Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms
Abstract
:1. Introduction
2. Material and Methods
2.1. Genomes and Proteomes Analyzed
2.2. Identification of Enzymes
Accuracy of the Promiscuous Enzymes
2.3. Statistical Analysis
3. Results and Discussion
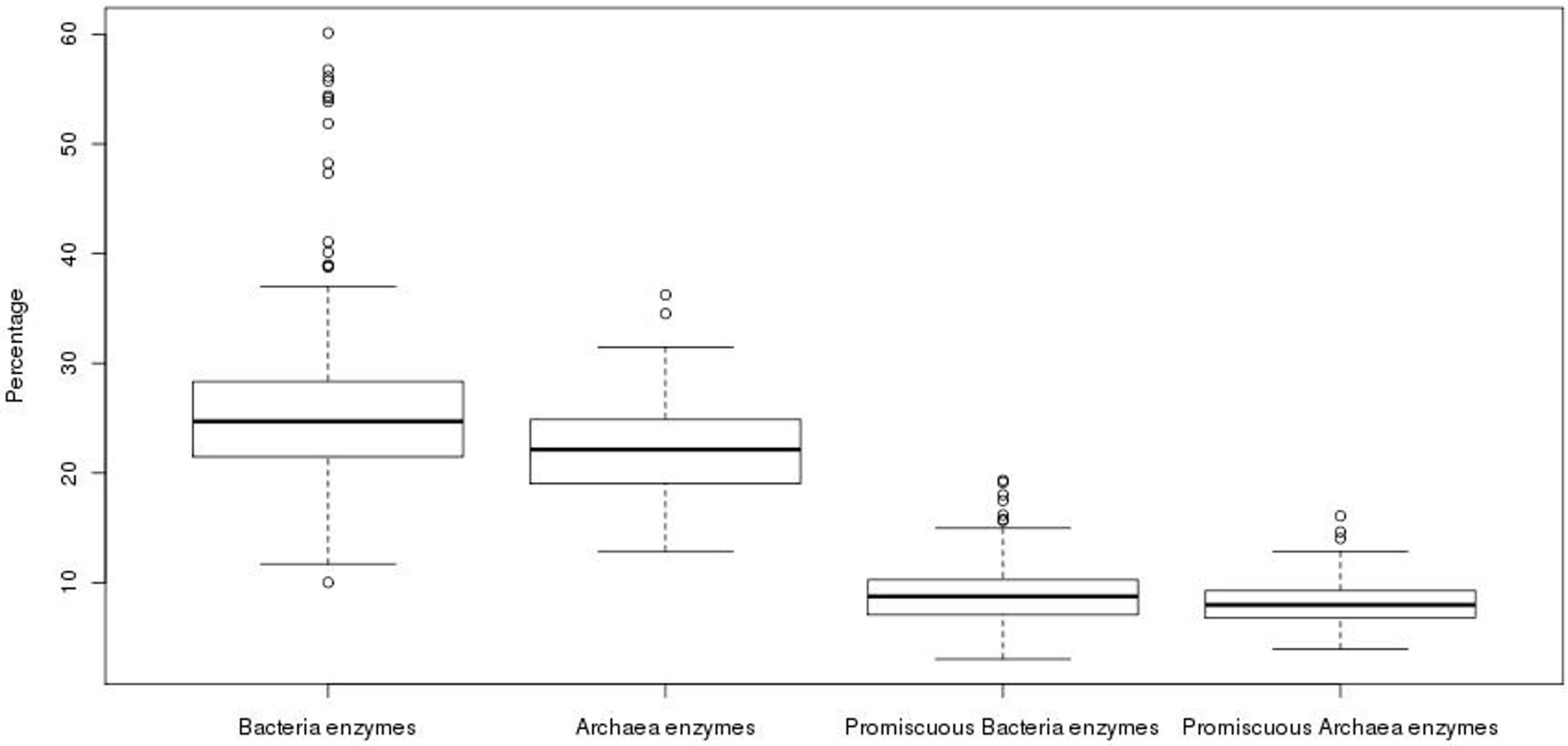
3.1. Archaea Exhibit a Lower Promiscuous Enzyme Fraction than Bacterial Genomes
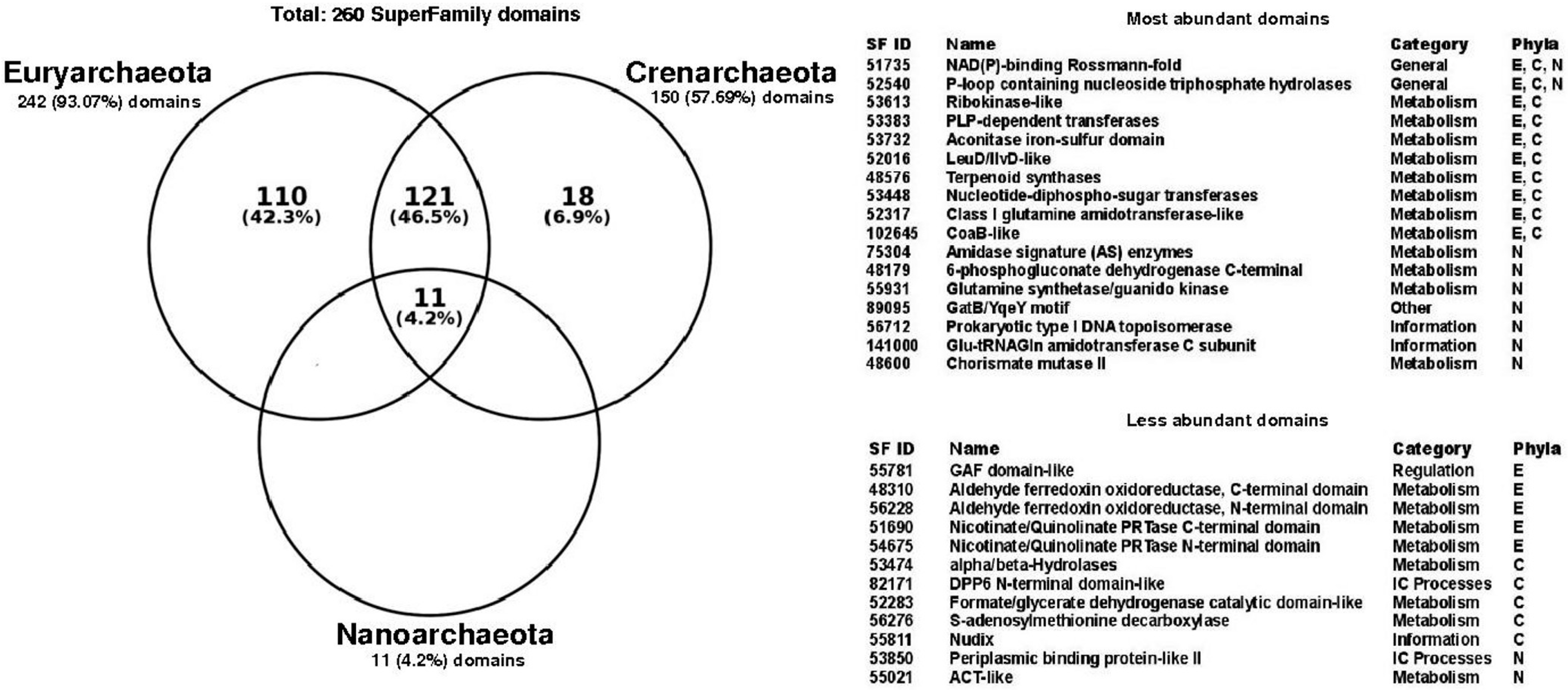
3.2. Structural Diversity in Promiscuous Enzymes is Higher than Specialist Enzymes
3.3. Functional Structural Diversity in Promiscuous Enzymes Exhibits a Non-Homogeneous Distribution
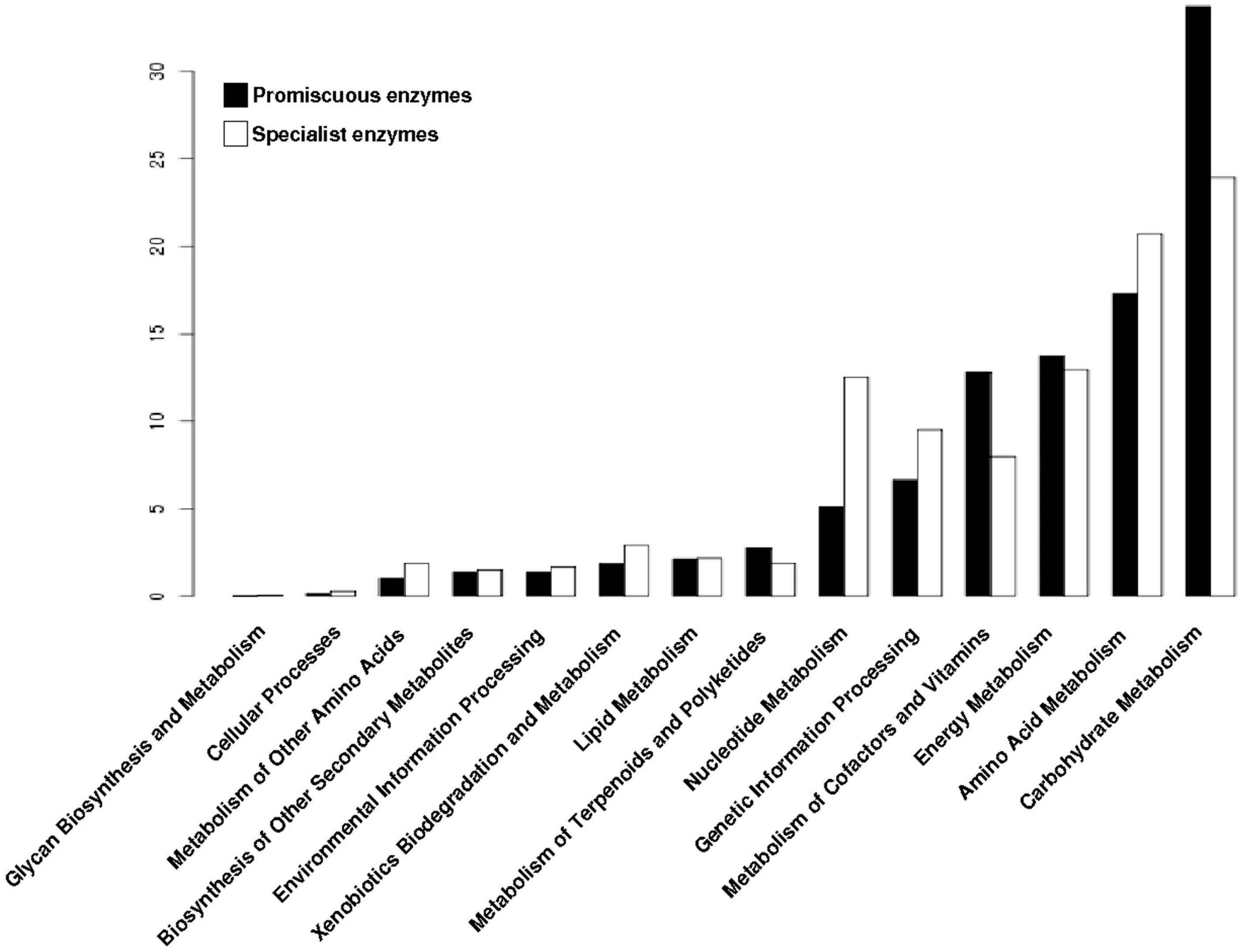
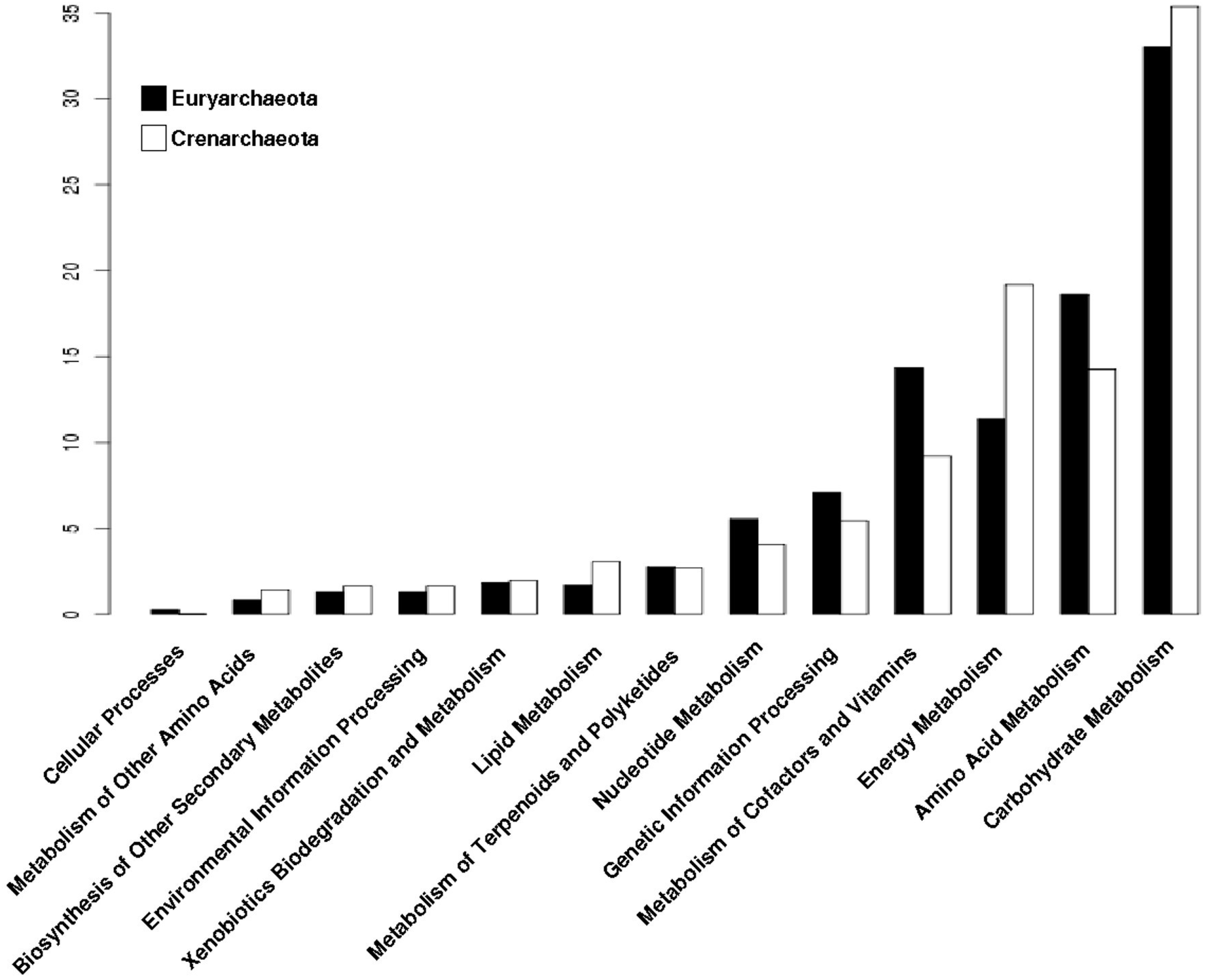
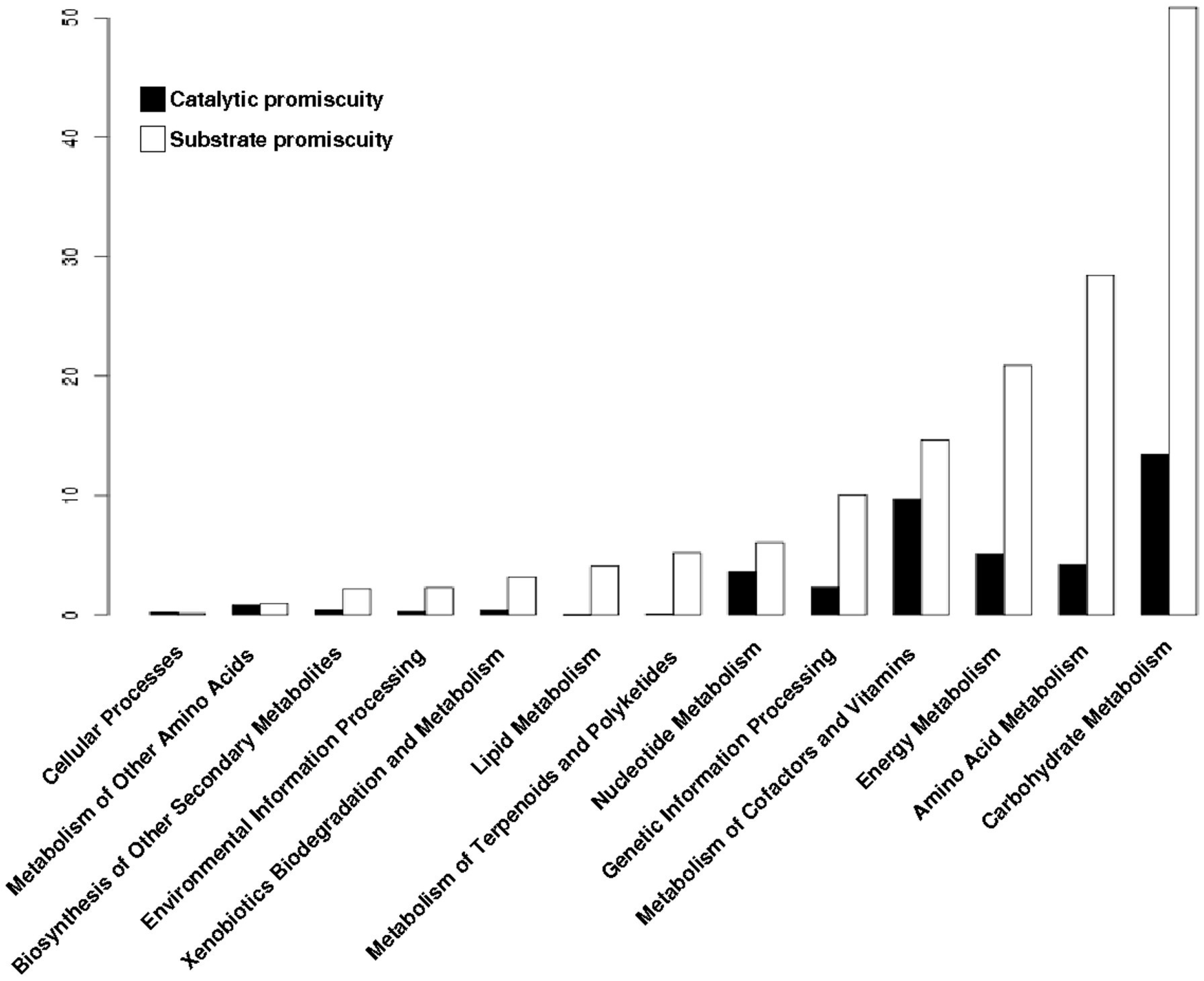
3.4. The Distribution of Promiscuous Enzymes between Functional Metabolic Categories is Variable
3.5. Promiscuous Enzymes Exhibit a High Enrichment of Substrate Promiscuity rather than Catalytic Promiscuity
3.6. Substrate Promiscuity is Represented to a Greater Extent in the Functional Metabolic Categories
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Nam, H.; Lewis, N.E.; Lerman, J.A.; Lee, D.H.; Chang, R.L.; Kim, D.; Palsson, B.O. Network context and selection in the evolution to enzyme specificity. Science 2012, 337, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Jia, B.; Cheong, G.W.; Zhang, S. Multifunctional enzymes in archaea: Promiscuity and moonlight. Extremophiles 2013, 17, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Ghosh, S.; Nagaraja, V. Moonlighting function of glutamate racemase from mycobacterium tuberculosis: Racemization and DNA gyrase inhibition are two independent activities of the enzyme. Microbiology 2008, 154, 2796–2803. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Huang, W.J.; Hu, S.C.; Zhang, H.L.; Wang, H.; Zhang, J.X.; Lin, H.H.; Chen, Y.Z.; Zou, Q.; Ji, Z.L. A global characterization and identification of multifunctional enzymes. PLoS ONE 2012, 7, e38979. [Google Scholar] [CrossRef] [PubMed]
- Huberts, D.H.; van der Klei, I.J. Moonlighting proteins: An intriguing mode of multitasking. Biochim. Biophys. Acta 2010, 1803, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Hult, K.; Berglund, P. Enzyme promiscuity: Mechanism and applications. Trends Biotechnol. 2007, 25, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R. Recent advances in enzyme promiscuity. Sustain. Chem. Process. 2016, 4, 2. [Google Scholar] [CrossRef]
- Khersonsky, O.; Roodveldt, C.; Tawfik, D.S. Enzyme promiscuity: Evolutionary and mechanistic aspects. Curr. Opin. Chem. Biol. 2006, 10, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Khersonsky, O.; Tawfik, D.S. The histidine 115-histidine 134 dyad mediates the lactonase activity of mammalian serum paraoxonases. J. Biol. Chem. 2006, 281, 7649–7656. [Google Scholar] [CrossRef] [PubMed]
- Suen, S.; Lu, H.H.; Yeang, C.H. Evolution of domain architectures and catalytic functions of enzymes in metabolic systems. Genome Biol. Evol. 2012, 4, 976–993. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A. Metabolic networks and their evolution. Adv. Exp. Med. Biol. 2012, 751, 29–52. [Google Scholar] [PubMed]
- Martinez-Nunez, M.A.; Poot-Hernandez, A.C.; Rodriguez-Vazquez, K.; Perez-Rueda, E. Increments and duplication events of enzymes and transcription factors influence metabolic and regulatory diversity in prokaryotes. PLoS ONE 2013, 8, e69707. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.; Hohn, M.J.; Ahel, I.; Graham, D.E.; Adams, M.D.; Barnstead, M.; Beeson, K.Y.; Bibbs, L.; Bolanos, R.; Keller, M.; et al. The genome of nanoarchaeum equitans: Insights into early archaeal evolution and derived parasitism. Proc. Natl. Acad. Sci. USA 2003, 100, 12984–12988. [Google Scholar] [CrossRef] [PubMed]
- Maeder, D.L.; Anderson, I.; Brettin, T.S.; Bruce, D.C.; Gilna, P.; Han, C.S.; Lapidus, A.; Metcalf, W.W.; Saunders, E.; Tapia, R.; et al. The methanosarcina barkeri genome: Comparative analysis with methanosarcina acetivorans and methanosarcina mazei reveals extensive rearrangement within methanosarcinal genomes. J. Bacteriol. 2006, 188, 7922–7931. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Douady, C.J.; Italia, M.J.; Marshall, W.E.; Stanhope, M.J. Universal trees based on large combined protein sequence data sets. Nat. Genet. 2001, 28, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Okuda, S.; Yamada, T.; Hamajima, M.; Itoh, M.; Katayama, T.; Bork, P.; Goto, S.; Kanehisa, M. KEGG atlas mapping for global analysis of metabolic pathways. Nucleic Acids Res. 2008, 36, W423–W426. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Pethica, R.; Zhou, Y.; Talbot, C.; Vogel, C.; Madera, M.; Chothia, C.; Gough, J. Superfamily--sophisticated comparative genomics, data mining, visualization and phylogeny. Nucleic Acids Res. 2009, 37, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, P.; Faulon, J.L. Molecular signatures-based prediction of enzyme promiscuity. Bioinformatics 2010, 26, 2012–2019. [Google Scholar] [CrossRef] [PubMed]
- R-programming. Development core team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Freilich, S.; Spriggs, R.V.; George, R.A.; Al-Lazikani, B.; Swindells, M.; Thornton, J.M. The complement of enzymatic sets in different species. J. Mol. Biol. 2005, 349, 745–763. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Anolles, G.; Yafremava, L.S.; Gee, H.; Caetano-Anolles, D.; Kim, H.S.; Mittenthal, J.E. The origin and evolution of modern metabolism. Int. J. Biochem. Cell Biol. 2009, 41, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Teichmann, S.A.; Rison, S.C.; Thornton, J.M.; Riley, M.; Gough, J.; Chothia, C. The evolution and structural anatomy of the small molecule metabolic pathways in Escherichia coli. J. Mol. Biol. 2001, 311, 693–708. [Google Scholar] [PubMed]
- Galperin, M.Y. Structural classification of bacterial response regulators: Diversity of output domains and domain combinations. J. Bacteriol. 2006, 188, 4169–4182. [Google Scholar] [CrossRef] [PubMed]
- Berquist, B.R.; Soneja, J.; DasSarma, S. Comparative genomic survey of information transfer systems in two diverse extremely halophilic archaea, Halobacterium sp. Strain NRC-1 and Haloarcula marismortui. In Adaptation to Life at High Salt Concentrations in Archaea, Bacteria, and Eukarya; Gunde-Cimerman, N., Oren, A., Plemenitas, A., Eds.; Springer: Dordrecht, The Netherlands, 2005; pp. 148–182. [Google Scholar]
- Peregrin-Alvarez, J.M.; Tsoka, S.; Ouzounis, C.A. The phylogenetic extent of metabolic enzymes and pathways. Genome Res. 2003, 13, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, C.; Kawai, S.; Murata, K. Nadp (h) phosphatase activities of archaeal inositol monophosphatase and eubacterial 3′-phosphoadenosine 5′-phosphate phosphatase. Appl. Environ. Microbiol. 2007, 73, 5447–5452. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, K.A.; Johnson, K.A.; Yang, H.; Roberts, M.F.; Seaton, B.A.; Head, J.F.; Stec, B. Crystal structure of a dual activity impase/fbpase (af2372) from Archaeoglobus fulgidus. The story of a mobile loop. J. Biol. Chem. 2002, 277, 22863–22874. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.; Urbanke, C.; Schonheit, P. Bifunctional phosphoglucose/phosphomannose isomerase from the hyperthermophilic archaeon Pyrobaculum aerophilum. Extremophiles 2004, 8, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Angelov, A.; Futterer, O.; Valerius, O.; Braus, G.H.; Liebl, W. Properties of the recombinant glucose/galactose dehydrogenase from the extreme thermoacidophile, Picrophilus torridus. FEBS J. 2005, 272, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Lamble, H.J.; Theodossis, A.; Milburn, C.C.; Taylor, G.L.; Bull, S.D.; Hough, D.W.; Danson, M.J. Promiscuity in the part-phosphorylative entner-doudoroff pathway of the archaeon Sulfolobus solfataricus. FEBS Lett. 2005, 579, 6865–6869. [Google Scholar] [CrossRef] [PubMed]
- Schonheit, P.; Schafer, T. Metabolism of hyperthermophiles. World J. Microbiol. Biotechnol. 1995, 11, 26–57. [Google Scholar] [CrossRef] [PubMed]
- Nunn, C.E.; Johnsen, U.; Schonheit, P.; Fuhrer, T.; Sauer, U.; Hough, D.W.; Danson, M.J. Metabolism of pentose sugars in the hyperthermophilic archaea Sulfolobus solfataricus and Sulfolobus acidocaldarius. J. Biol. Chem. 2010, 285, 33701–33709. [Google Scholar] [CrossRef] [PubMed]
- Blaut, M. Metabolism of methanogens. Antonie Van Leeuwenhoek 1994, 66, 187–208. [Google Scholar] [CrossRef] [PubMed]
- Deppenmeier, U. The unique biochemistry of methanogenesis. Prog. Nucleic Acid Res. Mol. Biol. 2002, 71, 223–283. [Google Scholar] [PubMed]
- Papke, R.T.; Douady, C.J.; Doolittle, W.F.; Rodriguez-Valera, F. Diversity of bacteriorhodopsins in different hypersaline waters from a single spanish saltern. Environ. Microbiol. 2003, 5, 1039–1045. [Google Scholar] [CrossRef] [PubMed]
- Arora, B.; Mukherjee, J.; Gupta, M.N. Enzyme promiscuity: Using the dark side of enzyme specificity in white biotechnology. Sustain. Chem. Proc. 2014, 2, 25. [Google Scholar] [CrossRef]
- Lamble, H.J.; Heyer, N.I.; Bull, S.D.; Hough, D.W.; Danson, M.J. Metabolic pathway promiscuity in the archaeon Sulfolobus solfataricus revealed by studies on glucose dehydrogenase and 2-keto-3-deoxygluconate aldolase. J. Biol. Chem. 2003, 278, 34066–34072. [Google Scholar] [CrossRef] [PubMed]
| Superfamily | Supfam ID | Functional Annotation | Phylum |
|---|---|---|---|
| Sulfolobus fructose-1,6-bisphosphatase-like superfamily (66) | 111249 | Metabolism | Euryarchaeota Crenarchaeota |
| Glu-tRNAGln amidotransferase C subunit superfamily (56) | 141000 | Information Translation | Euryarchaeota Crenarchaeota Nanoarchaeota |
| Siroheme synthase middle domains-like superfamily (51) | 75615 | Metabolism | Euryarchaeota Crenarchaeota |
| Heme-dependent peroxidases superfamily (18) | 48113 | Metabolism Redox | Euryarchaeota |
| Nitrous oxide reductase, N-terminal domain superfamily (4) | 50974 | Metabolism Redox | Euryarchaeota Crenarchaeota |
| Oxidoreductase molybdopterin-binding domain superfamily (3) | 56524 | Metabolism E- transfer | Euryarchaeota |
| Tropomyosin superfamily (2) | 57997 | Intra-Cellular processes Cell motility | Euryarchaeota |
| DNA breaking-rejoining enzymes superfamily (2) | 56349 | Information DNA replication/repair | Euryarchaeota Crenarchaeota |
| N-terminal domain of MutM-like DNA repair proteins superfamily (2) | 81624 | Information DNA replication/repair | Euryarchaeota |
| (Phosphotyrosine protein) phosphatases II superfamily (2) | 52799 | Regulation Kinases/phosphatases | Crenarchaeota |
| 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase, HPPK (7,8-dihydro-6-hydroxymethylpterin-pyrophosphokinase) superfamily (1) | 55083 | Metabolism Coenzyme metabolism and transport | Crenarchaeota |
| Eukaryotic DNA topoisomerase I, N-terminal DNA-binding fragment superfamily (1) | 56741 | Information DNA replication/repair | Crenarchaeota |
| Metabolic Categories (KEGG) | Substrate Promiscuity (%) | Catalytic Promiscuity (%) | ||
|---|---|---|---|---|
| Euryarchaeota | Crenarchaeota | Euryarchaeota | Crenarchaeota | |
| Carbohydrate metabolism | 33.21 | 36.12 | 32.26 | 34.79 |
| Amino acid metabolism | 21.06 | 14.69 | 10.36 | 9.94 |
| Energy metabolism | 11.87 | 18.81 | 10.04 | 19.59 |
| Metabolism of cofactors and vitamins | 10.94 | 7.34 | 25.96 | 17.83 |
| Genetic information processing | 7.04 | 5.9 | 6.94 | 2.63 |
| Nucleotide metabolism | 4.62 | 2.88 | 8.86 | 9.35 |
| Metabolism of terpenoids and polyketides | 3.53 | 3.36 | 0.21 | 0 |
| Lipid metabolism | 2.23 | 3.84 | 0 | 0 |
| Xenobiotics biodegradation and metabolism | 1.98 | 2.47 | 1.38 | 0 |
| Environmental information processing | 1.33 | 2.06 | 0.96 | 0 |
| Biosynthesis of other secondary metabolites | 1.61 | 1.23 | 0.21 | 3.5 |
| Metabolism of other amino acids | 0.4 | 1.23 | 2.02 | 2.33 |
| Cellular Processes | 0.12 | 0 | 0.74 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Núñez, M.A.; Rodríguez-Escamilla, Z.; Rodríguez-Vázquez, K.; Pérez-Rueda, E. Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms. Life 2017, 7, 30. https://doi.org/10.3390/life7030030
Martínez-Núñez MA, Rodríguez-Escamilla Z, Rodríguez-Vázquez K, Pérez-Rueda E. Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms. Life. 2017; 7(3):30. https://doi.org/10.3390/life7030030
Chicago/Turabian StyleMartínez-Núñez, Mario Alberto, Zuemy Rodríguez-Escamilla, Katya Rodríguez-Vázquez, and Ernesto Pérez-Rueda. 2017. "Tracing the Repertoire of Promiscuous Enzymes along the Metabolic Pathways in Archaeal Organisms" Life 7, no. 3: 30. https://doi.org/10.3390/life7030030





