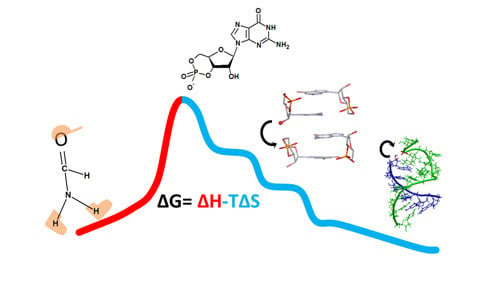
From Formamide to RNA, the Path Is Tenuous but Continuous
Abstract
:1. Introduction
2. The First Part of the Road Is Outlined
3. Nonenzymatic RNA Polymerization from 3',5' Cyclic Nucleotides


4. RNA Shows Properties of Auto-Catalytic Reactivity, Even in Its Simplest Sequences


5. Conclusions
Acknowledgments
Author Contributions
Appendix

Conflicts of Interest
References
- Lohrmann, R. Formation of nucleoside 5'-phosphoramidates under potentially prebiological conditions. J. Mol. Evol. 1977, 10, 137–154. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Ertem, G. Montmorillonite catalysis of RNA oligomer formation in aqueous solution. A model for the prebiotic formation of RNA. J. Am. Chem. Soc. 1993, 115, 12270–12275. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ferris, J.P. Kinetic and Mechanistic Analysis of Dinucleotide and Oligonucleotide Formation from the 5'-Phosphorimidazolide of Adenosine on Na(+)-Montmorillonite. J. Am. Chem. Soc. 1994, 116, 7564–7572. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, R.; Bartel, D.; Szostak, J.W. Non-enzymatic, Template-Directed Ligation of Oligoribonucleotides is Highly Regioselective for the Formation of 3'-5' Phosphodiester Bonds. J. Am. Chem. Soc. 1996, 118, 3340–3334. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.K.; Unrau, P.J.; Lawrence, M.S.; Glasner, M.E.; Bartel, D.P. RNA-Catalyzed RNA polymerization: Accurate and General RNA-Templated Primer Extension. Science 2001, 292, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Kanavarioti, A.; Monnard, P.A.; Deamer, D.W. Eutectic phases in ice facilitate nonenzymatic nucleic acid synthesis. Astrobiology 2001, 1, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Monnard, P.A.; Kanavarioti, A.; Deamer, D.W. Eutectic phase polymerization of activated ribonucleotide mixtures yields quasi-equimolar incorporation of purine and pyrimidine nucleobases. J. Am. Chem. Soc. 2003, 125, 13734–13740. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Ferris, J.P. Synthesis of 35–40 mers of RNA oligomers from unblocked monomers. A simple approach to the RNA world. Chem. Commun. 2003, 12, 1458–1459. [Google Scholar] [CrossRef]
- Kim, D.E.; Joyce, G.F. Cross-catalytic replication of an RNA ligase ribozyme. Chem. Biol. 2004, 11, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Ferris, J.P.; Joshi, P.C.; Wang, K.J.; Miyakawa, S.; Huang, W. Catalysis in prebiotic chemistry: Application to the synthesis of RNA oligomers. Adv. Space Res. 2004, 33, 100–105. [Google Scholar] [CrossRef]
- Mansy, S.S.; Schrum, J.P.; Krishnamurthy, M.; Tobé, S.; Treco, D.A.; Szostak, J.W. Template-directed synthesis of a genetic polymer in a model protocell. Nature 2008, 454, 122–125. [Google Scholar] [PubMed]
- Eckland, E.H.; Szostak, J.W.; Bartel, D.P. Structurally complex and highly active RNA ligases derived from random RNA sequences. Science 1995, 269, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Wochner, A.; Attwater, J.; Coulson, A.; Holliger, P. Ribozyme-catalyzed transcription of an active ribozyme. Science 2011, 332, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Lehman, N. Origin of life: Cold-hearted RNA heats up life. Nat. Chem. 2013, 12, 987–989. [Google Scholar] [CrossRef]
- Powner, M.W.; Gerland, B.; Sutherland, J.D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature 2009, 459, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Hirobe, M.; Higashiyama, K.; Takahashi, H.; Suzuki, K.T. Reaction mechanism for purine ring formation as studied by 13C-15N coupling. Tetrahedron Lett. 1978, 42, 4039–4044. [Google Scholar] [CrossRef]
- Yamada, H.; Hirobe, M.; Higashiyama, K.; Takahashi, H.; Suzuki, K.T. Detection of carbon-13-nitrogen-15 coupled units in adenine derived from doubly labeled hydrogen cyanide or formamide. J. Am. Chem. Soc. 1978, 100, 4617–4618. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Costanzo, G.; Negri, R.; di Mauro, E. A possible prebiotic synthesis of purine, adenine, cytosine, and 4(3H)-pyrimidinone from formamide: Implications for the origin of life. Biorg. Med. Chem. 2001, 9, 1249–1253. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; di Mauro, E. Genetics first or metabolism first? The formamide clue. Chem. Soc. Rev. 2012, 41, 5526–5565. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; di Mauro, E. Meteorites as Catalysts for Prebiotic Chemistry. Chemistry 2013, 19, 16916–16922. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Ciambecchini, U.; Crestini, C.; Costanzo, G.; Negri, R.; di Mauro, E. One-pot TiO2-Catalyzed Synthesis of Nucleic Bases and Acyclonucleosides from Formamide: Implications for the Origin of Life. Chembiochem 2003, 4, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Barks, H.L.; Buckley, R.; Grieves, G.A.; di Mauro, E.; Hud, N.V.; Orlando, T.M. Guanine, Adenine, and Hypoxanthine Production in UV-Irradiated Formamide Solutions: Relaxation of the Requirements for Prebiotic Purine Nucleobase Formation. Chembiochem 2010, 11, 1240–1243. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; di Mauro, E.; Kapralov, M.; Timoshenko, G.; Krasavin, E.; Rozanov, A. Production of Prebiotic Compounds by High-Energy Irradiation of formamide. JINR News 2013, 4, 16–19. [Google Scholar]
- Benner, S.A.; Kim, H.J.; Cardigan, M.A. Asphalt, water, and prebiotic synthesis of ribose, ribonucleosides, and RNA. Acc. Chem. Res. 2012, 45, 2025–2034. [Google Scholar] [CrossRef] [PubMed]
- Barras, C. Primeval soup: Creating life without water. NewScientist Life, 16 April 2014; 36–39. [Google Scholar]
- Schoffstall, A.M. Prebiotic phosphorylation of nucleosides in formamide. Orig. Life 1976, 7, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Schoffstall, A.M.; Mahone, S.M. Formate ester formation in amide solutions. Orig. Life Evol. Biosph. 1988, 18, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; di Mauro, E. Nucleoside phosphorylation by phosphate minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Pino, S.; Costanzo, G.; di Mauro, E. From formamide to RNA: The roles of formamide and water in the evolution of chemical information Special Issue on “The Origin of life and Microbiology”. Res. Microbiol. 2009, 160, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Pino, S.; Ciciriello, F.; di Mauro, E. Generation of long RNA chains in water. J. Biol. Chem. 2009, 284, 33206–33216. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Saladino, R.; Botta, G.; Giorgi, A.; Scipioni, A.; Pino, S.; di Mauro, E. Generation of RNA molecules by base catalyzed click-like reaction. Chembiochem 2012, 13, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Pino, S.; Costanzo, G.; Giorgi, A.; Šponer, J.; Šponer, J.E.; di Mauro, E. Ribozyme activity of RNA nonenzymatically polymerized from 3',5'-cyclicGMP. Entropy 2013, 15, 5362–5383. [Google Scholar] [CrossRef]
- Morasch, M.; Mast, C.B.; Langer, J.K.; Schilcher, P.; Braun, D. Dry polymerization of 3',5'-cyclic GMP to long strands of RNA. Chembiochem 2014, 15, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Van Holde, K. The Origins of Life and Evolution; Halvorson, H.O., van Holde, K.E., Eds.; Alan R. Liss, Inc.: New York, NY, USA, 1980; p. 31. [Google Scholar]
- Alberty, R.A. Thermodynamic properties of enzyme-catalyzed reactions involving guanine, xanthine, and their nucleosides and nucleotides. Biophys. Chem. 2006, 121, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Busiello, V.; Ciciriello, F.; Costamzo, G.; di Mauro, E. Origin of informational polymers: Differential stability of 3'- and 5'-phosphoester bonds in deoxy monomers and oligomers. J. Biol. Chem. 2005, 280, 35658–35669. [Google Scholar] [CrossRef] [PubMed]
- Ciciriello, F.; Costanzo, G.; Pino, S.; Crestini, C.; Saladino, R.; di Mauro, E. Molecular complexity favors the evolution of ribopolymers. Biochemistry 2008, 47, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciciriello, F.; di Mauro, E.; Costanzo, G. Origin of Informational Polymers: Differential Stability of Phosphoester Bonds in Ribo Monomers and Oligomers. J. Biol. Chem. 2006, 281, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F.; Orgel, L.E. The RNA World; Gesteland, R.F., Atkins, J.F., Eds.; Cold Spring Harbor Press: New York, NY, USA, 1999; p. 48. [Google Scholar]
- Gilbert, W. Origin of life: The RNA world. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Spirin, A.S. RNA world and its evolution. Mol. Biol. 2005, 39, 466–472. [Google Scholar] [CrossRef]
- Orgel, L.E. Some consequences of the RNA world hypothesis. Orig. Life Evol. Biosph. 2003, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, J.P.; Lazcano, A.; Miller, S.L. The roads to and from the RNA world. J. Theor. Biol. 2003, 222, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Lehman, N.; Joyce, G.F. Evolution in vitro of an RNA enzyme with altered metal dependence. Nature 1993, 361, 182–185. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.A.; Lehman, N. Generalized RNA-directed recombination of RNA. Chem. Biol. 2003, 10, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Diaz Arenas, C.; Lehman, N. Partitioning the fitness components of RNA populations evolving in vitro. PLoS One 2013, 8, e84454. [Google Scholar] [CrossRef] [PubMed]
- Lutay, A.V.; Grigoriev, I.V.; Zenkova, M.A.; Chernolovskaya, E.L.; Vlassov, V.V. Non-enzymatic recombination of RNA by means of transesterification. Russ. Chem. Bull. 2007, 56, 2499–2505. [Google Scholar] [CrossRef]
- Chetverina, H.V.; Demidenko, A.A.; Ugarov, V.I.; Chetverin, A.B. Spontaneous rearrangements in RNA sequences. FEBS Lett. 1999, 450, 89–94. [Google Scholar] [CrossRef]
- Chetverin, A.B.; Kopein, D.S.; Chetverina, H.V.; Demidenko, A.A.; Ugarov, V.I. Viral RNA-directed RNA polymerases use diverse mechanisms to promote recombination between RNA molecules. J. Biol. Chem. 2005, 280, 8748–8755. [Google Scholar] [CrossRef] [PubMed]
- Lutay, A.V.; Zenkova, M.A.; Vlassov, V.V. Nonenzymatic recombination of RNA: Possible mechanism for the formation of novel sequences. Chem. Biodivers. 2007, 4, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Striggles, J.C.; Martin, M.B.; Schmidt, F.J. Frequency of RNA-RNA interaction in a model of the RNA World. RNA 2006, 12, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Obermayer, B.; Krammer, H.; Braun, D.; Gerland, U. Emergence of information transmission in a prebiotic RNA reactor. Phys. Rev. Lett. 2011, 107, 018101. [Google Scholar] [CrossRef] [PubMed]
- Lehman, N.; Unrau, P.J. Recombination during in vitro evolution. J. Mol. Evol. 2005, 61, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Lehman, N. Enhancing the prebiotic relevance of a set of covalently self-assembling, autorecombining RNAs through in vitro selection. J. Mol. Evol. 2010, 70, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Díaz Arenas, C.; Lehman, N. Quasispecies-like behavior observed in catalytic RNA populations evolving in a test tube. BMC Evol. Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Rusterholtz, K.J.; Krummel, A.T.; Lehman, N. Detection of high levels of recombination generated during PCR amplification of RNA templates. Biotechniques 2006, 40, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.M.; Schneider, T.D. Features of spliceosome evolution and function inferred from an analysis of the information at human splice sites. J. Mol. Biol. 1992, 228, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Yonath, A. Polar bears, antibiotics, and the evolving ribosome (Nobel Lectures). Angew. Chem. Int. Ed. Engl. 2010, 49, 4341–4354. [Google Scholar] [CrossRef] [PubMed]
- Turk, R.M.; Chumachenko, N.V.; Yarus, M. Multiple translational products from a five-nucleotide ribozyme. Proc. Natl. Acad. Sci. USA 2010, 107, 4585–4589. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Costanzo, G.; di Mauro, E. On the Prebiotic Synthesis of Nucleobases, Nucleotides, Oligonucleotides, pre-RNA and pre-DNA Molecules. In Prebiotic Chemistry; Topics in Current Chemistry; Walde, P., Ed.; Springer: Berlin, Germany, 2005; Volume 259, pp. 29–68. [Google Scholar]
- Cleaves, H.J., II; Michalkova Scott, A.; Hill, F.C.; Leszczynski, J.; Sahai, N.; Hazen, R. Mineral-organic interfacial processes: Potential roles in the origins of life. Chem. Soc. Rev. 2012, 41, 5502–5525. [Google Scholar] [CrossRef] [PubMed]
- Adande, G.-R.; Woolf, N.-J.; Ziurys, L.-M. Observations of interstellar formamide: Availability of a prebiotic precursor in the galactic habitable zone. Astrobiology 2013, 13, 439–453. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, S.; Sponer, J.E.; Costanzo, G.; Saladino, R.; Mauro, E.D. From Formamide to RNA, the Path Is Tenuous but Continuous. Life 2015, 5, 372-384. https://doi.org/10.3390/life5010372
Pino S, Sponer JE, Costanzo G, Saladino R, Mauro ED. From Formamide to RNA, the Path Is Tenuous but Continuous. Life. 2015; 5(1):372-384. https://doi.org/10.3390/life5010372
Chicago/Turabian StylePino, Samanta, Judit E. Sponer, Giovanna Costanzo, Raffaele Saladino, and Ernesto Di Mauro. 2015. "From Formamide to RNA, the Path Is Tenuous but Continuous" Life 5, no. 1: 372-384. https://doi.org/10.3390/life5010372
APA StylePino, S., Sponer, J. E., Costanzo, G., Saladino, R., & Mauro, E. D. (2015). From Formamide to RNA, the Path Is Tenuous but Continuous. Life, 5(1), 372-384. https://doi.org/10.3390/life5010372