The Prevailing Catalytic Role of Meteorites in Formamide Prebiotic Processes
Abstract
:1. The Numerous Biogenic Functions of Meteorites
1.1. Meteorites Are Providers of Impact Energy during Their Passage through the Atmosphere
1.2. Meteorites as Agents for Geodynamism
1.3. As Providers of Simple Organic Biogenic Materials, the Role of Meteorites Varies Enormously
1.4. As Catalysts
2. State of the Art about the Synthetic Capacity of Formamide with Terrestrial Catalysts
3. The Catalytic Activity of Meteorites
3.1. Thermal Energy-Triggered Condensations Catalyzed by Iron, Stony-Iron, Chondrites, and Achondrites
3.2. Proton Irradiation-Triggered Condensations Catalyzed by Iron, Stony-Iron, Chondrites, and Achondrites
3.3. Heavy Ion-Triggered Condensations Catalyzed by Stony-Iron and Chondrites
3.4. Thermal Energy-Triggered Condensations Catalyzed by Chondrites in Formamide/Water Mixtures
3.5. Impact-Triggered Synthesis of Nucleobases and Their Precursors in the Presence of Meteorites
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules—An inventory for the Origin of Life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Nesvorny, D.; Sponer, J.; Kubelik, P.; Michalcikova, R.; Shestivska, V.; Sponer, J.E.; Civis, S. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 2015, 112, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Pietrucci, F.; Saitta, A.M.; Knizek, A.; Kubelik, P.; Wanek, O.; Shestivska, V.; Civis, S. Formation of nucleobases in a Miller-Urey reducing atmosphere. Proc. Natl. Acad. Sci. USA 2017, 114, 4306–4311. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Kubelik, P.; Knizek, A.; Pastorek, A.; Sutherland, J.; Civis, S. High energy radical chemistry formation of HCN-rich atmospheres on early Earth. Sci. Rep. 2017, 7, 6275–6283. [Google Scholar] [CrossRef] [PubMed]
- Greber, N.D.; Dauphas, N.; Bekker, A.; Ptacek, M.P.; Bindeman, I.N.; Hofman, A. Titanium isotopic evidence for felsic crust and plate tectonics 3.5 billion years ago. Science 2017, 357, 1271–1274. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, C.; Marchi, S.; Zhang, S.; Bottke, W. Impact-driven subduction on the Hadean Earth. Nat. Geosci. 2017, 10, 793–797. [Google Scholar] [CrossRef]
- Osinski, G.R.; Tornabene, L.L.; Banerjee, N.R.; Cockell, C.S.; Flemming, R.; Izawa, M.R.M.; McCutcheon, J.; Parnell, J.; Preston, L.J.; Pickersgill, A.E.; et al. Impact-generated hydrothermal systems on Earth and Mars. Icarus 2013, 224, 347–363. [Google Scholar] [CrossRef]
- Mulkidjanian, A.Y.; Bychkov, A.Y.; Dibrova, D.V.; Galperin, M.Y.; Koonin, E.V. Origin of first cells at terrestrial, anoxic geothermal fields. Proc. Natl. Acad. Sci. USA 2012, 109, E821–E830. [Google Scholar] [CrossRef] [PubMed]
- Djokic, T.; Van Kranendonk, M.J.; Campbell, K.A.; Walter, M.R.; Wald, C.R. Earliest signs of life on land preserved in ca. 3.5 Ga hot spring deposits. Nat. Commun. 2017, 8, 15263. [Google Scholar] [CrossRef]
- Schimitt-Kopplin, P. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.S.; Stern, J.C.; Elsila, J.E.; Glavin, D.P.; Dworkin, J.P. Understanding prebiotic chemistry through the analysis of extraterrestrial amino acids and nucleobases in meteorites. Chem. Soc. Rev. 2012, 41, 5459–5472. [Google Scholar] [CrossRef] [PubMed]
- Geach, J.E.; Papadopoulos, P.P. Molecular and atomic line surveys of galaxies. I: The dense, star-forming gas phase as a beacon. Astrophys. J. 2012, 757, 156. [Google Scholar] [CrossRef]
- Krocher, O.; Elsener, M.; Jacob, E. A model gas study of ammonium formate, methanamide and guanidinium formate as alternative ammonia precursor compounds for the selective catalytic reduction of nitrogen oxides in diesel exaust gas. Appl. Catal. B Environ. 2009, 88, 66–82. [Google Scholar] [CrossRef]
- Miller, S.L.; Urey, H.C. Organic compounds synthesis on the primitive Earth. Science 1959, 130, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Saitta, A.M.; Saija, F. Miller experiments in atomistic simulation. Proc. Natl. Acad. Sci. USA 2014, 111, 13768–13773. [Google Scholar] [CrossRef] [PubMed]
- Adande, G.R.; Woolf, N.J.; Ziurys, L.M. Observations of interstellar formamide: Availability of a prebiotic precursor in the galactic habitable zone. Astrobiology 2013, 13, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sepulcre, A.; Jaber, A.A.; Mendoza, E.; Lefloch, B.; Ceccarelli, C.; Vastel, C.; Bachiller, R.; Cernicaro, J.; Codella, C.; Kahane, C.; et al. Shedding light on the formation of the pre-biotic molecule formamide with ASAI. Mon. Not. R. Astron. Soc. 2015, 449, 2438–2458. [Google Scholar] [CrossRef]
- Rubin, R.H.; Swenson, G.W.; Benson, R.C.; Tigelaar, H.L.; Flygare, W.H. Microwave detection of interstellar space. Astrophys. J. 1971, 169, L39. [Google Scholar] [CrossRef]
- Biver, N.; Bockelée-Morvan, D.; Debout, V.; Crovisier, J.; Boissier, J.; Lis, D.C.; Dello Russo, N.; Moreno, R.; Colom, P.; Paubert, G.; et al. Complex organic molecules in comets C/2012 F6 (Lemmon) and C/2013 R1 (Lovejoy): Detection of ethylene glycol and formamide. Astron. Astrophys. 2014, 566, L5. [Google Scholar] [CrossRef]
- Niether, D.; Afanasenkan, D.; Dhont, J.K.G.; Wiegand, S. Accumulation of formamide in hydrothermal pores to form prebiotic nucleobases. Proc. Natl. Acad. Sci. USA 2016, 113, 4272–4277. [Google Scholar] [CrossRef] [PubMed]
- Niether, D.; Wiegand, S. Heuristic Approach to Understanding the Accumulation Process in Hydrothermal Pores. Entropy 2017, 19, 33. [Google Scholar] [CrossRef]
- Rotelli, L.; Trigo-Rodriguez, J.P.; Moyano-Cambero, C.E.; Carota, E.; Botta, L.; Di Mauro, E.; Saladino, R. The key role of meteorites in the formation of relevant prebiotic molecules in a formamide/water environment. Sci. Rep. 2017, 6, 38888. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Saladino, R.; Bizzarri, B.M.; Cobucci-Ponzano, B.; Iacono, R.; Avino, R.; Caliro, S.; Carandente, A.; Lorenzini, F.; Tortora, A.; et al. Formamide-based prebiotic chemistry in the Phlegrean fields. Adv. Space Res. 2017. [Google Scholar] [CrossRef]
- Carota, E.; Botta, G.; Rotelli, L.; Di Mauro, E.; Saladino, R. Current advances in prebiotic chemistry under space conditions. Curr. Org. Chem. 2015, 19, 1963–1979. [Google Scholar] [CrossRef]
- Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; Di Mauro, E. Genetics first or metabolism first? The formamide clue. Chem. Soc. Rev. 2012, 41, 5526–5565. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Pino, S.; Costanzo, G.; Di Mauro, E. Formamide and the origin of life. Phys. Life Rev. 2012, 9, 84–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slebocka-Tilk, H.; Sauriol, F.; Monette, M.; Brown, M.S. Aspects of the hydrolysis of formamide: Revisitation of the water reaction and determination of the solvent deuterium kinetic isotope effect in base. Can. J. Chem. 2002, 80, 1343–1350. [Google Scholar] [CrossRef]
- Costanzo, G.; Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E. Nucleoside phosphorylation by phosphate minerals. J. Biol. Chem. 2007, 282, 16729–16735. [Google Scholar] [CrossRef] [PubMed]
- Schoffstall, A.M.; Barto, R.J.; Ramos, D.L. Nucleosides and deoxynucleoside phosphorylation in formamide solutions. Orig. Life 1982, 12, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, G.; Saladino, R.; Botta, L.; Giorgi, A.; Scipioni, A.; Pino, S.; Di Mauro, E. Generation of RNA molecules by base catalyzed click-like reaction. ChemBioChem 2012, 13, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Schmandt, B.; Jacobsen, S.D.; Becker, T.W.; Liu, Z.; Dueker, K.G. Dehydration melting at the top of the lower mantle. Science 2014, 344, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A.; Ricardo, A.; Carrigan, M.A. Is there a common chemical model for life in the universe? Curr. Opin. Chem. Biol. 2004, 8, 672–689. [Google Scholar] [CrossRef] [PubMed]
- Benner, S.A. Paradoxes in the origin of life. Orig. Life Evol. Biosph. 2014, 44, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Busiello, V.; Ciciriello, F.; Costanzo, G.; Di Mauro, E. Origin of informational polymers: Differential stability of 3′- and 5′-phosphoester bonds in deoxy monomers and oligomers. J. Biol. Chem. 2005, 280, 35658–35669. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Di Mauro, E.; Costanzo, G. Origin of Informational Polymers: Differential Stability of Phosphoester Bonds in Ribo Monomers and Oligomers. J. Biol. Chem. 2006, 281, 5790–5796. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Bizzarri, B.; Di Mauro, E.; Garcia Ruiz, J.M. A global scale scenario for prebiotic chemistry: Silica-based self-assembled mineral structures and formamide. Biochemistry 2016, 55, 2806–2811. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Crestini, C.; Cossetti, C.; Di Mauro, E.; Deamer, D. Catalytic effects of Murchison material: Prebiotic synthesis and degradation of RNA precursors. Orig. Life Evol. Biosph. 2011, 41, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; Di Mauro, E. Meteorites as Catalysts for Prebiotic Chemistry. Chem. Eur. J. 2013, 19, 16916–16922. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; Kapralov, M.; Timoshenko, G.N.; Rozanov, A.; Krasavin, E.; Di Mauro, E. Meteorite-catalyzed syntheses of nucleosides and of other prebiotic compounds from formamide under proton irradiation. Proc. Natl. Acad. Sci. USA 2015, 112, E2746–E2755. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Carota, E.; Botta, G.; Kapralov, M.; Timoshenko, G.; Rozanov, A.; Krasavin, E.; Di Mauro, E. First evidence on the role of heavy ion irradiation of meteorites and formamide in the origin of biomolecules. Orig. Life Evol. Biosph. 2016, 4, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Bizzarri, B.M.; Botta, L.; Sponer, J.; Sponer, J.E.; Georgelin, T.; Jaber, M.; Rigaud, B.; Kapralov, M.; Timoshenko, G.N.; et al. Proton irradiation: A key to the challenge of N-glycosidic bond formation in a prebiotic context. Sci. Rep. 2017, 7, 14709. [Google Scholar] [CrossRef] [PubMed]
- Lane, N.; Martin, W. The origin of membrane bioenergetics. Cell 2012, 151, 1406–1416. [Google Scholar] [CrossRef] [PubMed]
- Airapetian, V.S.; Glocer, A.; Gronoff, G.; Hébrard, E.; Danchi, W. Prebiotic chemistry and atmospheric warming of early Earth by an active young Sun. Nat. Geosci. 2016, 9, 452–455. [Google Scholar] [CrossRef]
- Nuevo, M.; Chen, Y.-J.; Hu, W.-J.; Qiu, J.-M.; Wu, S.-R.; Fung, H.-S.; Chu, C.-C.; Yih, T.-S.; Ip, W.-H.; Wu, C.-Y.R. Irradiation of pyrimidine in pure H2O ice with high-energy ultraviolet photons. Astrobiology 2014, 14, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Reeves, H.; Fowler, W.A.; Hoyle, F. Galactic cosmic ray Origin of Li, Be and B in stars. Nature 1970, 226, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.G.W.; Colgate, S.A.; Grossman, L. Cosmic abundance of Boron. Nature 1973, 243, 204–207. [Google Scholar] [CrossRef]
- Chausidon, M.; Robert, F. Nucleosynthesis of 11B-rich boron in the pre-solar cloud recorded in meteoritic chondrules. Nature 1995, 374, 337–339. [Google Scholar] [CrossRef]
- Ferus, M.; Michalčíková, R.; Shestivská, V.; Šponer, J.; Šponer, J.E.; Civiš, S. High-energy chemistry of formamide: A simpler way for nucleobase formation. J. Phys. Chem. A 2014, 118, 719–736. [Google Scholar] [CrossRef] [PubMed]

| Products | Ref. | Reaction Type | Meteorite Type |
|---|---|---|---|
| Nucleobases and their analogs: uracil, cytosine, adenine, guanine, isocytosine, dihydrouracil and hypoxanthine. Aminoacids: glycine, alanine, valine, leucine, phenylalanine. Carboxylic acids: from oxalic acid (C-2) up to nonanoic acid (C-9). Condensing agents: carbodiimide and urea. | [38] | Condensation process under thermal energy conditions in formamide | Iron: Canyon-Diablo, Campo-del-Cielo, and Sikhote-Alin. Stony-Iron: Seymchan and NWA 4482. Chondrites: NWA 2828, Gold Basin, Dhofar 959, Murchison, and NWA 1465 Achondrites: NWA 5357, Al-Haggounia. |
| Nucleosides: cytidine, uridine, adenosine, and thymidine. Nucleobases and their analogs: uracil, thymine, cytosine, adenine, guanine, isocytosine, hypoxanthine, 2,6-diaminopurine, and orotic acid (among others). Sugars: ribose, 2-deoxyribose, glucose, 2-deoxyglucose (among others). Aminoacids: glycine, alanine, and proline. Carboxylic acids: from oxalic acid (C-2) up to arachidic acid (C-20). | [39] | Formamide irradiation by high-energy proton beams | Iron: Canyon Diablo and Campo del Cielo. Stony-Iron: NWA 4482. Chondrites: NWA 2828, Gold Basin, Dhofar 959, NWA 1465, Chelyabinsk, and Orgueil. Achondrites: NWA 5357 and Al-Haggounia. |
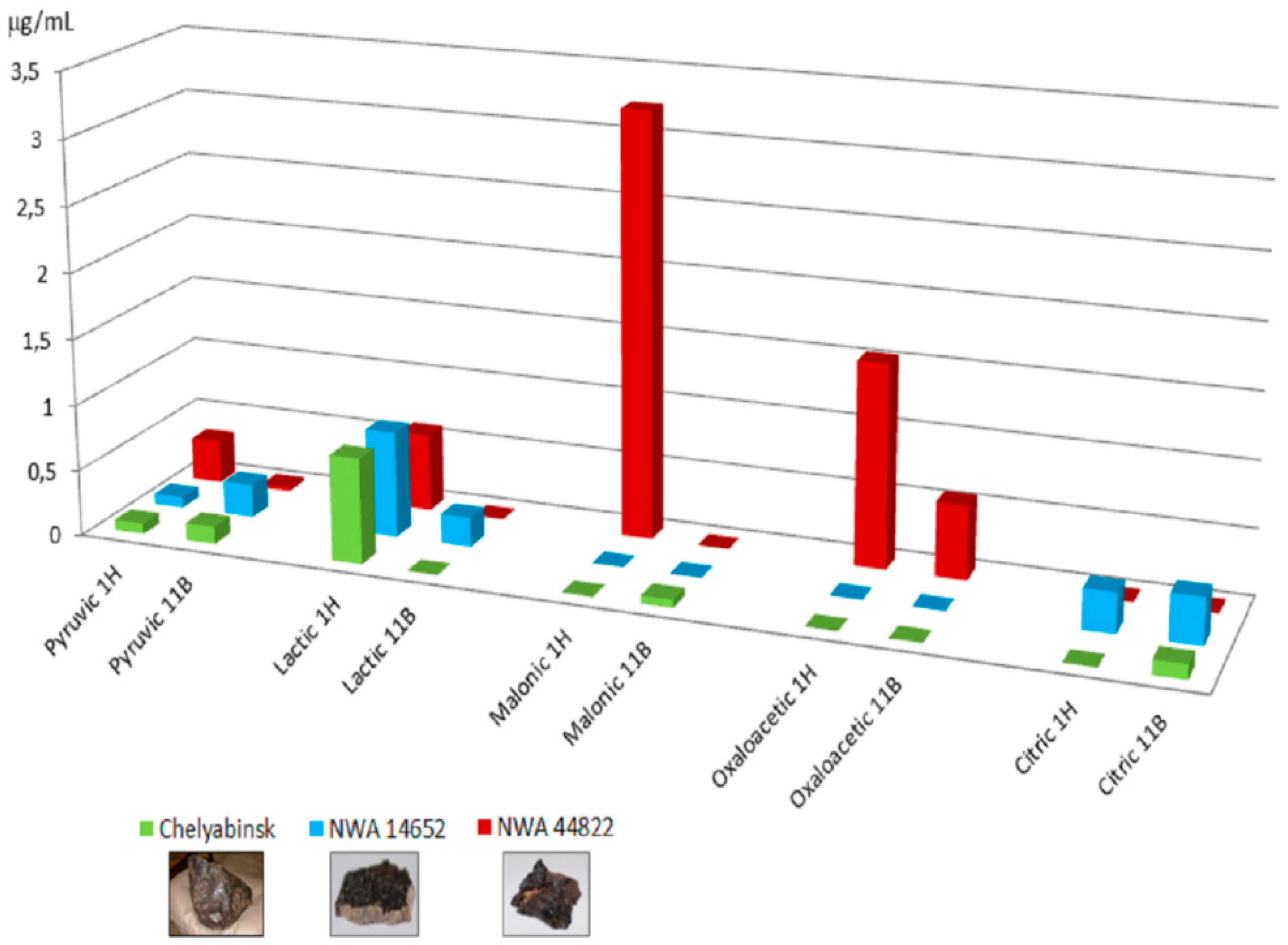
| Nucleobases and their analogs: uracil, cytosine, adenine, guanine, isocytosine, hypoxanthine, 4,6-diamonipurine, 2,4-diamonopyrimidine, 4-amino imidazole carbonitrile, 2,4-dihydroxy pyrimidine, and orotic acid. Carboxylic acids: pyruvic acid, lactic acid, maleic acid, oxaloacetic acid, citric acid. | [40] | Formamide irradiation by high-energy 11B boron beams | Stony-Iron: NWA 4482. Chondrites: Dhofar 959, Gold Basin, Chelyabinsk, NWA 1465. |
| Nucleobases and their analogs: uracil, adenine, guanine, hypoxanthine, isocytosine, purine, 4(3H)-pyrimidinone. Sugars: fructose and ribose. Aminoacids: glycine and N-formyl glycine. Carboxylic acids: glycolic acid, oxalic acid, pyruvic acid, lactic acid, malic acid, and succinic acid. | [23] | Condensation process under thermal energy conditions in formamide/thermal water mixture | Stony-Iron: Seymchan and NWA4482. Chondrites: NWA2028. Achondrites: Al-Haggounia and NWA5357. |
| Nucleobases and their analogs: uracil, adenine, guanine, hypoxanthine, isocytosine, 2,6-diamino purine, purine, 4(3H)-pyrimidinone, orotic acid, and 2,4-diamino-6 hydroxypyrimidine. Aminoacids: glycine, N-formyl glycine, and alanine. Carboxylic acids: glycolic acid, oxalic acid, pyruvic acid, lactic acid, malic acid, succinic acid, oxaloacetic acid, fumaric acid, ketoglutaric acid, citric acid, palmitic acid, and stearic acid. | [24] | Condensation process under thermal energy conditions in formamide/sea and thermal water mixture | Chondrites: ALH 84028, EET 92042, MIL 05024, LAR 04318, GRO 95551, and GRO 95566. |
| Nucleobases: uracil, adenine, guanine, thymine, cytosine. Aminoacids: glycine. | [48] | Formamide energy impact-triggered synthesis | Iron: Campo del Cielo. Chondrites: NWA 6472. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saladino, R.; Botta, L.; Di Mauro, E. The Prevailing Catalytic Role of Meteorites in Formamide Prebiotic Processes. Life 2018, 8, 6. https://doi.org/10.3390/life8010006
Saladino R, Botta L, Di Mauro E. The Prevailing Catalytic Role of Meteorites in Formamide Prebiotic Processes. Life. 2018; 8(1):6. https://doi.org/10.3390/life8010006
Chicago/Turabian StyleSaladino, Raffaele, Lorenzo Botta, and Ernesto Di Mauro. 2018. "The Prevailing Catalytic Role of Meteorites in Formamide Prebiotic Processes" Life 8, no. 1: 6. https://doi.org/10.3390/life8010006






