Exposed Areas Above Sea Level on Earth >3.5 Gyr Ago: Implications for Prebiotic and Primitive Biotic Chemistry
Abstract
:1. Introduction
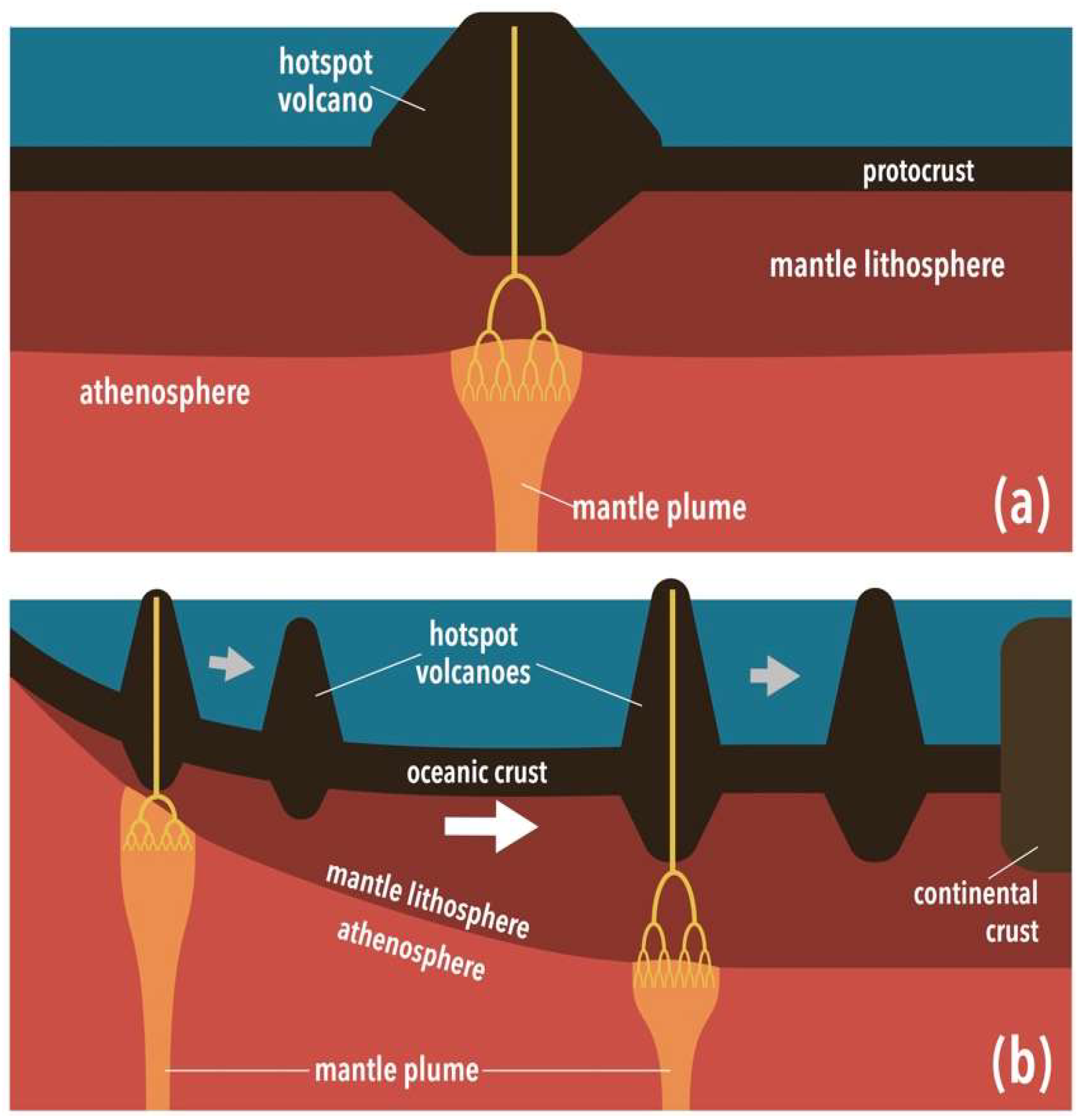
2. Early Exposed Areas Above Sea Level
3. WLPs Chemistry and Prebiotic Chemistry
4. Lake Waiau: A Modern Volcanic Island WLP?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peretó, J.; Bada, J.L.; Lazcano, A. Charles Darwin and the origin of life. Orig. Life Evol. Biosph. 2009, 39, 395–406. [Google Scholar] [PubMed]
- Pearce, B.K.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Nat. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradley, B.; Matthew, P.; Maheen, G.; Brian, J.C.; Francisco, V.; Nicholas, V.H.; César, M.S. Darwin’s warm little pond: A one-pot reaction for prebiotic phosphorylation and the mobilization of phosphate from minerals in a urea-based solvent. Angew. Chem. Int. Ed. Engl. 2016, 55, 13249–13253. [Google Scholar]
- Damer, B. A field trip to the Archaean in search of Darwin’s warm little pond. Life 2016, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Follmann, H.; Brownson, C. Darwin’s warm little pond revisited: From molecules to the origin of life. Naturwissenschaften 2009, 96, 1265–1292. [Google Scholar] [CrossRef] [PubMed]
- Deamer, D. Darwin’s prescient guess. Proc. Nat. Acad. Sci. USA 2017, 114, 11264–11265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buick, R. The earliest records of life on Earth. In Planets and Life: The Emerging Science of Astrobiology; Woodruff, T.S., III, John, B., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 237–264. [Google Scholar]
- Bell, E.A.; Boehnke, P.; Harrison, T.M.; Mao, W.L. Potentially biogenic carbon preserved in a 4.1 billion-year-old zircon. Proc. Nat. Acad. Sci. USA 2015, 112, 14518–14521. [Google Scholar] [PubMed]
- Stribling, R.; Miller, S.L. Energy yields for hydrogen cyanide and formaldehyde syntheses: The HCN and amino acid concentrations in the primitive ocean. Orig. Life Evol. Biosph. 1987, 17, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Bada, J.L. Survival of amino acids in micrometeorites during atmospheric entry. Astrobiology 2001, 1, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Chris Mehta, C.; Perez, A.; Thompson, G.; Pasek, M.A. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Sleep, N. Hotspots and mantle plumes: Some phenomenology. J. Geophys. Res. 1990, 95, 6715–6736. [Google Scholar] [CrossRef]
- Saito, M.A.; Sigman, D.M.; Morel, F.M. The bioinorganic chemistry of the ancient ocean: The co-evolution of cyanobacterial metal requirements and biogeochemical cycles at the Archean–Proterozoic boundary? Inorg. Chim. Acta 2003, 356, 308–318. [Google Scholar] [CrossRef]
- Armstrong, R.L. Radiogenic isotopes: The case for crustal recycling on a near-steady-state no-continental-growth Earth. Philos. Trans. R. Soc. Lond. A 1987, 301, 443–472. [Google Scholar] [CrossRef]
- Patchett, P.J.; Arndt, N.T. Nd isotopes and tectonics of 1.9–1.7 Ga crustal genesis. Earth Planet. Sci. Lett. 1986, 78, 329–338. [Google Scholar] [CrossRef]
- McCulloch, M.T.; Bennett, V.C. Evolution of the early Earth: Constraints from 143Nd-142Nd isotopic systematics. Lithos 1993, 30, 237–255. [Google Scholar] [CrossRef]
- Campbell, I.H. Constraints on continental growth models from Nb/U ratios in the 3.5 Ga Barberton and other Archaean basalt-komatiite suites. Am. J. Sci. 2003, 303, 319–351. [Google Scholar] [CrossRef]
- Condie, K.C.; Aster, R.C. Episodic zircon age spectra of orogenic granitoids: The supercontinent connection and continental growth. Precambrian Res. 2010, 180, 227–236. [Google Scholar] [CrossRef]
- Korenaga, J. Estimating the formation age distribution of continental crust by unmixing zircon age data. Earth Planet. Sci. Lett. 2018, 482, 388–395. [Google Scholar] [CrossRef]
- Korenaga, J.; Planavsky, N.J.; Evans, D.A.D. Global water cycle and the coevolution of Earth’s interior and surface environment. Philos. Trans. R. Soc. A 2017, 375, 20150393. [Google Scholar] [CrossRef] [PubMed]
- Arndt, N. Why was flood volcanism on submerged continental platforms so common in the Precambrian? Precambrian Res. 1999, 97, 155–164. [Google Scholar] [CrossRef]
- Bindeman, I.N.; Zakharov, D.O.; Palandri, J.; Greber, N.D.; Dauphas, N.; Retallack, G.J.; Hofmann, A.; Lackey, J.S.; Bekker, A. Rapid emergence of subaerial landmasses and onset of a modern hydrologic cycle 2.5 billion years ago. Nature 2018, 557, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Ito, E.; Harris, D.M.; Anderson, A.T. Alteration of oceanic crust and geologic cycling of chlorine and water. Geochim. Cosmochim. Acta 1983, 47, 1613–1624. [Google Scholar] [CrossRef]
- Sleep, N.H. Geological and geochemical constraints on the origin and evolution of life. Astrobiology 2018, 18, 1199–1219. [Google Scholar] [CrossRef] [PubMed]
- Wilde, S.A.; Valley, J.W.; Peck, W.H.; Graham, C.M. Evidence from detrital zircons for the existence of continental crust and oceans on the Earth 4.4 Gyr ago. Nature 2001, 409, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth’s surface 4300 Myr ago. Nature 2001, 409, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, J. Initiation and evolution of plate tectonics on Earth: Theories and observations. Annu. Rev. Earth Planet. Sci. 2013, 41, 117–151. [Google Scholar] [CrossRef]
- Condie, K.C.; Pease, V. When Did Plate Tectonics Begin on Planet. Earth? Geological Society of America: Boulder, CO, USA, 2008. [Google Scholar]
- Hopkins, M.D.; Harrison, T.M.; Manning, C.E. Constraints on Hadean geodynamics from mineral inclusions in >4 Ga zircons. Earth Planet. Sci. Lett. 2010, 298, 367–376. [Google Scholar] [CrossRef]
- Rosas, J.C.; Korenaga, J. Rapid crustal growth and efficient crustal recycling in the early {Earth}: Implications for Hadean and Archean geodynamics. Earth Planet. Sci. Lett. 2018, 494, 42–49. [Google Scholar] [CrossRef]
- Korenaga, J. Crustal evolution and mantle dynamics through Earth history. Philos. Trans. R. Soc. A 2018, 376, 20170408. [Google Scholar] [CrossRef] [PubMed]
- Solomatov, V.S. Scaling of temperature- and stress-dependent viscosity convection. Phys. Fluids 1995, 7, 266–274. [Google Scholar] [CrossRef]
- Halevy, I.; Bachan, A. The geologic history of seawater pH. Science 2017, 335, 1069–1071. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Todd, Z.R.; Sutherland, J.D.; Sasselov, D.D. Sulfidic anion concentration on early Earth for surficial origins-of-life chemistry. Astrobiology 2018, 18, 1023–1040. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, J. Can mantle convection be self-regulated? Sci. Adv. 2016, 2, E1601168. [Google Scholar] [CrossRef] [PubMed]
- Korenaga, J. Pitfalls in modeling mantle convection with internal heating. J. Geophys. Res. 2017, 122, 4064–4085. [Google Scholar] [CrossRef]
- Nimmo, F. Energetics of the core. In Treatise on Geophysics, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 8, pp. 27–55. [Google Scholar]
- King, S.D.; Adam, C. Hotspot swells revisited. Phys. Earth Planet. Inter. 2014, 235, 66–83. [Google Scholar] [CrossRef]
- Korenaga, J. Archean geodynamics and the thermal evolution of Earth. In Archean Geodynamics and Environments; American Geophysical Union: Washington, DC, USA, 2006; pp. 7–32. [Google Scholar]
- Korenaga, J. Plate tectonics, flood basalts, and the evolution of Earth’s oceans. Terra Nova 2008, 20, 419–439. [Google Scholar] [CrossRef]
- Korenaga, J. How does small-scale convection manifest in surface heat flux? Earth Planet. Sci. Lett. 2009, 287, 329–332. [Google Scholar] [CrossRef]
- DePaolo, D.J.; Manga, M. Deep origin of hotspots--the mantle plume model. Science 2003, 300, 920–921. [Google Scholar] [CrossRef] [PubMed]
- Zahnle, K.; Schaefer, L.; Fegley, B. Earth’s earliest atmospheres. Cold Spring Harb. Perspect. Biol. 2010, 2, A004895. [Google Scholar] [CrossRef] [PubMed]
- Cruz, J.V.; Antunes, P.; Amaral, C.; França, Z.; Nunes, J.C. Volcanic lakes of the Azores archipelago (Portugal): Geological setting and geochemical characterization. J. Volcanol. Geotherm. Res. 2006, 156, 135–157. [Google Scholar] [CrossRef]
- Evan, M.S.; Steven, B.S.; Fabrizio, N.; Emma, S.B.; Jianhua, W.; Stephen, H.R.; Wuyi, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar]
- Hilton, D.R.; McMurtry, G.H.; Kreulen, R. Evidence for extensive degassing of the Hawaiian mantle plume from helium-carbon relationships at Kilauea volcano. Geophy. Res. Letts. 1997, 24, 3065–3068. [Google Scholar] [CrossRef]
- Griffin, W.L.; Huang, J.X.; Thomassot, E.; Gain, S.E.M.; Toledo, V.; Suzanne, Y.O. Super-reducing conditions in ancient and modern volcanic systems: Sources and behaviour of carbon-rich fluids in the lithospheric mantle. Mineral. Petrol. 2018, 112, 1–14. [Google Scholar] [CrossRef]
- Johnson, A.P.; Cleaves, H.J.; Dworkin, J.P.; Glavin, D.P.; Lazcano, A.; Bada, J.L. The Miller volcanic spark discharge experiment. Science 2008, 322, 404. [Google Scholar] [CrossRef] [PubMed]
- Bada, J.L. New insights into prebiotic chemistry from Stanley Miller’s spark discharge experiments. Chem. Soc. Rev. 2013, 42, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Scheu, B.; Dingwell, D.B.; Cimarelli, C.; Bada, J.; Chalmers, J.H.; Burton, A.S. Prebiotic Synthesis in Volcanic Discharges: Exposing Ash to Volcanic/Primordial Gas Atmospheres. In Proceedings of the American Geophysical Union Fall Meeting, New Orleans, LA, USA, 11–15 December 2017. [Google Scholar]
- Woodcock, A.H. Hawaiian alpine lake level, rainfall trends, and spring flow. Pac. Sci. 1980, 34, 195–209. [Google Scholar]
- Woodcock, A.H.; Rubin, M.; Duce, R.A. Deep layer of sediments in alpinelake in the tropical mid-Pacific. Science 1966, 154, 647–648. [Google Scholar] [CrossRef] [PubMed]
- Delparte, D.M.; Belt, M.; Nishioka, C.; Turner, N.R.; Richardson, R.T.; Ericksen, T. Monitoring tropical alpine lake levels in a culturallysensitive environment utilizing 3D technological approaches. Arct. Antarct. Alp. Res. 2014, 46, 709–718. [Google Scholar] [CrossRef]
- Massey, J.E. Physicochemical influences on phytoplankton production in a tropical alpine lake. Archiv. Hydrobiol. 1981, 91, 133–143. [Google Scholar]
- Becker, S.; Schneider, C.; Okamura, H.; Crisp, A.; Amatov, T.; Dejmek, M.; Carell, T. Wet-dry cycles enable the parallel origin of canonical and non-canonical nucleosides by continuous synthesis. Nat. Commun. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dott, R.H., Jr.; Dalziel., W.D. Darwin the geologist in southern South America. Earth Sci. Hist. 2016, 35, 303–345. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bada, J.L.; Korenaga, J. Exposed Areas Above Sea Level on Earth >3.5 Gyr Ago: Implications for Prebiotic and Primitive Biotic Chemistry. Life 2018, 8, 55. https://doi.org/10.3390/life8040055
Bada JL, Korenaga J. Exposed Areas Above Sea Level on Earth >3.5 Gyr Ago: Implications for Prebiotic and Primitive Biotic Chemistry. Life. 2018; 8(4):55. https://doi.org/10.3390/life8040055
Chicago/Turabian StyleBada, Jeffrey L., and Jun Korenaga. 2018. "Exposed Areas Above Sea Level on Earth >3.5 Gyr Ago: Implications for Prebiotic and Primitive Biotic Chemistry" Life 8, no. 4: 55. https://doi.org/10.3390/life8040055




