Formation and Stability of Prebiotically Relevant Vesicular Systems in Terrestrial Geothermal Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Collection of Water Samples from Hot Springs
2.2.2. Formation of Fatty Acid Vesicles
2.2.3. Dehydration-Rehydration (DH-RH) Experiments
2.2.4. Geochemical Analysis of Hot Spring Water Samples
3. Results
3.1. Formation of Mixed Fatty Acid Vesicles in Hot Spring Water
3.2. Effect of Chain Length and Saturation on the Vesicle Forming Ability of Fatty Acid and Its Derivatives in the Hot Spring Water Samples
3.3. Temperature Stability of Vesicles Containing a Mixture of OA and GMO
3.4. Stability of Mixed Fatty Acid Vesicles under Dehydration-Rehydration (DH-RH) Conditions
3.5. Geochemical Analysis of the Hot Spring Water Samples
4. Discussion
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Distinguishing Between Oil Droplets and Vesicles Under DIC Microscopy
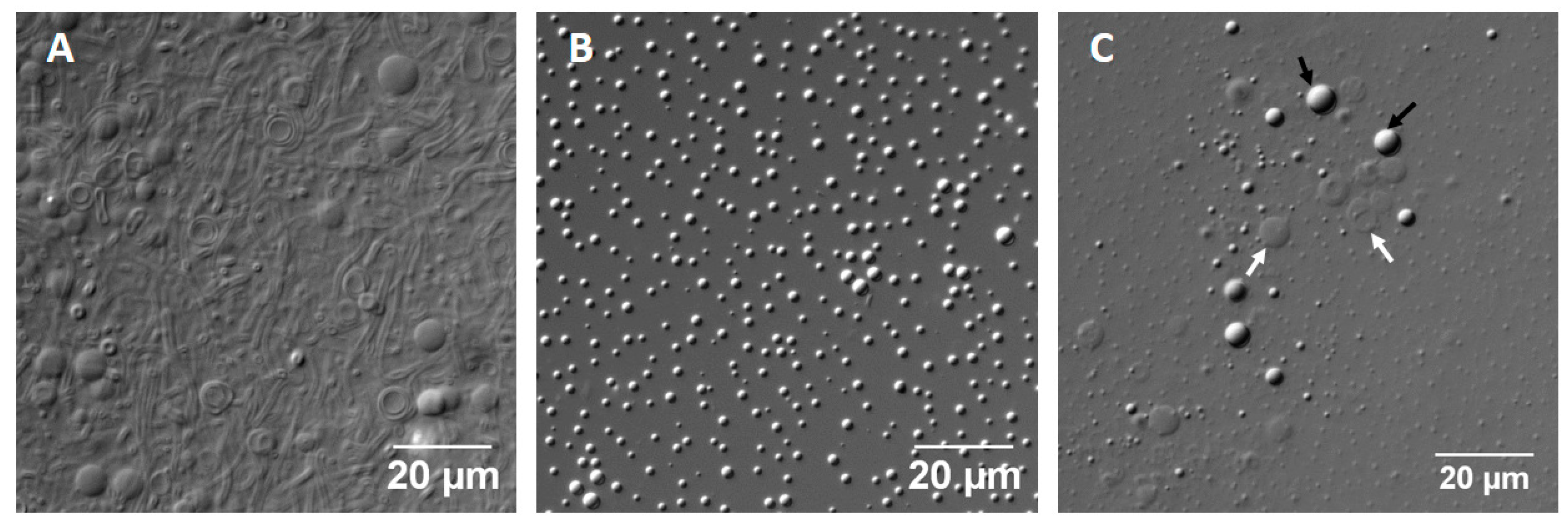
References
- Domagal-Goldman, S.D.; Wright, K.E.; Adamala, K.; Arina de la Rubia, L.; Bond, J.; Dartnell, L.R.; Goldman, A.D.; Lynch, K.; Naud, M.-E.; Paulino-Lima, I.G.; et al. The Astrobiology Primer v2.0. Astrobiology 2016, 16, 561–653. [Google Scholar] [CrossRef] [PubMed]
- Corliss, J.B.; Baross, J.; Hoffman, S. An Hypothesis Concerning the Relationships Between Submarine Hot Springs and the Origin of Life on Earth. In Proceedings of the 26th International Geological Congress, Oceanologica Acta, Paris, France, 7 July 1980; pp. 59–69. [Google Scholar]
- Monnard, P.-A.; Apel, C.L.; Kanavarioti, A.; Deamer, D.W. Influence of Ionic Inorganic Solutes on Self-Assembly and Polymerization Processes Related to Early Forms of Life: Implications for a Prebiotic Aqueous Medium. Astrobiology 2002, 2, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Bada, J.L. Primordial Ocean Chemistry and its Compatibility with the RNA World. Orig. Life Evol. Biosph. 2011, 41, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Dose, K. Peptides and amino acids in the primordial hydrosphere. Biosystems 1975, 6, 224–228. [Google Scholar] [CrossRef]
- De Duve, C. Blueprint for a Cell: The Nature and Origin of Life; Neil Patterson Publishers: Burlington, CA, USA, 1991. [Google Scholar]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. The Role of Lipid Membranes in Life’s Origin. Life 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Damer, B.; Deamer, D. Coupled Phases and Combinatorial Selection in Fluctuating Hydrothermal Pools: A Scenario to Guide Experimental Approaches to the Origin of Cellular Life. Life 2015, 5, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Rajamani, S.; Vlassov, A.; Benner, S.; Coombs, A.; Olasagasti, F.; Deamer, D. Lipid-assisted Synthesis of RNA-like Polymers from Mononucleotides. Orig. Life Evol. Biosph. 2008, 38, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Mungi, C.V.; Rajamani, S. Characterization of RNA-Like Oligomers from Lipid-Assisted Nonenzymatic Synthesis: Implications for Origin of Informational Molecules on Early Earth. Life 2015, 5, 65–84. [Google Scholar] [CrossRef] [PubMed]
- Szostak, J.W.; Bartel, D.P.; Luisi, P.L. Synthesizing life. Nature 2001, 409, 387–390. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.H.; Singh, K.V.; Ahmad, S. A Report on the expedition of NASA spaceward bound India Programme, Ladakh, India, 9 August 2016. J. Palaeontol. Soc. India 2016, 61, 301–302. [Google Scholar]
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Ward, C.R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef] [PubMed]
- Mansy, S.S.; Szostak, J.W. Thermostability of model protocell membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 13351–13355. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, G.R.; Goswami, V.; Singh, S.K.; Chakrapani, G.J. Temporal variations in Sr and 87Sr/86Sr of the Ganga headwaters: Estimates of dissolved Sr flux to the mainstream. Hydrol. Process. 2010, 24, 1159–1171. [Google Scholar] [CrossRef]
- McCollom, T.M.; Ritter, G.; Simoneit, B.R. Lipid synthesis under hydrothermal conditions by Fischer-Tropsch-type reactions. Orig. Life Evol. Biosph. 1999, 29, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Yuen, G.U. Quantification of monocarboxylic acids in the Murchison carbonaceous meteorite. Nature 1979, 282, 396–398. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Danino, D.; Raghavan, S.R. Polymerizable Vesicles Based on a Single-Tailed Fatty Acid Surfactant: A Simple Route to Robust Nanocontainers. Langmuir 2009, 25, 1566–1571. [Google Scholar] [CrossRef] [PubMed]
- Kasting, J.F. Earth’s Early Atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Rai, S.K.; Bartarya, S.K.; Gupta, A.K.; Negi, M. Stable isotopes (δ13CDIC, δD, δ18O) and geochemical characteristics of geothermal springs of Ladakh and Himachal (India): Evidence for CO2 discharge in northwest Himalaya. Geothermics 2016, 64, 314–330. [Google Scholar] [CrossRef]
- Craig, J.; Absar, A.; Bhat, G.; Cadel, G.; Hafiz, M.; Hakhoo, N.; Kashkari, R.; Moore, J.; Ricchiuto, T.E.; Thurow, J.; et al. Hot springs and the geothermal energy potential of Jammu & Kashmir State, N.W. Himalaya, India. Earth-Sci. Rev. 2013, 126, 156–177. [Google Scholar] [CrossRef]
- Namani, T.; Walde, P. From Decanoate Micelles to Decanoic Acid/Dodecylbenzenesulfonate Vesicles. Langmuir 2005, 21, 6210–6219. [Google Scholar] [CrossRef] [PubMed]
- Rendón, A.; Carton, D.G.; Sot, J.; García-Pacios, M.; Montes, L.-R.; Valle, M.; Arrondo, J.-L.R.; Goñi, F.M.; Ruiz-Mirazo, K. Model Systems of Precursor Cellular Membranes: Long-Chain Alcohols Stabilize Spontaneously Formed Oleic Acid Vesicles. Biophys. J. 2012, 102, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Derry, L.A.; Anderson, S.P.; France-Lanord, C. Hydrothermal source of radiogenic Sr to Himalayan rivers. Geology 2001, 29, 803. [Google Scholar] [CrossRef]
- Thompson, J.M.; DeMonge, J.M. Chemical Analyses of Hot Springs, Pools, and Geysers from Yellowstone National Park, Wyoming, and Vicinity; Open-File Report 96-68, 1-67; U.S. Department of the Interior; U.S. Geological Survey: Denver, CO, USA, 1996.





| A | ||||||
| Combination of Fatty Acid and Its Derivatives | Presence or Absence of Vesicles in the Various Fatty Acid Systems Studied | |||||
| 0.2 M Bicine Buffer | Puga | |||||
| DA (C10:0) System | UDA (C11:1) System | OA (C18:1) System | DA (C10:0) System | UDA (C11:1) System | OA (C18:1) System | |
| only acid | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| acid + alcohol (2:1 ratio) | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ |
| acid + glyceride (2:1 ratio) | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| acid + alcohol + glyceride (4:1:1 ratio) | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ |
| B | ||||||
| Combination of Fatty Acid and Its Derivatives | Presence or Absence of Vesicles in the Various Fatty Acid Systems Studied | |||||
| Chumathang | Panamic | |||||
| DA (C10:0) System | UDA (C11:1) System | OA (C18:1) System | DA (C10:0) System | UDA (C11:1) System | OA (C18:1) System | |
| only acid | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| acid + alcohol (2:1 ratio) | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| acid + glyceride (2:1 ratio) | ✓ | ✓ | ✓ | ✓ | ✓ # | ✓ # |
| acid + alcohol + glyceride (4:1:1 ratio) | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ |
| Hot Spring Water | pH | Major Cations | Major Anions | TZ+ | TZ− | TZ+/TZ− | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Ca2+ | Mg2+ | Li+ | HCO3− | Cl− | SO42− | |||||
| All Values in mM | µE | |||||||||||
| Puga | 8.48 | 26.14 | 2.08 | 0.89 | 0 | 1.26 | 14.57 | 11.83 | 1.27 | 31258 | 28936 | 1.08 |
| Chumathang | 8.64 | 15.54 | 0.55 | 0.22 | 0.005 | 0.44 | 10.2 | 2.99 | 2.53 | 16980 | 18248 | 0.93 |
| Panamic | 8.37 | 6.76 | 0.14 | 0.36 | 0.002 | 0.05 | 5.68 | 0.26 | 0.99 | 7665 | 7918 | 0.97 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, M.P.; Samanta, A.; Tripathy, G.R.; Rajamani, S. Formation and Stability of Prebiotically Relevant Vesicular Systems in Terrestrial Geothermal Environments. Life 2017, 7, 51. https://doi.org/10.3390/life7040051
Joshi MP, Samanta A, Tripathy GR, Rajamani S. Formation and Stability of Prebiotically Relevant Vesicular Systems in Terrestrial Geothermal Environments. Life. 2017; 7(4):51. https://doi.org/10.3390/life7040051
Chicago/Turabian StyleJoshi, Manesh Prakash, Anupam Samanta, Gyana Ranjan Tripathy, and Sudha Rajamani. 2017. "Formation and Stability of Prebiotically Relevant Vesicular Systems in Terrestrial Geothermal Environments" Life 7, no. 4: 51. https://doi.org/10.3390/life7040051
APA StyleJoshi, M. P., Samanta, A., Tripathy, G. R., & Rajamani, S. (2017). Formation and Stability of Prebiotically Relevant Vesicular Systems in Terrestrial Geothermal Environments. Life, 7(4), 51. https://doi.org/10.3390/life7040051






