Profiling Murchison Soluble Organic Matter for New Organic Compounds with APPI- and ESI-FT-ICR MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. ESI Analyses
2.2. APPI Analysis
2.3. Data Treatment
3. Results and Discussion
3.1. (−) ESI FT-ICR MS Analysis
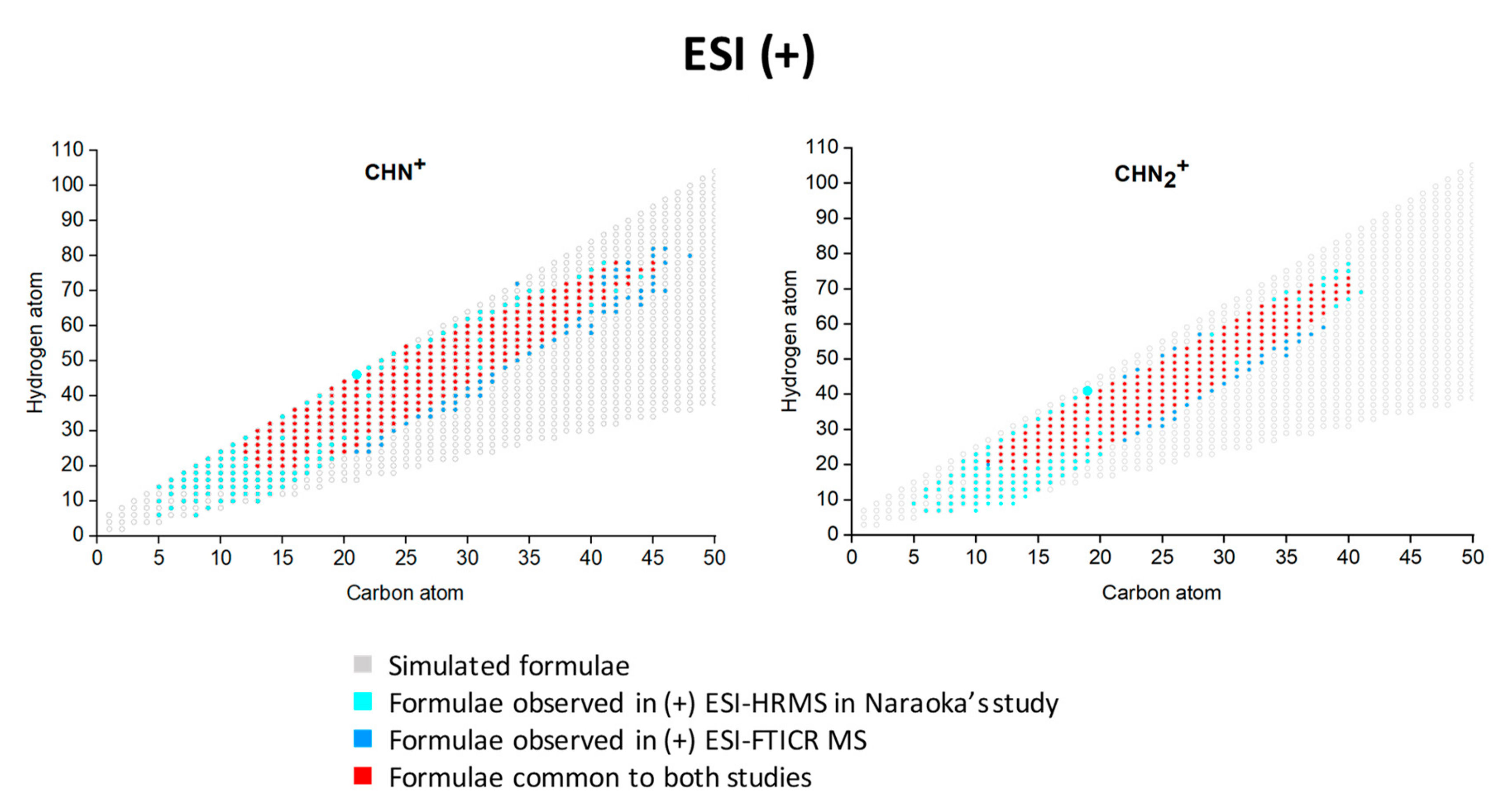
3.2. (+) ESI FT-ICR MS Analysis
3.3. Contribution of (+) APPI FT-ICR MS Analysis
3.4. Comparison of the Achieved Data
3.4.1. Contribution of the (−) ESI FT-ICR MS Analysis
3.4.2. Contribution of the (+) ESI FT-ICR MS Analysis
3.4.3. Contribution of the (+) APPI FT-ICR MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lauretta, D.S.; McSween, H.Y. Meteorites and the Early Solar System II; University of Arizona Press: Tucson, AZ, USA, 2006. [Google Scholar]
- Davis, A.M. Meteorites, Comets, and Planets: Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, M.P.; Burton, A.S.; Elsila, J.E.; Baker, E.M.; Smith, K.E.; Glavin, D.P.; Dworkin, J.P. A search for amino acids and nucleobases in the Martian meteorite Roberts Massif 04262 using liquid chromatography-mass spectrometry. Meteorit. Planet. Sci. 2013, 48, 786–795. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.P.; Middleditch, B.S.; Oró, J. Polycyclic aromatic hydrocarbons in the Murchison meteorite. Org. Geochem. 1984, 5, 211–216. [Google Scholar] [CrossRef]
- Callahan, M.P.; Martin, M.G.; Burton, A.S.; Glavin, D.P.; Dworkin, J.P. Amino acid analysis in micrograms of meteorite sample by nanoliquid chromatography–high-resolution mass spectrometry. J. Chromatogr. A 2014, 1332, 30–34. [Google Scholar] [CrossRef]
- Pering, K.L.; Ponnamperuma, C. Aromatic Hydrocarbons in the Murchison Meteorite. Science 1971, 173, 237–239. [Google Scholar] [CrossRef]
- Hertkorn, N.; Harir, M.; Schmitt-Kopplin, P. Nontarget analysis of Murchison soluble organic matter by high-field NMR spectroscopy and FTICR mass spectrometry. Magn. Reson. Chem. 2015, 53, 754–768. [Google Scholar] [CrossRef]
- Gardinier, A.; Derenne, S.; Robert, F.; Behar, F.; Largeau, C.; Maquet, J. Solid state CP/MAS C-13 NMR of the insoluble organic matter of the Orgueil and Murchison meteorites: Quantitative study. Earth Planet. Sci. Lett. 2000, 184, 9–21. [Google Scholar] [CrossRef]
- Cody, G.D.; Alexander, C.M.O.; Tera, F. Solid-state (H-1 and C-13) nuclear magnetic resonance spectroscopy of insoluble organic residue in the Murchison meteorite: A self-consistent quantitative analysis. Geochim. Cosmochim. Acta 2002, 66, 1851–1865. [Google Scholar] [CrossRef]
- Cronin, J.R.; Chang, S. Organic Matter in Meteorites: Molecular and Isotopic Analyses of the Murchison Meteorite. In The Chemistry of Life’s Origins; Greenberg, J.M., Mendoza-Gómez, C.X., Pirronello, V., Eds.; NATO ASI Series; Springer Netherlands: Dordrecht, The Netherlands, 1993; pp. 209–258. [Google Scholar]
- Pizzarello, S.; Shock, E. The Organic Composition of Carbonaceous Meteorites: The Evolutionary Story Ahead of Biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Hertkorn, N.; Frommberger, M.; Witt, M.; Koch, B.P.; Schmitt-Kopplin, P.; Perdue, E.M. Natural Organic Matter and the Event Horizon of Mass Spectrometry. Anal. Chem. 2008, 80, 8908–8919. [Google Scholar] [CrossRef]
- Hertzog, J.; Carré, V.; Le Brech, Y.; Mackay, C.L.; Dufour, A.; Mašek, O.; Aubriet, F. Combination of electrospray ionization, atmospheric pressure photoionization and laser desorption ionization Fourier transform ion cyclotronic resonance mass spectrometry for the investigation of complex mixtures—Application to the petroleomic analysis of bio-oils. Anal. Chim. Acta 2017, 969, 26–34. [Google Scholar]
- Hertzog, J.; Carré, V.; Jia, L.; Mackay, C.L.; Pinard, L.; Dufour, A.; Mašek, O.; Aubriet, F. Catalytic Fast Pyrolysis of Biomass over Microporous and Hierarchical Zeolites: Characterization of Heavy Products. ACS Sustain. Chem. Eng. 2018, 6, 4717–4728. [Google Scholar] [CrossRef]
- Lababidi, S.; Schrader, W. Online normal-phase high-performance liquid chromatography/Fourier transform ion cyclotron resonance mass spectrometry: Effects of different ionization methods on the characterization of highly complex crude oil mixtures. Rapid Commun. Mass Spectrom. 2014, 28, 1345–1352. [Google Scholar] [CrossRef]
- Jiang, B.; Liang, Y.; Xu, C.; Zhang, J.; Hu, M.; Shi, Q. Polycyclic Aromatic Hydrocarbons (PAHs) in Ambient Aerosols from Beijing: Characterization of Low Volatile PAHs by Positive-Ion Atmospheric Pressure Photoionization (APPI) Coupled with Fourier Transform Ion Cyclotron Resonance. Environ. Sci. Technol. 2014, 48, 4716–4723. [Google Scholar] [CrossRef]
- Bae, E.; Na, J.-G.; Chung, S.H.; Kim, H.S.; Kim, S. Identification of about 30 000 Chemical Components in Shale Oils by Electrospray Ionization (ESI) and Atmospheric Pressure Photoionization (APPI) Coupled with 15 T Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (FT-ICR MS) and a Comparison to Conventional Oil. Energy Fuels 2010, 24, 2563–2569. [Google Scholar]
- Ruf, A.; Kanawati, B.; Hertkorn, N.; Yin, Q.-Z.; Moritz, F.; Harir, M.; Lucio, M.; Michalke, B.; Wimpenny, J.; Shilobreeva, S.; et al. Previously unknown class of metalorganic compounds revealed in meteorites. Proc. Natl. Acad. Sci. USA 2017, 114, 2819–2824. [Google Scholar] [CrossRef] [Green Version]
- Kanawati, B.; Bader, T.M.; Wanczek, K.-P.; Li, Y.; Schmitt-Kopplin, P. Fourier transform (FT)-artifacts and power-function resolution filter in Fourier transform mass spectrometry. Rapid Commun. Mass Spectrom. 2017, 31, 1607–1615. [Google Scholar] [CrossRef]
- Tziotis, D.; Hertkorn, N.; Schmitt-Kopplin, P. Kendrick-analogous network visualisation of ion cyclotron resonance Fourier transform mass spectra: Improved options for the assignment of elemental compositions and the classification of organic molecular complexity. Eur. J. Mass Spectrom. Chichester Engl. 2011, 17, 415–421. [Google Scholar] [CrossRef]
- Naraoka, H.; Hashiguchi, M. In situ organic compound analysis on a meteorite surface by desorption electrospray ionization coupled with an Orbitrap mass spectrometer. Rapid Commun. Mass Spectrom. 2018, 32, 959–964. [Google Scholar] [CrossRef]
- Naraoka, H.; Yamashita, Y.; Yamaguchi, M.; Orthous-Daunay, F.-R. Molecular Evolution of N-Containing Cyclic Compounds in the Parent Body of the Murchison Meteorite. ACS Earth Space Chem. 2017, 1, 540–550. [Google Scholar] [CrossRef]
- Hashiguchi, M.; Naraoka, H. High-mass resolution molecular imaging of organic compounds on the surface of Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 452–468. [Google Scholar] [CrossRef]
- Yamashita, Y.; Naraoka, H. Two homologous series of alkylpyridines in the Murchison meteorite. Geochem. J. 2014, 48, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Oró, J.; Gibert, J.; Lichtenstein, H.; Wikstrom, S.; Flory, D.A. Amino-acids, Aliphatic and Aromatic Hydrocarbons in the Murchison Meteorite. Nature 1971, 230, 105. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Feng, X.; Epstein, S.; Cronin, J. Isotopic Analyses of Nitrogenous Compounds from the Murchison Meteorite—Ammonia, Amines, Amino-Acids, and Polar Hydrocarbons. Geochim. Cosmochim. Acta 1994, 58, 5579–5587. [Google Scholar] [CrossRef]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochim. Cosmochim. Acta 2014, 141, 331–345. [Google Scholar] [CrossRef] [Green Version]
- Swamy, K.S.K. Physics of Comets; World Scientific: Singapore, 2010. [Google Scholar]
- Osinski, G.R.; Kring, D.A. Large Meteorite Impacts and Planetary Evolution V; Geological Society of America: Boulder, CO, USA, 2015. [Google Scholar]
- Pizzarello, S. Hydrogen Cyanide in the Murchison Meteorite. Astrophys. J. 2012, 754, L27. [Google Scholar] [CrossRef]
- Dickens, J.E.; Irvine, W.M.; DeVries, C.H.; Ohishi, M. Hydrogenation of Interstellar Molecules: A Survey for Methylenimine (CH2NH). Astrophys. J. 1997, 479, 307–312. [Google Scholar] [CrossRef]
- Shimoyama, A. Complex organics in meteorites. Adv. Space Res. 1997, 19, 1045–1052. [Google Scholar] [CrossRef]
- Bandurski, E.L.; Nagy, B. The polymer-like organic material in the Orgueil meteorite. Geochim. Cosmochim. Acta 1976, 40, 1397–1406. [Google Scholar] [CrossRef]
- Danger, G.; Borget, F.; Chomat, M.; Duvernay, F.; Theulé, P.; Guillemin, J.-C.; d’Hendecourt, L.L.S.; Chiavassa, T. Experimental investigation of aminoacetonitrile formation through the Strecker synthesis in astrophysical-like conditions: Reactivity of methanimine (CH2NH), ammonia (NH3), and hydrogen cyanide (HCN). Astron. Astrophys. 2011, 535, A47. [Google Scholar] [CrossRef]
- Saladino, R.; Crestini, C.; Ciciriello, F.; Costanzo, G.; Di Mauro, E. About a Formamide-Based Origin of Informational Polymers: Syntheses of Nucleobases and Favourable Thermodynamic Niches for Early Polymers. Orig. Life Evol. Biosph. 2006, 36, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Saladino, R.; Botta, G.; Delfino, M.; Di Mauro, E. Meteorites as Catalysts for Prebiotic Chemistry. Chem. Eur. J. 2013, 19, 16916–16922. [Google Scholar] [CrossRef] [PubMed]
- Rotelli, L.; Trigo-Rodríguez, J.M.; Moyano-Cambero, C.E.; Carota, E.; Botta, L.; Di Mauro, E.; Saladino, R. The key role of meteorites in the formation of relevant prebiotic molecules in a formamide/water environment. Sci. Rep. 2016, 6, 38888. [Google Scholar] [CrossRef] [PubMed]
- Ferus, M.; Nesvorny, D.; Sponer, J.; Kubelik, P.; Michalcikova, R.; Shestivska, V.; Sponer, J.E.; Civis, S. High-energy chemistry of formamide: A unified mechanism of nucleobase formation. Proc. Natl. Acad. Sci. USA 2015, 112, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Purcell, J.M.; Hendrickson, C.L.; Rodgers, R.P.; Marshall, A.G. Atmospheric Pressure Photoionization Proton Transfer for Complex Organic Mixtures Investigated by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2007, 18, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Yassine, M.M.; Harir, M.; Dabek-Zlotorzynska, E.; Schmitt-Kopplin, P. Structural characterization of organic aerosol using Fourier transform ion cyclotron resonance mass spectrometry: Aromaticity equivalent approach. Rapid Commun. Mass Spectrom. 2014, 28, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, I.; Pillinger, C. Isotopic Compositions of Individual Polycyclic Aromatic-Hydrocarbons from the Murchison Meteorite. Mon. Not. R. Astron. Soc. 1994, 269, 235–240. [Google Scholar] [CrossRef]
- Sephton, M.A.; Love, G.D.; Watson, J.S.; Verchovsky, A.B.; Wright, I.P.; Snape, C.E.; Gilmour, I. Hydropyrolysis of insoluble carbonaceous matter in the Murchison meteorite: New insights into its macromolecular structure11Associate editor: G. D. Cody. Geochim. Cosmochim. Acta 2004, 68, 1385–1393. [Google Scholar] [CrossRef]
- Krishnamurthy, R.V.; Epstein, S.; Cronin, J.R.; Pizzarello, S.; Yuen, G.U. Isotopic and molecular analyses of hydrocarbons and monocarboxylic acids of the Murchison meteorite. Geochim. Cosmochim. Acta 1992, 56, 4045–4058. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Aliphatic hydrocarbons of the Murchison meteorite. Geochim. Cosmochim. Acta 1990, 54, 2859–2868. [Google Scholar] [CrossRef]
- Deamer, D.W. Polycyclic aromatic hydrocarbons: Primitive pigment systems in the prebiotic environment. Adv. Space Res. 1992, 12, 183–189. [Google Scholar] [CrossRef]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Interstellar polycyclic aromatic hydrocarbons—The infrared emission bands, the excitation/emission mechanism, and the astrophysical implications. Astrophys. J. Suppl. Ser. 1989, 71, 733–775. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Kopplin, P.; Englmann, M.; Rossello-Mora, R.; Schiewek, R.; Brockmann, K.J.; Benter, T.; Schmitz, O.J. Combining chip-ESI with APLI (cESILI) as a multimode source for analysis of complex mixtures with ultrahigh-resolution mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 2803–2809. [Google Scholar] [CrossRef] [Green Version]




| Heteroatom Class | ESI (−) | ESI (+) | APPI (+) |
|---|---|---|---|
| CH | 12 (0.1%) | 4 (0.1%) | 402 (9.2%) |
| CHO | 1530 (13.3%) | 6 (0.1%) | 1368 (31.4%) |
| CHONa | - | 285 (6.4%) | - |
| CHOS | 2544 (22%) | 3 (0.1%) | 286 (6.5%) |
| CHN | 150 (1.3%) | 747 (16.8%) | 421 (9.7%) |
| CHON | 3544 (30.7%) | 2104 (47.2%) | 1685 (38.6%) |
| CHONS | 3618 (31.3%) | 524 (11.8%) | 198 (4.5%) |
| CHOMg | 151 (1.3%) | 786 (17.6%) | - |
| TOTAL ASSIGNED FEATURES | 11549 | 4459 | 4360 |
| Weighted average O/C | 0.20 | 0.13 | 0.08 |
| Weighted average H/C | 1.67 | 1.74 | 1.71 |
| Weighted average N/C | 0.04 | 0.09 | 0.03 |
| Weighted average S/C | 0.04 | 0.01 | 0.00 |
| Weighted average mass | 320.60 | 444.37 | 353.15 |
| Raw Formula | Putative Compound(s) | |
|---|---|---|
| CH species | C13H14 | Trimethylnaphthalene |
| C14H12 | Methylfluorene | |
| C14H16 | Hexahydrophenanthrene/Hexyhydroanthracene | |
| C15H12 | Methylphenanthrene | |
| C16H16 | Hexyahydropyrene | |
| C16H14 | Dimethlyphenanthrene | |
| C16H10 | Fluoranthene/Pyrene | |
| C17H12 | Methylpyrene/Benzofluorene | |
| C18H12 | Chrysene | |
| C18H14 | Dimethylpyrene | |
| C18H18 | Hexahydrochrysene | |
| C19H14 | Methylchrysene | |
| C19H16 | Trimethylpyrene | |
| C20H12 | Perylene/Benzofluoranthene/Benzopyrene | |
| CHO species | C13H8O | Fluorenone |
| C13H10O | Benzophenone | |
| C14H10O | Anthracenone | |
| C14H8O2 | Anthracenedione | |
| C17H10O | Benzanthrone/Benzofluorenone | |
| C18H10O2 | Benzoanthracenedione |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hertzog, J.; Naraoka, H.; Schmitt-Kopplin, P. Profiling Murchison Soluble Organic Matter for New Organic Compounds with APPI- and ESI-FT-ICR MS. Life 2019, 9, 48. https://doi.org/10.3390/life9020048
Hertzog J, Naraoka H, Schmitt-Kopplin P. Profiling Murchison Soluble Organic Matter for New Organic Compounds with APPI- and ESI-FT-ICR MS. Life. 2019; 9(2):48. https://doi.org/10.3390/life9020048
Chicago/Turabian StyleHertzog, Jasmine, Hiroshi Naraoka, and Philippe Schmitt-Kopplin. 2019. "Profiling Murchison Soluble Organic Matter for New Organic Compounds with APPI- and ESI-FT-ICR MS" Life 9, no. 2: 48. https://doi.org/10.3390/life9020048






