Probing Saltern Brines with an Oxygen Electrode: What Can We Learn about the Community Metabolism in Hypersaline Systems?
Abstract
:1. Introduction
2. Dissolved Oxygen Concentrations in Crystallizer Brines
3. Methods for Monitoring Oxygen Concentration Changes in Saltern Crystallizer Brines for the Estimation of Microbial Activities
4. Effects of Selected Carbon Compounds on the Community Respiration in Saltern Crystallizer Ponds
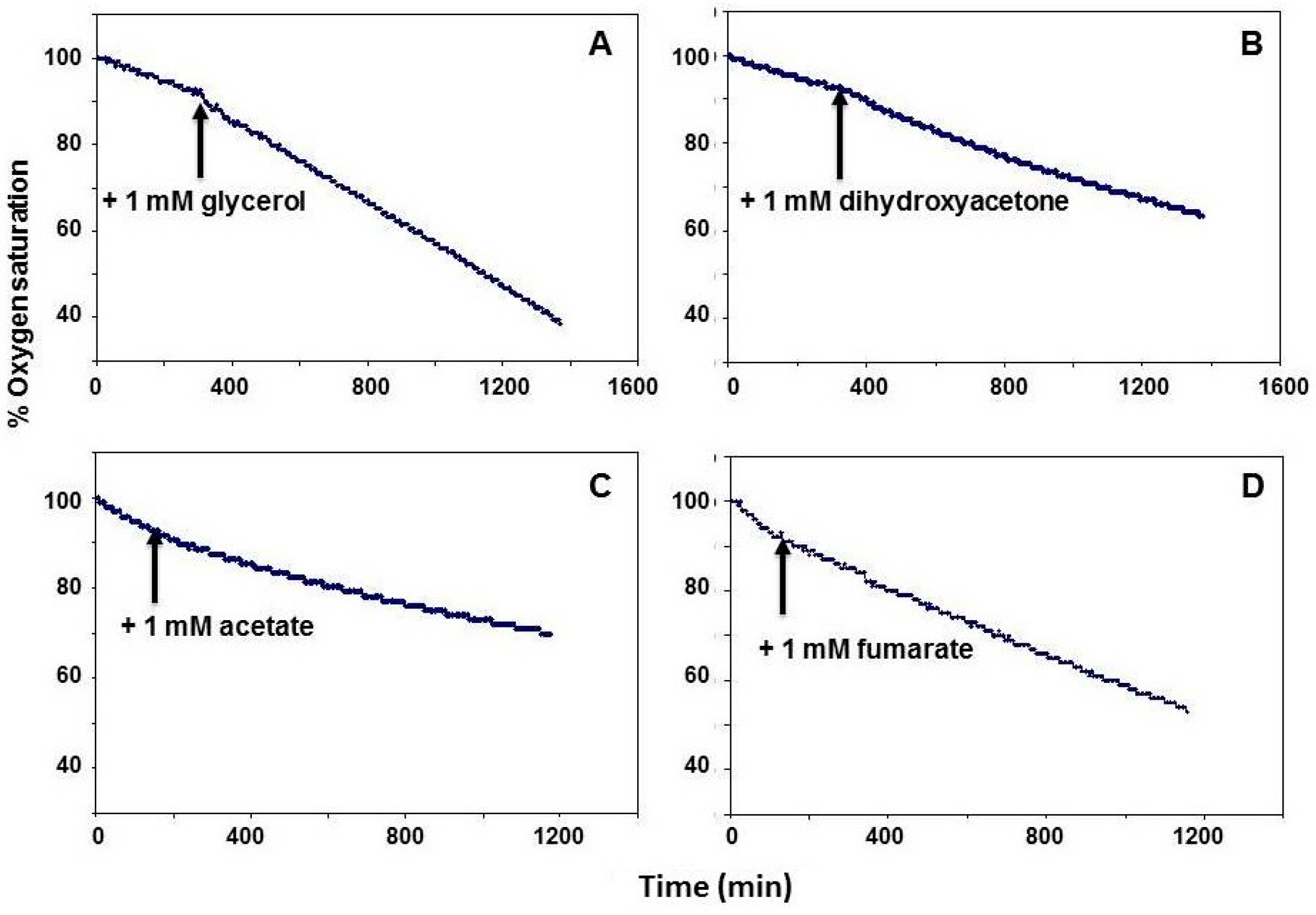
- Significant stimulation of oxygen uptake was observed when brine samples were enriched with 1 mM of glycerol, dihydroxyacetone or pyruvate.
- No or little stimulation was obtained following addition of 10 mg/L yeast extract.
- No stimulation or even a slight inhibition of community respiration was found after addition of 1 mM Na-acetate or 1 mM succinate.
- The oxygen uptake rate was significantly inhibited (up to 50%) following the addition of 1 mM fumarate.
4.1. Glycerol
4.2. Dihydroxyacetone
4.3. Pyruvate
4.4. Acetate
4.5. Fumarate
5. Use of Oxygen Electrodes to Assess Primary Productivity in Saltern Crystallizer Ponds
6. The Possible Effect of Bacteriorhodopsin and Other Light-Driven Proton Pumps on the Community Respiration in Saltern Crystallizer Ponds
7. Final Comments
Acknowledgments
Conflicts of Interest
References
- Oren, A. The ecology of Dunaliella in high-salt environments. J. Biol. Res. Thessalon. 2014, 21, 23. [Google Scholar] [CrossRef] [PubMed]
- Javor, B. Hypersaline Environments. Microbiology and Biogeochemistry; Springer-Verlag: Berlin, Germany, 1989. [Google Scholar]
- Javor, B.J. Planktonic standing crop and nutrients in a saltern ecosystem. Limnol. Oceanogr. 1983, 28, 153–159. [Google Scholar] [CrossRef]
- Oren, A. Halophilic Microorganisms and Their Environments; Kluwer Scientific Publishers: Dordrecht, The Netherlands, 2002. [Google Scholar]
- Antón, J.; Oren, A.; Benlloch, S.; Rodríguez-Valera, F.; Amann, R.; Rosselló-Mora, R. Salinibacter ruber gen. nov., sp. nov., a novel, extreme halophilic member of the Bacteria from saltern crystallizer ponds. Int. J. Syst. Evolut. Microbiol. 2002, 52, 485–491. [Google Scholar] [CrossRef]
- Antón, J.; Rosselló-Mora, R.; Rodríguez-Valera, F.; Amann, R. Extremely halophilic Bacteria in crystallizer ponds from solar salterns. Appl. Environ. Microbiol. 2000, 66, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- Elevi Bardavid, R.; Ionescu, D.; Oren, A.; Rainey, F.A.; Hollen, B.J.; Bagaley, D.R.; Small, A.M.; McKay, C.M. Selective enrichment, isolation and molecular detection of Salinibacter and related extremely halophilic Bacteria from hypersaline environments. Hydrobiologia 2007, 576, 3–13. [Google Scholar] [CrossRef]
- Oren, A.; Rodríguez-Valera, F. The contribution of Salinibacter species to the red coloration of saltern crystallizer ponds. FEMS Microbiol. Ecol. 2001, 36, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.E.; Stagnitti, F.; Kokkinn, M.J.; Williams, W.D. Dissolved oxygen concentrations in hypersaline waters. Limnol. Oceanogr. 1991, 36, 235–250. [Google Scholar] [CrossRef]
- Sherwood, J.E.; Stagnitti, F.; Kokkinn, M.J.; Williams, W.D. A standard table for predicting equilibrium dissolved oxygen concentrations in salt lakes dominated by sodium chloride. Int. J. Salt Lake Res. 1982, 1, 1–6. [Google Scholar] [CrossRef]
- Tindall, B.J.; Trüper, H.G. Ecophysiology of the aerobic halophilic archaebacteria. Syst. Appl. Microbiol. 1986, 7, 202–212. [Google Scholar] [CrossRef]
- Rodriguez-Valera, F.; Ventosa, A.; Juez, G.; Imhoff, J.F. Variation of environmental features and microbial populations with salt concentration in a multi-pond saltern. Microb. Ecol. 1985, 11, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, P.; Markova, K.; Tanev, S.; Davis, J.S. Observations on a solar saltworks near Burgas, Bulgaria. Int. J. Salt Lake Res. 1998, 7, 357–368. [Google Scholar] [CrossRef]
- Sammy, N. Biological systems in north-western Australian solar salt fields. In Sixth International Symposium on Salt; Schreiber, B.C., Harner, H.L., Eds.; The Salt Institute: Toronto, ON, Canada, 1983; Volume 1, pp. 207–215. [Google Scholar]
- Pedrós-Alió, C.; Calderón-Paz, J.I.; MacLean, M.H.; Medina, G.; Marrasé, C.; Gasol, J.M.; Guixa-Boixereu, N. The microbial food web along salinity gradients. FEMS Microbiol. Ecol. 2000, 32, 143–155. [Google Scholar] [CrossRef]
- Warkentin, M.; Schumann, R.; Oren, A. Community respiration studies in saltern crystallizer ponds. Aquat. Microb. Ecol. 2009, 56, 255–261. [Google Scholar] [CrossRef]
- Oren, A. Life at high salt and low oxygen: How do the Halobacteriaceae cope with low oxygen concentrations in their environment? In Polyextremophiles—Organisms Living under Multiple Forms of Stress; Seckbach, J., Oren, A., Stan-Lotter, H., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 533–548. [Google Scholar]
- Oren, A.; Abu-Ghosh, S.; Argov, T.; Kara-Ivanov, E.; Shitrit, D.; Volpert, A.; Horwitz, R. Expression and functioning of retinal-based proton pumps in a saltern crystallizer brine. Extremophiles 2016, 20, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Elevi Bardavid, R.; Khristo, P.; Oren, A. Interrelationships between Dunaliella and halophilic prokaryotes in saltern crystallizer ponds. Extremophiles 2008, 12, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Availability, uptake, and turnover of glycerol in hypersaline environments. FEMS Microbiol. Ecol. 1993, 12, 15–23. [Google Scholar] [CrossRef]
- Oren, A.; Gurevich, P. Production of D-lactate, acetate, and pyruvate from glycerol in communities of halophilic archaea in the Dead Sea and in saltern crystallizer ponds. FEMS Microbiol. Ecol. 1994, 14, 147–156. [Google Scholar] [CrossRef]
- Burns, D.G.; Janssen, P.H.; Itoh, T.; Kamekura, M.; Li, Z.; Jensen, G.; Rodríguez-Valera, F.; Bolhuis, H.; Dyall-Smith, M.L. Haloquadratum walsbyi gen. nov., sp. nov., the square haloarchaeon of Walsby, isolated from saltern crystallizers in Australia and Spain. Int. J. Syst. Evolut. Microbiol. 2007, 57, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Rosselló-Mora, R.; Lee, N.; Antón, J.; Wagner, M. Substrate uptake in extremely halophilic microbial communities revealed by microautoradiography and fluorescence in situ hybridization. Extremophiles 2003, 7, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, H.; Palm, P.; Wende, A.; Falb, M.; Rampp, M.; Rodriguez-Valera, F.; Pfeiffer, F.; Oesterhelt, D. The genome of the square archaeon Haloquadratum walsbyi: Life at the limits of water activity. BMC Genom. 2006, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Legault, B.A.; Lopez-Lopez, A.; Alba-Casado, J.C.; Doolittle, W.F.; Bolhuis, H.; Rodriguez-Valera, F.; Papke, R.T. Environmental genomics of “Haloquadratum walsbyi” in a saltern crystallizer indicates a large pool of accessory genes in an otherwise coherent species. BMC Genom. 2006, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Sher, J.; Elevi, R.; Mana, L.; Oren, A. Glycerol metabolism in the extremely halophilic bacterium Salinibacter ruber. FEMS Microbiol. Lett. 2004, 232, 211–215. [Google Scholar] [CrossRef]
- Oren, A. The role of glycerol in the nutrition of halophilic archaeal communities: A study of respiratory electron transport. FEMS Microbiol. Ecol. 1995, 16, 281–290. [Google Scholar] [CrossRef]
- Elevi Bardavid, R.; Oren, A. Dihydroxyacetone metabolism in Salinibacter ruber and in Haloquadratum walsbyi. Extremophiles 2008, 12, 125–131. [Google Scholar] [CrossRef] [PubMed]
- López-Pérez, M.; Ghai, R.; Leon, M.J.; Rodríguez-Olmos, A.; Copa-Patiño, J.L.; Soliveri, J.; Sanchez-Porro, C.; Ventosa, A.; Rodriguez-Valera, F. Genomes of “Spiribacter“, a streamlined halophilic bacterium. BMC Genom. 2013, 14, 787. [Google Scholar] [CrossRef] [PubMed]
- León, M.J.; Fernández, A.B.; Ghai, R.; Sánchez-Porro, C.; Rodriguez-Valera, F.; Ventosa, A. From metagenomics to pure culture: Isolation and characterization of the moderately halophilic bacterium Spiribacter salinus gen. nov., sp. nov. Appl. Environ. Microbiol. 2014, 80, 3850–3857. [Google Scholar] [CrossRef]
- Vreeland, R.H.; Straight, S.; Krammes, J.; Dougherty, K.; Rosenzweig, W.D.; Kamekura, M. Halosimplex carlsbadense gen. nov., sp. nov. a unique halophilic archaeon, with three 16S rRNA genes, that grows only in defined medium with glycerol and acetate or pyruvate. Extremophiles 2002, 6, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Pyruvate: A key nutrient in hypersaline environments? Microorganisms 2015, 3, 407–416. [Google Scholar] [CrossRef]
- Oren, A. Uptake and turnover of acetate in hypersaline environments. FEMS Microbiol. Ecol. 1995, 18, 75–84. [Google Scholar] [CrossRef]
- Oren, A. Anaerobic growth of halophilic archaeobacteria by reduction of fumarate. J. Gen. Microbiol. 1991, 137, 1387–1390. [Google Scholar] [CrossRef]
- Mongodin, E.F.; Nelson, K.E.; Daugherty, S.; DeBoy, R.T.; Wister, J.; Khouri, H.; Weidman, J.; Walsh, D.A.; Papke, R.T.; Sanchez Perez, G.; et al. The genome of Salinibacter ruber: Convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc. Natl. Acad. Sci. USA 2005, 102, 18147–18152. [Google Scholar] [CrossRef] [PubMed]
- Joint, I.; Henriksen, P.; Garde, K.; Riemann, B. Primary production, nutrient assimilation and microzooplankton grazing along a hypersaline gradient. FEMS Microbiol. Ecol. 2002, 39, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Saltern evaporation ponds as model systems for the study of primary production processes under hypersaline conditions. Aquat. Microb. Ecol. 2009, 56, 193–204. [Google Scholar] [CrossRef]
- Balashov, S.P.; Imasheva, E.S.; Boichenko, V.A.; Antón, J.; Wang, J.M.; Lanyi, J.K. Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science 2005, 309, 2061–2064. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Krippahl, G. Light inhibition of respiration in Halobacterium halobium. FEBS Lett. 1973, 36, 72–76. [Google Scholar] [CrossRef]
- Boichenko, V.A.; Wang, J.M.; Antón, J.; Lanyi, J.K.; Balashov, S.P. Functions of carotenoids in xanthorhodopsin and archaeorhodopsin, from action spectra of photoinhibition of cell respiration. Biochim. Biophys. Acta 2006, 1757, 1649–1656. [Google Scholar] [CrossRef] [PubMed]
- Oren, A.; Shilo, M. Bacteriorhodopsin in a bloom of halobacteria in the Dead Sea. Arch. Microbiol. 1981, 130, 185–187. [Google Scholar] [CrossRef]
- Lobasso, S.; Lopalco, P.; Angelini, R.; Pollice, A.; Laera, G.; Milano, F.; Agostiano, A.; Corcelli, A. Isolation of Squarebop I bacteriorhodopsin from biomass of coastal salterns. Protein Expr. Purif. 2012, 84, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Lobasso, S.; Lopalco, P.; Vitale, R.; Saponetti, M.S.; Capitanio, G.; Mangini, V.; Milano, F.; Trotta, M.; Corcelli, A. The light-activated proton pump Bop I of the archaeon Haloquadratum walsbyi. Photochem. Photobiol. 2012, 88, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Sørensen, K.B.; Oren, A. Biogeochemistry of a gypsum-encrusted microbial ecosystem. Geobiology 2004, 2, 133–150. [Google Scholar] [CrossRef]
- Hammer, U.T. Primary production in saline lakes. A review. Hydrobiologia 1981, 81, 47–57. [Google Scholar] [CrossRef]
- Danon, A.; Caplan, S.R. CO2 fixation by Halobacterium halobium. FEBS Lett. 1977, 74, 255–258. [Google Scholar] [CrossRef]
- Oren, A. Bacteriorhodopsin-mediated CO2 photoassimilation in the Dead Sea. Limnol. Oceanogr. 1983, 28, 33–41. [Google Scholar] [CrossRef]


© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oren, A. Probing Saltern Brines with an Oxygen Electrode: What Can We Learn about the Community Metabolism in Hypersaline Systems? Life 2016, 6, 23. https://doi.org/10.3390/life6020023
Oren A. Probing Saltern Brines with an Oxygen Electrode: What Can We Learn about the Community Metabolism in Hypersaline Systems? Life. 2016; 6(2):23. https://doi.org/10.3390/life6020023
Chicago/Turabian StyleOren, Aharon. 2016. "Probing Saltern Brines with an Oxygen Electrode: What Can We Learn about the Community Metabolism in Hypersaline Systems?" Life 6, no. 2: 23. https://doi.org/10.3390/life6020023





