Leclerc, L.; Jourdain, M.; Racadot, A.; Chagnon, J.L.; Rime, A.; Chopin, C. Sex steroid hormones in circulatory shock, sepsis syndrome, and septic shock. Circ. Shock 1994, 43, 171–178. [Google Scholar] [PubMed]
- Sharma, A.C.; Bosmann, H.B.; Motew, S.J.; Hales, K.H.; Hales, D.B.; Ferguson, J.L. Steroid hormone alterations following induction of chronic intraperitoneal sepsis in male rats. Shock 1996, 6, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Kasperk, C.H.; Wergedal, J.E.; Farley, J.R.; Linkhart, T.A.; Turner, R.T.; Baylink, D.J. Androgens directly stimulate proliferation of bone cells in vitro. Endocrinology 1989, 124, 1576–1578. [Google Scholar] [CrossRef] [PubMed]
- Kasperk, C.H.; Wakley, G.K.; Hierl, T.; Ziegler, R. Gonadal and adrenal androgens are potent regulators of human bone cell metabolism in vitro. J. Bone Miner. Res. 1997, 12, 464–471. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Hicok, K.C.; Khosla, S. Effects of gonadal and adrenal androgens in a novel androgen-responsive human osteoblastic cell line. J. Cell Biochem. 1998, 71, 96–108. [Google Scholar] [CrossRef]
- Chin, K.Y.; Ima-Nirwana, S. The effects of orchidectomy and supraphysiological testosterone administration on trabecular bone structure and gene expression in rats. Aging Male 2015, 18, 60–66. [Google Scholar] [CrossRef]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef]
- Nagy, V.; Penninger, J.M. The RANKL-RANK Story. Gerontology 2015, 61, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Wein, M.N.; Kronenberg, H.M. Regulation of Bone Remodeling by Parathyroid Hormone. Cold Spring Harb. Perspect. Med. 2018, 8, a031237. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chavez-Arom, V.; Han, J.J.; Yeh, B.Y. High Rates of Vitamin D Deficiency in Acute Rehabilitation Patients. Arch. Rehabil. Res. Clin. Transl. 2021, 3, 100137. [Google Scholar] [CrossRef]
- Khundmiri, S.J.; Murray, R.D.; Lederer, E. PTH and Vitamin D. Compr. Physiol. 2016, 6, 561–601. [Google Scholar]
- Rolvien, T.; Amling, M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2022, 110, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Miner. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef]
- Ackers, I.; Malgor, R. Interrelationship of canonical and non-canonical Wnt signalling pathways in chronic metabolic diseases. Diab Vasc. Dis. Res. 2018, 15, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.; Yorgan, T.A.; Rolvien, T.; Ulsamer, L.; Koehne, T.; Liao, N.; Keller, D.; Vollersen, N.; Teufel, S.; Neven, M.; et al. Wnt1 is an Lrp5-independent bone-anabolic Wnt ligand. Sci. Transl. Med. 2018, 10, eaau7137. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, L.; Nookaew, I.; Mannen, E.; Silva, M.J.; Almeida, M.; Xiong, J. Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 2019, 8, e49631. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, J.I.; Plotkin, L.I.; Stewart, S.A.; Weinstein, R.S.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Miner. Res. 2006, 21, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Cabahug-Zuckerman, P.; Frikha-Benayed, D.; Majeska, R.J.; Tuthill, A.; Yakar, S.; Judex, S.; Schaffler, M.B. Osteocyte Apoptosis Caused by Hindlimb Unloading is Required to Trigger Osteocyte RANKL Production and Subsequent Resorption of Cortical and Trabecular Bone in Mice Femurs. J. Bone Miner. Res. 2016, 31, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.D.; Jiang, L.S.; Dai, L.Y. Effects of spinal cord injury on osteoblastogenesis, osteoclastogenesis and gene expression profiling in osteoblasts in young rats. Osteoporos. Int. 2007, 18, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic response to severe burn injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Randall, S.M.; Fear, M.W.; Wood, F.M.; Rea, S.; Boyd, J.H.; Duke, J.M. Long-term musculoskeletal morbidity after adult burn injury: A population-based cohort study. BMJ Open 2015, 5, e009395. [Google Scholar] [CrossRef] [PubMed]
- Roshanzamir, S.; Dabbaghmanesh, M.H.; Dabbaghmanesh, A.; Nejati, S. Autonomic dysfunction and osteoporosis after electrical burn. Burns 2016, 42, 583–588. [Google Scholar] [CrossRef]
- Rousseau, A.F.; Kerschan-Schindl, K.; Scherkl, M.; Amrein, K. Bone metabolism and fracture risk during and after critical illness. Curr. Opin. Crit. Care 2020, 26, 379–385. [Google Scholar] [CrossRef]
- Klein, G.L. The role of the musculoskeletal system in post-burn hypermetabolism. Metabolism 2019, 97, 81–86. [Google Scholar] [CrossRef]
- Yu, L.; Wang, L.; Chen, S. Endogenous toll-like receptor ligands and their biological significance. J. Cell Mol. Med. 2010, 14, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Ogunbileje, J.O.; Porter, C.; Herndon, D.N.; Chao, T.; Abdelrahman, D.R.; Papadimitriou, A.; Chondronikola, M.; Zimmers, T.A.; Reidy, P.T.; Rasmussen, B.B.; et al. Hypermetabolism and hypercatabolism of skeletal muscle accompany mitochondrial stress following severe burn trauma. Am. J. Physiol. Endocrinol. Metab. 2016, 311, E436–E448. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Sousse, L.E.; Irick, R.; Schryver, E.; Klein, G.L. Interactions of Phosphate Metabolism With Serious Injury, Including Burns. JBMR Plus 2017, 1, 59–65. [Google Scholar] [CrossRef]
- Mangum, L.H.; Avila, J.J.; Hurtgen, B.J.; Lofgren, A.L.; Wenke, J.C. Burn and thoracic trauma alters fracture healing, systemic inflammation, and leukocyte kinetics in a rat model of polytrauma. J. Orthop. Surg. Res. 2019, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Jaglal, S.B.; Donescu, O.S.; Bansod, V.; Laprade, J.; Thorpe, K.; Hawker, G.; Majumdar, S.R.; Meadows, L.; Cadarette, S.M.; Papaioannou, A.; et al. Impact of a centralized osteoporosis coordinator on post-fracture osteoporosis management: A cluster randomized trial. Osteoporos. Int. 2012, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. CMAJ 2010, 182, 1864–1873. [Google Scholar] [CrossRef]
- Oftadeh, R.; Perez-Viloria, M.; Villa-Camacho, J.C.; Vaziri, A.; Nazarian, A. Biomechanics and mechanobiology of trabecular bone: A review. J. Biomech. Eng. 2015, 137, 0108021–01080215. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Amling, M.; Ignatius, A. Calcium and vitamin D in bone fracture healing and post-traumatic bone turnover. Eur. Cell Mater. 2018, 35, 365–385. [Google Scholar] [CrossRef]
- Gifre, L.; Vidal, J.; Carrasco, J.; Portell, E.; Puig, J.; Monegal, A.; Guañabens, N.; Peris, P. Incidence of skeletal fractures after traumatic spinal cord injury: A 10-year follow-up study. Clin. Rehabil. 2014, 28, 361–369. [Google Scholar] [CrossRef]
- Egeberg, A.; Schwarz, P.; Harsløf, T.; Andersen, Y.M.F.; Pottegård, A.; Hallas, J.; Thyssen, J.P. Association of Potent and Very Potent Topical Corticosteroids and the Risk of Osteoporosis and Major Osteoporotic Fractures. JAMA Dermatol. 2021, 157, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, N. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Banefelt, J.; Åkesson, K.E.; Spångéus, A.; Ljunggren, O.; Karlsson, L.; Ström, O.; Ortsäter, G.; Libanati, C.; Toth, E. Risk of imminent fracture following a previous fracture in a Swedish database study. Osteoporos. Int. 2019, 30, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Lips, P.; van Schoor, N.M. The effect of vitamin D on bone and osteoporosis. Best. Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 585–591. [Google Scholar] [CrossRef]
- Cândido, F.G.; Bressan, J. Vitamin D: Link between osteoporosis, obesity, and diabetes? Int. J. Mol. Sci. 2014, 15, 6569–6591. [Google Scholar] [CrossRef]
- Fischer, V.; Haffner-Luntzer, M.; Prystaz, K.; Vom Scheidt, A.; Busse, B.; Schinke, T.; Amling, M.; Ignatius, A. Calcium and vitamin-D deficiency marginally impairs fracture healing but aggravates posttraumatic bone loss in osteoporotic mice. Sci. Rep. 2017, 7, 7223. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Liedert, A.; Ignatius, A. Mechanobiology of bone remodeling and fracture healing in the aged organism. Innov. Surg. Sci. 2016, 1, 57–63. [Google Scholar] [CrossRef]
- Holick, M.F.; Siris, E.S.; Binkley, N.; Beard, M.K.; Khan, A.; Katzer, J.T.; Petruschke, R.A.; Chen, E.; de Papp, A.E. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J. Clin. Endocrinol. Metab. 2005, 90, 3215–3224. [Google Scholar] [CrossRef]
- Grant, A.M.; Avenell, A.; Campbell, M.K.; McDonald, A.M.; MacLennan, G.S.; McPherson, G.C.; Anderson, F.H.; Cooper, C.; Francis, R.M.; Donaldson, C.; et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): A randomised placebo-controlled trial. Lancet 2005, 365, 1621–1628. [Google Scholar]
- Kahwati, L.C.; Weber, R.P.; Pan, H.; Gourlay, M.; LeBlanc, E.; Coker-Schwimmer, M.; Viswanathan, M. Vitamin D, Calcium, or Combined Supplementation for the Primary Prevention of Fractures in Community-Dwelling Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 319, 1600–1612. [Google Scholar] [CrossRef]
- Kanis, J.A.; Johnell, O.; De Laet, C.; Johansson, H.; Oden, A.; Delmas, P.; Eisman, J.; Fujiwara, S.; Garnero, P.; Kroger, H.; et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004, 35, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Johnell, O.; Oden, A.; Sembo, I.; Redlund-Johnell, I.; Dawson, A.; De Laet, C.; Jonsson, B. Long-term risk of osteoporotic fracture in Malmö. Osteoporos. Int. 2000, 11, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Prins, J.T.H.; Van Lieshout, E.M.M.; Reijnders, M.R.L.; Verhofstad, M.H.J.; Wijffels, M.M.E. Rib fractures after blunt thoracic trauma in patients with normal versus diminished bone mineral density: A retrospective cohort study. Osteoporos. Int. 2020, 31, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.A.; Elbejjani, M.; Gudnason, V.; Sigurdsson, G.; Lang, T.; Sigurdsson, S.; Aspelund, T.; Meirelles, O.; Siggeirsdottir, K.; Launer, L.; et al. Proximal Femur Volumetric Bone Mineral Density and Mortality. 13 Years of Follow-Up of the AGES-Reykjavik Study. J. Bone Miner. Res. 2017, 32, 1237–1242. [Google Scholar] [CrossRef]
- Lim, W.H.; Ng, C.H.; Ow, Z.G.W.; Ho, O.T.W.; Tay, P.W.L.; Wong, K.L.; Tan, E.X.X.; Tang, S.Y.; Teo, C.M.; Muthiah, M.D. A systematic review and meta-analysis on the incidence of osteoporosis and fractures after liver transplant. Transpl. Int. 2021, 34, 1032–1043. [Google Scholar] [CrossRef]
- Glatt, V.; Canalis, E.; Stadmeyer, L.; Bouxsein, M.L. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007, 22, 1197–1207. [Google Scholar] [PubMed]
- Cooper, M.S. Our approach to osteoporosis screening and treatment needs to change. CMAJ 2008, 178, 1683–1684. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, U.; Piccirilli, E.; Fantini, M.; Baldi, J.; Gasbarra, E.; Bei, R. Sarcopenia and fragility fractures: Molecular and clinical evidence of the bone-muscle interaction. J. Bone Joint Surg. Am. 2015, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Ciuffi, S.; Donati, S.; Marini, F.; Palmini, G.; Luzi, E.; Brandi, M.L. Circulating MicroRNAs as Novel Biomarkers for Osteoporosis and Fragility Fracture Risk: Is There a Use in Assessment Risk? Int. J. Mol. Sci. 2020, 21, 6927. [Google Scholar] [CrossRef]
- Watts, N.B. Postmenopausal Osteoporosis: A Clinical Review. J. Womens Health 2018, 27, 1093–1096. [Google Scholar] [CrossRef]
- Gerasimaviciute, V.; Mathur, R.; Mansfield, K.E.; McDermott, M.P.; Neasham, D.E.; O’Kelly, J.L. Osteoporosis-related characteristics in care home residents in England: A retrospective cohort study. BJGP Open 2023, 7, BJGPO.2022.0142. [Google Scholar] [CrossRef] [PubMed]
- Vondracek, S.F.; Linnebur, S.A. Diagnosis and management of osteoporosis in the older senior. Clin. Interv. Aging 2009, 4, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Faienza, M.F.; Ventura, A.; Marzano, F.; Cavallo, L. Postmenopausal osteoporosis: The role of immune system cells. Clin. Dev. Immunol. 2013, 2013, 575936. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J. Bone Miner. Res. 1996, 11, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Leslie, W.D.; Schousboe, J.T.; Morin, S.N.; Martineau, P.; Lix, L.M.; Johansson, H.; McCloskey, E.V.; Harvey, N.C.; Kanis, J.A. Fracture risk following high-trauma versus low-trauma fracture: A registry-based cohort study. Osteoporos. Int. 2020, 31, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Crandall, C.J.; Larson, J.C.; LaCroix, A.Z.; Robbins, J.A.; Wactawski-Wende, J.; Johnson, K.C.; Sattari, M.; Stone, K.L.; Weitlauf, J.C.; Gure, T.R.; et al. Risk of Subsequent Fractures in Postmenopausal Women After Nontraumatic vs Traumatic Fractures. JAMA Intern. Med. 2021, 181, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Di, Y.P.; Chang, M.; Huang, X.; Chen, Q.; Hong, N.; Kahkonen, B.A.; Di, M.E.; Yu, C.; Keller, E.T.; et al. Cigarette smoke-associated inflammation impairs bone remodeling through NFκB activation. J. Transl. Med. 2021, 19, 163. [Google Scholar] [CrossRef]
- Mendoza, E.S.; Lopez, A.A.; Valdez, V.A.; Mercado-Asis, L.B. Osteoporosis and Prevalent Fractures among Adult Filipino Men Screened for Bone Mineral Density in a Tertiary Hospital. Endocrinol. Metab. 2016, 31, 433–438. [Google Scholar] [CrossRef]
- Ovadja, Z.N.; Snel, C.Y.; Lapid, O.; Van Lieshout, J. Avascular Necrosis with Cystic Changes in the Triquetrum after Trauma in Combination with Heavy Smoking and Local Corticosteroid Injections. J. Hand Microsurg. 2020, 12 (Suppl. S1), S58–S60. [Google Scholar] [CrossRef]
- Brooke-Wavell, K.; Skelton, D.A.; Barker, K.L.; Clark, E.M.; De Biase, S.; Arnold, S.; Paskins, Z.; Robinson, K.R.; Lewis, R.M.; Tobias, J.H.; et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br. J. Sports Med. 2022, 56, 837–846. [Google Scholar] [CrossRef]
- Madhuchani, D.; Seneviratne, S.N.; Ward, L.M. Bone health in childhood and adolescence: An overview on dual-energy X-ray absorptiometry scanning, fracture surveillance and bisphosphonate therapy for low-middle-income countries. Front. Endocrinol. 2023, 14, 1082413. [Google Scholar] [CrossRef] [PubMed]
- Hartley, G.W.; Roach, K.E.; Nithman, R.W.; Betz, S.R.; Lindsey, C.; Fuchs, R.K.; Avin, K.G. Physical Therapist Management of Patients with Suspected or Confirmed Osteoporosis: A Clinical Practice Guideline From the Academy of Geriatric Physical Therapy. J. Geriatr. Phys. Ther. 2022, 44, E106–E119. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.J.; Brophy, R.H.; Demetrakopoulos, D.; Koob, J.; Hong, R.; Rana, A.; Lin, J.T.; Lane, J.M. Interventions to improve osteoporosis treatment following hip fracture: A prospective, randomized trial. J. Bone Joint Surg. Am. 2005, 87, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, A.; Saag, K.G.; Danila, M.I. Improving drug adherence in osteoporosis: An update on more recent studies. Ther. Adv. Musculoskelet. Dis. 2018, 10, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.N.; Burden, A.M.; Cadarette, S.M. The impact of pharmacist interventions on osteoporosis management: A systematic review. Osteoporos. Int. 2011, 22, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Norton, N.; Harvey, N.C.; Jacobson, T.; Johansson, H.; Lorentzon, M.; McCloskey, E.V.; Willers, C.; Borgström, F. SCOPE 2021: A new scorecard for osteoporosis in Europe. Arch. Osteoporos. 2021, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.M.; Selby, P.L. Osteomalacia. Baillieres Clin. Endocrinol. Metab. 1997, 11, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.M.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.; Ladeira, K.; Pinho, F.; Saraiva, N.; Bonito, N.; Pinto, L.; Goncalves, F. Bone Metastases: An Overview. Oncol. Rev. 2017, 11, 321. [Google Scholar]
- Madkhali, T.; Alhefdhi, A.; Chen, H.; Elfenbein, D. Primary hyperparathyroidism. Ulus. Cerrahi Derg. 2016, 32, 58–66. [Google Scholar] [CrossRef]
- Mattia, C.; Coluzzi, F.; Celidonio, L.; Vellucci, R. Bone pain mechanism in osteoporosis: A narrative review. Clin. Cases Miner. Bone Metab. 2016, 13, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.Q.; Huang, J.; Lin, J.L.; Song, C.L. Pathophysiological mechanism of acute bone loss after fracture. J. Adv. Res. 2023, 49, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; Plaisance, E.P.; Fisher, G. Weight loss and bone mineral density. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Powderly, W.G. Osteoporosis and bone health in HIV. Curr. HIV/AIDS Rep. 2012, 9, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis: Definition and clinical presentation. Spine 1997, 22 (Suppl. S24), 12s–16s. [Google Scholar] [CrossRef]
- Aibar-Almazán, A.; Voltes-Martínez, A.; Castellote-Caballero, Y.; Afanador-Restrepo, D.F.; Carcelén-Fraile, M.D.C.; López-Ruiz, E. Current Status of the Diagnosis and Management of Osteoporosis. Int. J. Mol. Sci. 2022, 23, 9465. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, G.R.; Vijayaraghavan, P.V. Urinary N-telopeptide: The New Diagnostic Test for Osteoporosis. Surg. J. 2019, 5, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Hans, D.; Métrailler, A.; Gonzalez Rodriguez, E.; Lamy, O.; Shevroja, E. Quantitative Ultrasound (QUS) in the Management of Osteoporosis and Assessment of Fracture Risk: An Update. Adv. Exp. Med. Biol. 2022, 1364, 7–34. [Google Scholar] [PubMed]
- Neuerburg, C.; Mittlmeier, L.; Schmidmaier, R.; Kammerlander, C.; Böcker, W.; Mutschler, W.; Stumpf, U. Investigation and management of osteoporosis in aged trauma patients: A treatment algorithm adapted to the German guidelines for osteoporosis. J. Orthop. Surg. Res. 2017, 12, 86. [Google Scholar] [CrossRef]
- Gertz, B.J.; Holland, S.D.; Kline, W.F.; Matuszewski, B.K.; Freeman, A.; Quan, H.; Lasseter, K.C.; Mucklow, J.C.; Porras, A.G. Studies of the oral bioavailability of alendronate. Clin. Pharmacol. Ther. 1995, 58, 288–298. [Google Scholar] [CrossRef]
- Eriksen, E.F.; Lyles, K.W.; Colón-Emeric, C.S.; Pieper, C.F.; Magaziner, J.S.; Adachi, J.D.; Hyldstrup, L.; Recknor, C.; Nordsletten, L.; Lavecchia, C.; et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J. Bone Miner. Res. 2009, 24, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Tsourdi, E.; Makras, P.; Rachner, T.D.; Polyzos, S.; Rauner, M.; Mandanas, S.; Hofbauer, L.C.; Anastasilakis, A.D. Denosumab effects on bone density and turnover in postmenopausal women with low bone mass with or without previous treatment. Bone 2019, 120, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Tholey, G.; Ghandour, M.S.; Bloch, S.; Ledig, M.; Mandel, P. Glutamine synthetase and energy metabolism enzymes in cultivated chick neurons and astrocytes: Modulation by serum and hydrocortisone. Brain Res. 1987, 428, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Dijkink, S.; Meier, K.; Krijnen, P.; Yeh, D.D.; Velmahos, G.C.; Schipper, I.B. Malnutrition and its effects in severely injured trauma patients. Eur. J. Trauma. Emerg. Surg. 2020, 46, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Gordon, C.M.; Janz, K.F.; Kalkwarf, H.J.; Lappe, J.M.; Lewis, R.; O’Karma, M.; Wallace, T.C.; Zemel, B.S. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporos. Int. 2016, 27, 1281–1386. [Google Scholar] [CrossRef] [PubMed]
- Lewiecki, E.M.; Binkley, N.; Clark, P.; Kim, S.; Leslie, W.D.; Morin, S.N. Core principles for fracture prevention: North American Consensus from the National Osteoporosis Foundation, Osteoporosis Canada, and Academia Nacional de Medicina de Mexico. Osteoporos. Int. 2020, 31, 2073–2076. [Google Scholar] [CrossRef]
- Milat, F.; Ebeling, P.R. Osteoporosis treatment: A missed opportunity. Med. J. Aust. 2016, 205, 185–190. [Google Scholar] [CrossRef]
- Firoozabadi, R.; Harnden, E.; Krieg, J.C. Immediate weight-bearing after ankle fracture fixation. Adv. Orthop. 2015, 2015, 491976. [Google Scholar] [PubMed]
- Tu, K.N.; Lie, J.D.; Wan, C.K.V.; Cameron, M.; Austel, A.G.; Nguyen, J.K.; Van, K.; Hyun, D. Osteoporosis: A Review of Treatment Options. Pharm. Ther. 2018, 43, 92–104. [Google Scholar]
- Abrahamsen, B.; Osmond, C.; Cooper, C. Life Expectancy in Patients Treated for Osteoporosis: Observational Cohort Study Using National Danish Prescription Data. J. Bone Miner. Res. 2015, 30, 1553–1559. [Google Scholar] [CrossRef]
- Dionyssiotis, Y.; Skarantavos, G.; Papagelopoulos, P. Modern rehabilitation in osteoporosis, falls, and fractures. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2014, 7, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, M.; Sinaki, M.; Geusens, P.; Boonen, S.; Preisinger, E.; Minne, H.W. Musculoskeletal rehabilitation in osteoporosis: A review. J. Bone Miner. Res. 2004, 19, 1208–1214. [Google Scholar] [CrossRef] [PubMed]
- Sweet, M.G.; Sweet, J.M.; Jeremiah, M.P.; Galazka, S.S. Diagnosis and treatment of osteoporosis. Am. Fam. Physician 2009, 79, 193–200. [Google Scholar] [PubMed]
- Paul, K.; Cohen, A.; Nelson, S.; Becker, B.; Cho, M.; Gibert, J.; Janson, E.; Kaur, H.; Kavanagh, M.; Kintner, J.; et al. Osteoporosis Screening, Diagnosis, and Treatment Guideline; Kaiser Foundation Health Plan of Washington: Oakland, CA, USA, 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, M.B.; Syed, S.A.; Whiteson, H.Z.; Hirani, R.; Etienne, M.; Tiwari, R.K. Navigating Post-Traumatic Osteoporosis: A Comprehensive Review of Epidemiology, Pathophysiology, Diagnosis, Treatment, and Future Directions. Life 2024, 14, 561. https://doi.org/10.3390/life14050561
Weiss MB, Syed SA, Whiteson HZ, Hirani R, Etienne M, Tiwari RK. Navigating Post-Traumatic Osteoporosis: A Comprehensive Review of Epidemiology, Pathophysiology, Diagnosis, Treatment, and Future Directions. Life. 2024; 14(5):561. https://doi.org/10.3390/life14050561
Chicago/Turabian StyleWeiss, Matthew B., Shoaib A. Syed, Harris Z. Whiteson, Rahim Hirani, Mill Etienne, and Raj K. Tiwari. 2024. "Navigating Post-Traumatic Osteoporosis: A Comprehensive Review of Epidemiology, Pathophysiology, Diagnosis, Treatment, and Future Directions" Life 14, no. 5: 561. https://doi.org/10.3390/life14050561




