Evidence Supporting the Uptake and Genomic Incorporation of Environmental DNA in the “Ancient Asexual” Bdelloid Rotifer Philodina roseola
Abstract
:1. Introduction
- Bdelloid rotifers (represented here by P. roseola) should internalize environmental DNA more readily than the monogonont rotifers (represented by Brachionus rubens), which cannot withstand desiccation (except as sexually produced resting eggs).
- Incorporation of environmental DNA into the genome of P. roseola can only occur in conjunction with a desiccation event and should result in heritable changes in the untreated F1 generation (e.g., changes in fitness).
2. Materials and Methods
2.1. Rotifer and Algal Cultures
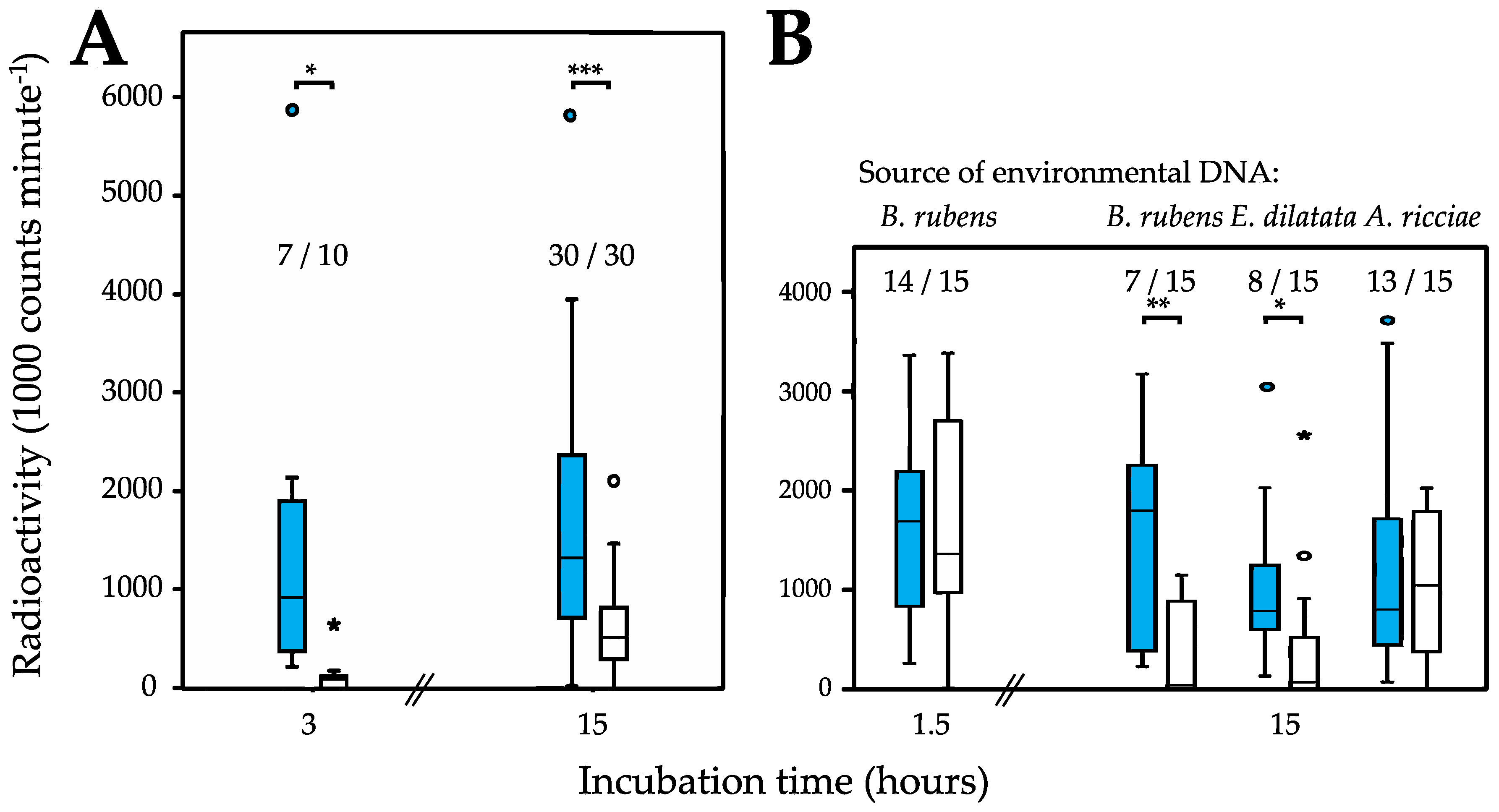
2.2. Quantifying DNA Uptake
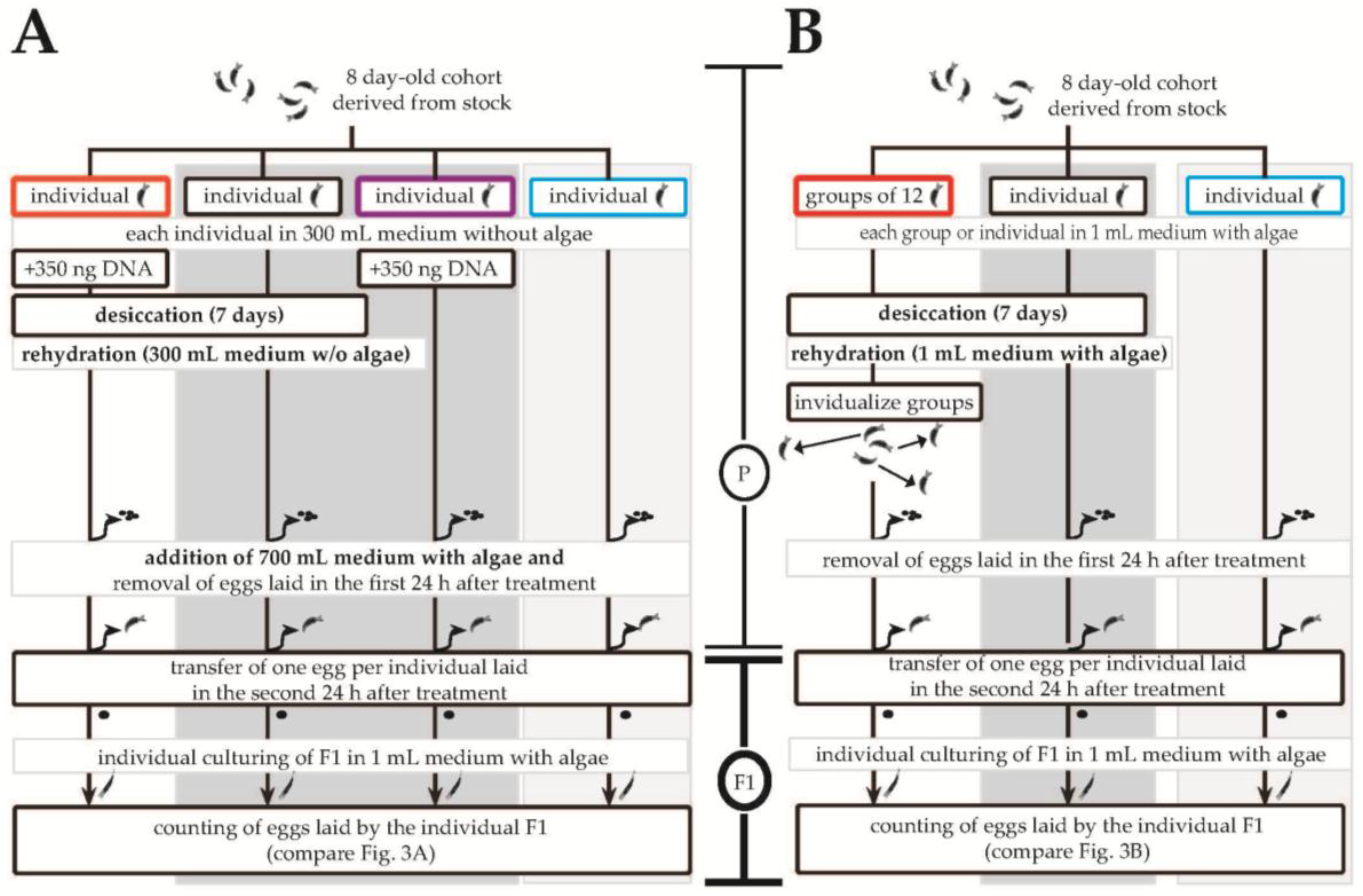
2.3. Quantifying Reproductive Output
3. Results
3.1. Uptake of Environmental DNA
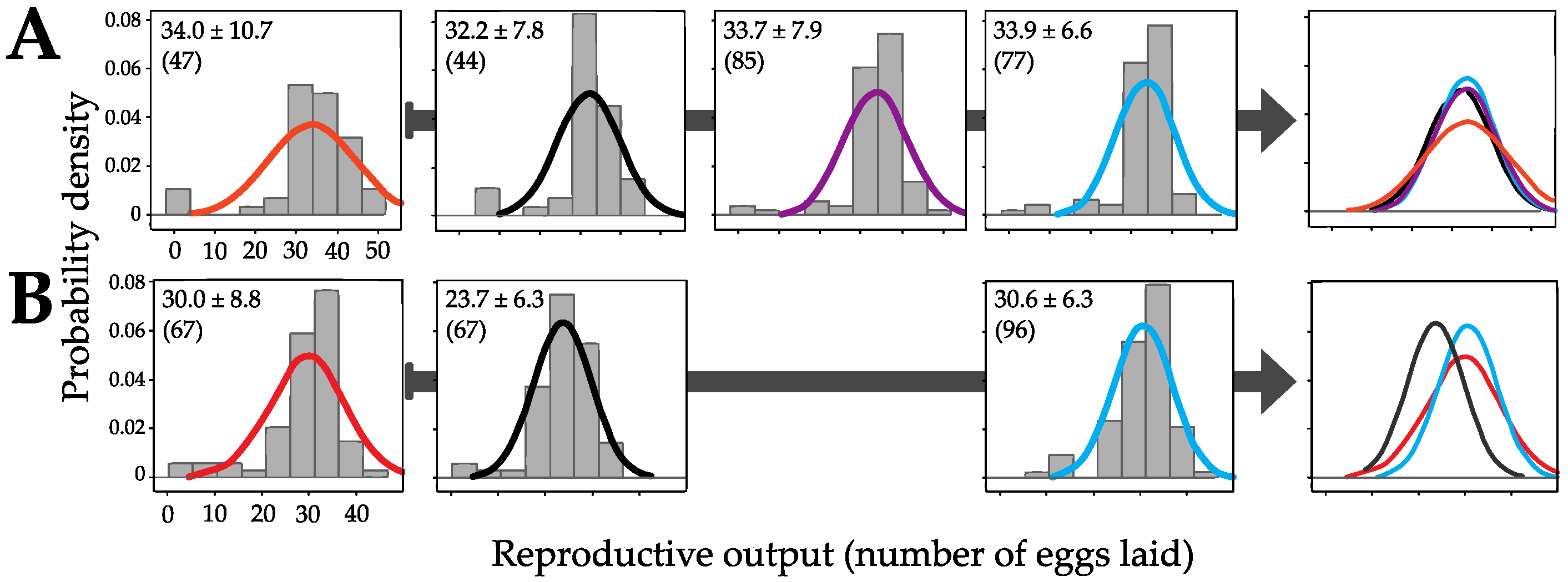
3.2. Genomic Incorporation and Fitness Effects
3.3. Differential Effects between Desiccation in Groups Versus in Isolation
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sielaff, M.; Schmidt, H.; Struck, T.H.; Rosenkranz, D.; Mark Welch, D.B.; Hankeln, T.; Herlyn, H. Phylogeny of Syndermata (syn. Rotifera): Mitochondrial gene oder verifies epizoic Seisonidea as sister to endoparasitic Acanthocephala within monophyletic Hemirotifera. Mol. Phylogenet Evol. 2016, 96, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Segers, H. Annotated checklist of the rotifers (phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 2007, 1564, 1–104. [Google Scholar]
- Maynard Smith, J. Evolution: Contemplating life without sex. Nature 1986, 324, 300–301. [Google Scholar] [CrossRef]
- Schurko, A.M.; Neiman, M.; Logsdon, J.M., Jr. Signs of sex: What we know and how we know it. Trends Ecol. Evol. 2009, 24, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. The evolutionary advantage of recombination. Genetics 1974, 78, 737–756. [Google Scholar] [PubMed]
- Gabriel, W.; Lynch, M.; Bürger, R. Muller’s ratchet and mutational meltdowns. Evolution 1993, 47, 1744–1757. [Google Scholar] [CrossRef] [Green Version]
- Ricci, C.; Fontaneto, D. The importance of being a bdelloid: Ecological and evolutionary consequences of dormancy. Ital. J. Zool. 2009, 76, 240–249. [Google Scholar] [CrossRef]
- Welch, D.B.M.; Ricci, C.; Meselson, M. Bdelloid rotifers: Progress in understanding the success of an evolutionary scandal. In Lost Sex; Schön, I., Martens, K., Dijk, P., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 259–279. [Google Scholar]
- Fontaneto, D.; Herniou, E.A.; Boschetti, C.; Caprioli, M.; Melone, G.; Ricci, C.; Barraclough, T.G. Independently evolving species in asexual bdelloid rotifers. PLoS Biol. 2007, 5, 914–921. [Google Scholar] [CrossRef] [PubMed]
- Poinar, G.O.; Ricci, C. Bdelloid rotifers in dominican amber: Evidence for parthenogenetic continuity. Cell. Mol. Life Sci. 1992, 48, 408–410. [Google Scholar] [CrossRef]
- Eyres, I.; Boschetti, C.; Crisp, A.; Smith, T.P.; Fontaneto, D.; Tunnacliffe, A.; Barraclough, T.G. Horizontal gene transfer in bdelloid rotifers is ancient, ongoing and more frequent in species from desiccating habitats. BMC Biol. 2015, 13, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, C. Anhydrobiotic capabilities of bdelloid rotifers. Hydrobiologia 1998, 387, 321–326. [Google Scholar] [CrossRef]
- Rice, W.R.; Friberg, U. Genomic clues to an ancient asexual scandal. Genome Biol. 2007, 8, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Caprioli, M.; Ricci, C. Recipes for successful anhydrobiosis in bdelloid rotifers. Hydrobiologia 2001, 446, 13–17. [Google Scholar] [CrossRef]
- Bohonak, A.J.; Jenkins, D.G. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecol. Lett. 2003, 6, 783–796. [Google Scholar] [CrossRef]
- Wilson, C.G.; Sherman, P.W. Anciently asexual bdelloid rotifers escape lethal fungal parasites by drying up and blowing away. Science 2010, 327, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Boothby, T.C.; Tenlen, J.R.; Smith, F.W.; Wang, J.R.; Patanella, K.A.; Osborne Nishimura, E.; Tintori, S.C.; Li, Q.; Jones, C.D.; Yandell, M.; et al. Evidence for extensive horizontal gene transfer from the draft genome of a tardigrade. Proc. Natl. Acad. Sci. USA 2015, 112, 15976–15981. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Golovina, E.A.; Buitink, J. Mechanisms of plant desiccation tolerance. Trends Plant Sci. 2001, 6, 431–438. [Google Scholar] [CrossRef]
- Leopold, A.C.; Musgrave, M.E.; Williams, K.M. Solute leakage resulting from leaf desiccation. Plant Physiol. 1981, 68, 1222–1225. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, F.A.; Wolkers, W.F.; Buitink, J.; Golovina, E.A.; Crowe, J.H.; Crowe, L.M. Membrane stabilization in the dry state. Comp. Biochem. Physiol. A Physiol. 1997, 117, 335–341. [Google Scholar] [CrossRef]
- Gladyshev, E.; Meselson, M. Extreme resistance of bdelloid rotifers to ionizing radiation. Proc. Natl. Acad. Sci. USA 2008, 105, 5139–5144. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, E.A.; Arkhipova, I.R. Genome structure of bdelloid rotifers: Shaped by asexuality or desiccation? J. Hered. 2010, 101, S85–S93. [Google Scholar] [CrossRef] [PubMed]
- Hespeels, B.; Knapen, M.; Hanot-Mambres, D.; Heuskin, A.C.; Pineux, F.; Lucas, S.; Koszul, R.; van Doninck, K. Gateway to genetic exchange? DNA double-strand breaks in the bdelloid rotifer Adineta vaga submitted to desiccation. J. Evol. Biol. 2014, 27, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Debortoli, N.; Li, X.; Eyres, I.; Fontaneto, D.; Hespeels, B.; Tang, C.Q.; Flot, J.-F.; Van Doninck, K. Genetic exchange among bdelloid rotifers is more likely due to horizontal gene transfer than to meiotic sex. Curr. Biol. 2016, 26, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Boschetti, C.; Pouchkina-Stantcheva, N.; Hoffmann, P.; Tunnacliffe, A. Foreign genes and novel hydrophilic protein genes participate in the desiccation response of the bdelloid rotifer Adineta ricciae. J Exp. Biol. 2012, 214, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Flot, J.-F.; Hespeels, B.; Li, X.; Noel, B.; Arkhipova, I.; Danchin, E.G.J.; Hejnol, A.; Henrissat, B.; Koszul, R.; Aury, J.-M.; et al. Genomic evidence for ameiotic evolution in the bdelloid rotifer Adineta vaga. Nature 2013, 500, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Wiedenbeck, J.; Cohan, F.M. Origins of bacterial diversity through horizontal genetic transfer and adaptation to new ecological niches. FEMS Microbiol. Rev. 2011, 35, 957–976. [Google Scholar] [CrossRef] [PubMed]
- Signorovitch, A.; Hur, J.; Gladyshev, E.; Meselson, M. Allele sharing and evidence for sexuality in a mitochondrial clade of bdelloid rotifers. Genetics 2015, 200, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Koutsovoulos, G.; Kumar, S.; Laetsch, D.R.; Stevens, L.; Daub, J.; Conlon, C.; Maroon, H.; Thomas, F.; Aboobaker, A.; Blaxter, M. No evidence for extensive horizontal gene transfer in the genome of the tardigrade Hypsibius dujardini. Proc. Natl. Acad. Sci. USA 2016, 113, 5053–5058. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M. Asexual evolution: Can species exist without sex? Curr. Biol. 2007, 17, R543–R544. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.B.M.; Cummings, M.P.; Hillis, D.M.; Meselson, M. Divergent gene copies in the asexual class Bdelloidea (Rotifera) separated before the bdelloid radiation or within bdelloid families. Proc. Natl. Acad. Sci. USA 2004, 101, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, E.A.; Meselson, M.; Arkhipova, I.R. Massive horizontal gene transfer in bdelloid rotifers. Science 2008, 320, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Kilham, S.; Kreeger, D.; Lynn, S.; Goulden, C.; Herrera, L. COMBO: A defined freshwater culture medium for algae and zooplankton. Hydrobiologia 1998, 377, 147–159. [Google Scholar] [CrossRef]
- Ricci, C.; Melone, G.; Santo, N.; Caprioli, M. Morphological response of a bdelloid rotifer to desiccation. J. Morphol. 2003, 257, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Becks, L.; Agrawal, A.F. The effect of sex on the mean and variance of fitness in facultatively sexual rotifers. J. Evol. Biol. 2011, 24, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Becks, L.; Agrawal, A.F. The evolution of sex is favoured during adaptation to new environments. PLoS Biol. 2012, 10, e1001317. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, R.D.; Murphy, K.E.; Morrow, J.B.; Cole, K.D. Trophic transfer of nanoparticles in a simplified invertebrate food web. Nat. Nanotechnol. 2008, 3, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Caprioli, M. Anhydrobiosis in bdelloid species, populations and individuals. Integr. Comp. Biol. 2005, 45, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Caprioli, M.; Fontaneto, D. Stress and fitness in parthenogens: Is dormancy a key feature for bdelloid rotifers? BMC Evol. Biol. 2007, 7, S9. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Covino, C. Anhydrobiosis of Adineta ricciae: Costs and benefits. Hydrobiologia 2005, 546, 307–314. [Google Scholar] [CrossRef]
- Ricci, C.; Santo, N.; Elena Radaelli, E.; Bolzern, A.M. Epigenetic inheritance systems in bdelloid rotifers. I. Maternal-age-related biochemical effects. Ital. J. Zool. 1999, 66, 333–339. [Google Scholar] [CrossRef]
- Hur, J.H.; Van Doninck, K.; Mandigo, M.L.; Meselson, M. Degenerate tetraploidy was established before bdelloid rotifer families diverged. Mol. Biol. Evol. 2009, 26, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.B.M.; Welch, J.L.M.; Meselson, M. Evidence for degenerate tetraploidy in bdelloid rotifers. Proc. Natl. Acad. Sci. USA 2008, 105, 5145–5149. [Google Scholar] [CrossRef] [PubMed]
- Marotta, R.; Leasi, F.; Uggetti, A.; Ricci, C.; Melone, G. Dry and survive: Morphological changes during anhydrobiosis in a bdelloid rotifer. J. Struct. Biol. 2010, 171, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Snell, T.W.; Hicks, D.G. Assessing toxicity of nanoparticles using Brachionus manjavacas (Rotifera). Environ. Toxicol. 2011, 26, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Bock, R. The give-and-take of DNA: Horizontal gene transfer in plants. Trends Plant Sci. 2010, 15, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Boto, L. Horizontal gene transfer in evolution: Facts and challenges. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunning Hotopp, J.C. Horizontal gene transfer between bacteria and animals. Trends Genet. 2011, 27, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Belshaw, R.; Quicke, D.L.J.; Volkl, W.; Godfray, H.C.J. Molecular markers indicate rare sex in a predominantly asexual parasitoid wasp. Evolution 1999, 53, 1189–1199. [Google Scholar] [CrossRef]
- Normark, B.B. Evolution in a putatively ancient asexual aphid lineage: Recombination and rapid karyotype change. Evolution 1999, 53, 1458–1469. [Google Scholar] [CrossRef]
- Lee, S.C.; Ni, M.; Li, W.; Shertz, C.; Heitman, J. The evolution of sex: A perspective from the fungal kingdom. Microbiol. Mol. Biol. Rev. 2010, 74, 298–340. [Google Scholar] [CrossRef] [PubMed]
- Croll, D.; Sanders, I.R. Recombination in Glomus intraradices, a supposed ancient asexual arbuscular mycorrhizal fungus. BMC Evol. Biol. 2009, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Lahr, D.J.G.; Parfrey, L.W.; Mitchell, E.A.D.; Katz, L.A.; Lara, E. The chastity of amoebae: Re-evaluating evidence for sex in amoeboid organisms. Proc. R. Soc. Lond. B Biol. Sci. 2011, 278, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Dunthorn, M.; Katz, L.A. Secretive ciliates and putative asexuality in microbial eukaryotes. Trends Microbiol. 2010, 18, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Boto, L. Horizontal gene transfer in the acquisition of novel traits by metazoans. Proc. R. Soc. Lond. B Biol. Sci. 2014, 281, 20132450. [Google Scholar] [CrossRef] [PubMed]
| Philodina roseola Treatment | Environmental DNA Source Species | Incubation Time [Hours] | Paired t-Test (Two-Sided) of the Amount of Radioactive DNA Taken up | Contingency Table for Proportion of Trials in Which DNA Was Taken up (df = 1 in All Cases) |
|---|---|---|---|---|
| Desiccated | P. roseola | 3 | t = 2.416, df = 9, P = 0.039 | 7/10, χ2 = 3.529, P = 0.060 |
| 15 | t = 4.352, df = 29, P < 0.001 | 30/30, χ2 = 0, P = 1.000 | ||
| Hydrated | B. rubens | 1.5 | t = −0.725, df = 14, P = 0.480 | 14/15, χ2 = 1.035, P = 0.309 |
| 15 | t = 3.855, df = 14, P = 0.002 | 7/15, χ2 = 10.909, P < 0.001 | ||
| E. dilatata | 15 | t = 2.333, df = 14, P = 0.035 | 8/15, χ2 = 9.130, P = 0.003 | |
| A. ricciae | 15 | t = 0.589, df = 14, P = 0.565 | 13/15, χ2 = 2.143, P = 0.143 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bininda-Emonds, O.R.P.; Hinz, C.; Ahlrichs, W.H. Evidence Supporting the Uptake and Genomic Incorporation of Environmental DNA in the “Ancient Asexual” Bdelloid Rotifer Philodina roseola. Life 2016, 6, 38. https://doi.org/10.3390/life6030038
Bininda-Emonds ORP, Hinz C, Ahlrichs WH. Evidence Supporting the Uptake and Genomic Incorporation of Environmental DNA in the “Ancient Asexual” Bdelloid Rotifer Philodina roseola. Life. 2016; 6(3):38. https://doi.org/10.3390/life6030038
Chicago/Turabian StyleBininda-Emonds, Olaf R. P., Claus Hinz, and Wilko H. Ahlrichs. 2016. "Evidence Supporting the Uptake and Genomic Incorporation of Environmental DNA in the “Ancient Asexual” Bdelloid Rotifer Philodina roseola" Life 6, no. 3: 38. https://doi.org/10.3390/life6030038






