Gingival Crevicular Fluid as a Novel Potential Source of Biomarkers Distinguishes Pubertal from Post-Pubertal Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Subjects
2.3. Collection of GCF
2.4. Sample Preparation
2.5. MALDI-TOF Analysis
2.6. Data Processing and Statistics
3. Results
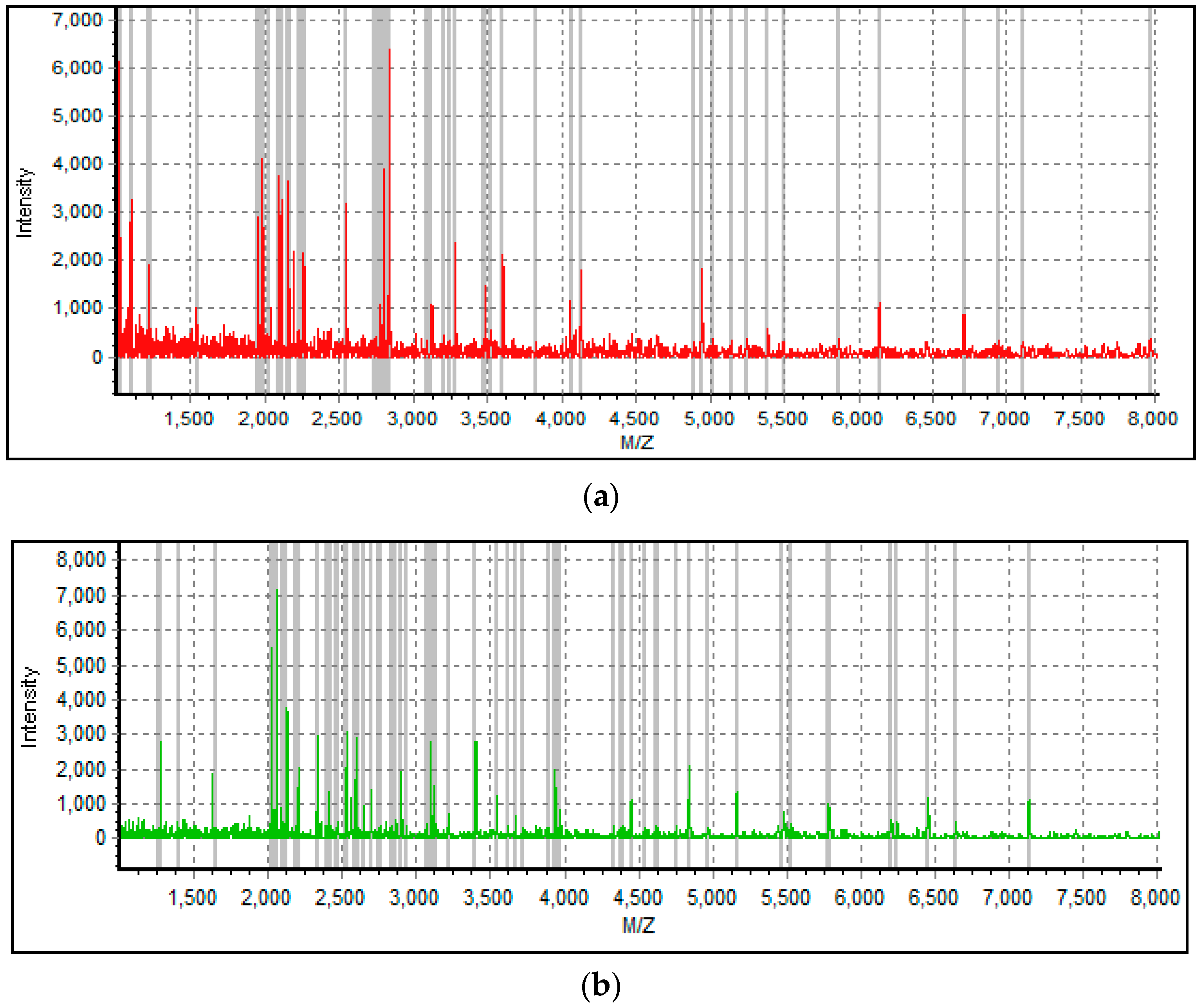
3.1. Subject Information and MALDI-TOF Spectra
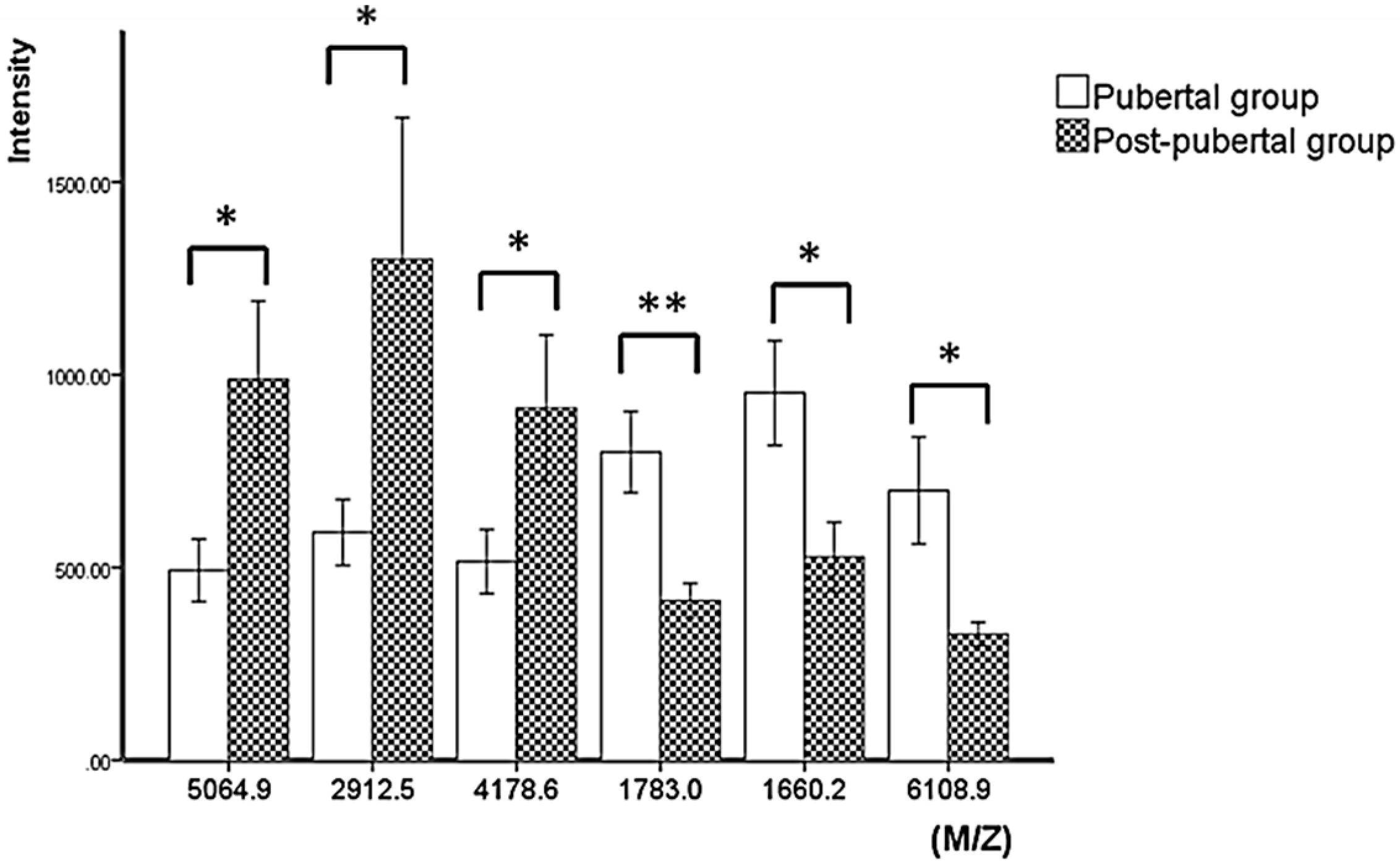
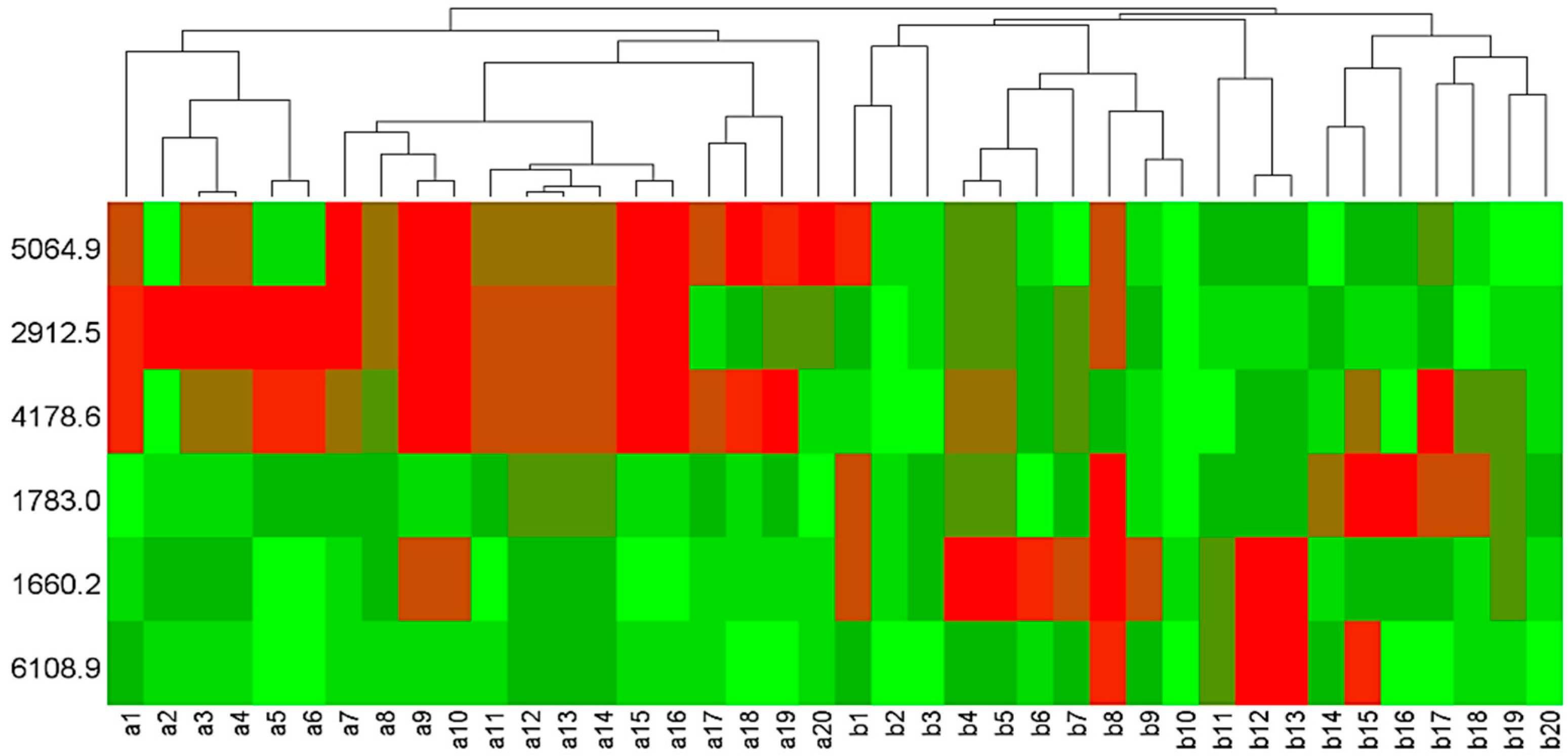
3.2. Statistical Analysis of Mass Peaks
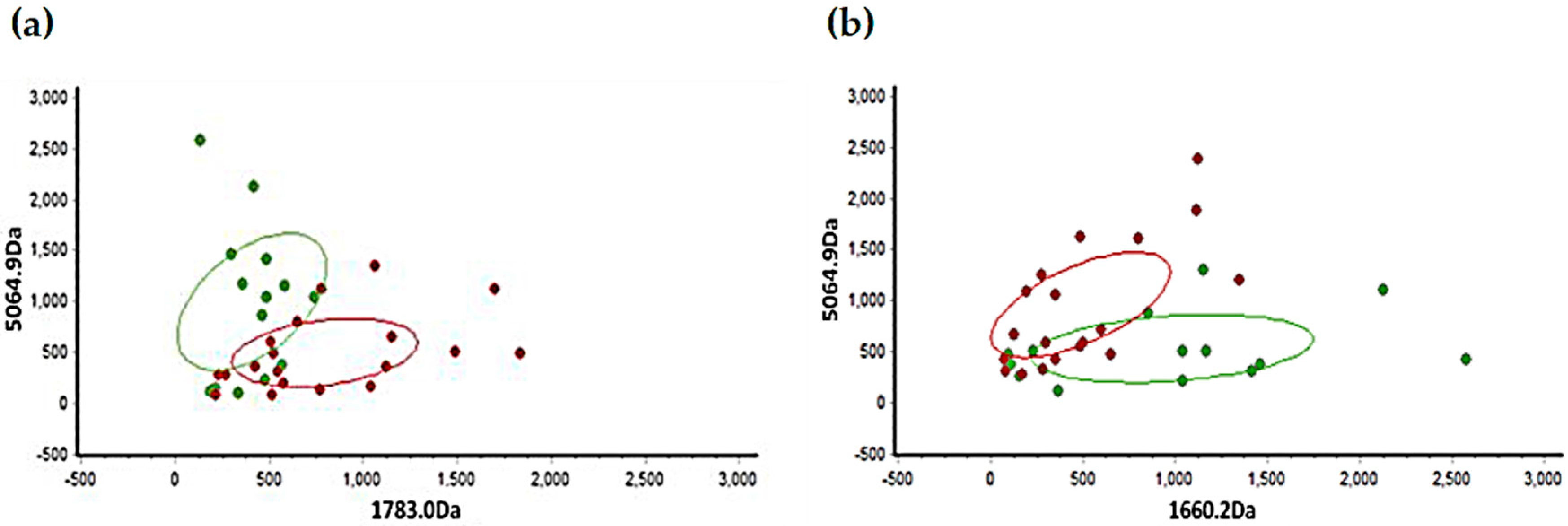
3.3. Validation of Diagnostic Model
4. Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Perinetti, G.; Westphalen, G.H.; Biasotto, M.; Salgarello, S.; Contardo, L. The diagnostic performance of dental maturity for identification of the circumpubertal growth phases: A meta-analysis. Prog. Orthod. 2013, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; Di Lenarda, R.; Contardo, L. Diagnostic performance of combined canine and second molar maturity for identification of growth phase. Prog. Orthod. 2013, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.B.; Southard, K.A.; Qian, F.; Marshall, S.D.; Franciscus, R.G.; Southard, T.E. Cervical vertebrae maturation method: Poor reproducibility. Am. J. Orthod. Dentofac. 2009, 136, 478–480. [Google Scholar] [CrossRef]
- Nestman, T.S.; Marshall, S.D.; Qian, F.; Holton, N.; Franciscus, R.G.; Southard, T.E. Cervical vertebrae maturation method morphologic criteria: Poor reproducibility. Am. J. Orthod. Dentofac. 2011, 140, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; Franchi, L.; Castaldo, A.; Contardo, L. Gingival crevicular fluid protein content and alkaline phosphatase activity in relation to pubertal growth phase. Angle Orthod. 2012, 82, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; Baccetti, T.; Contardo, L.; Di Lenarda, R. Gingival crevicular fluid alkaline phosphatase activity as a non-invasive biomarker of skeletal maturation. Orthod. Craniofac. Res. 2011, 14, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; Baccetti, T.; Di Leonardo, B.; Di Lenarda, R.; Contardo, L. Dentition phase and chronological age in relation to gingival crevicular fluid alkaline phosphatase activity in growing subjects. Prog. Orthod. 2011, 12, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, G.S. Formation, collection and significance of gingival crevice fluid. Periodontology 2003, 31, 32–42. [Google Scholar] [CrossRef]
- Uitto, V.J. Gingival crevice fluid—An introduction. Periodontology 2003, 31, 9–11. [Google Scholar] [CrossRef]
- Chapple, I.L.; Garner, I.; Saxby, M.S.; Moscrop, H.; Matthews, J.B. Prediction and diagnosis of attachment loss by enhanced chemiluminescent assay of crevicular fluid alkaline phosphatase levels. J. Clin. Periodontol. 1999, 26, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Kunjappu, J.J.; Mathew, V.B.; Hegde, S.; Kashyap, R.; Hosadurga, R. Assessment of the alkaline phosphatase level in gingival crevicular fluid, as a biomarker to evaluate the effect of scaling and root planing on chronic periodontitis: An in vivo study. J. Oral Maxillofac. Pathol. 2012, 16, 54–57. [Google Scholar] [PubMed]
- Babur, C.; Ozcan, G.; Cebi, D.U.; Pervane, B.; Ozdemir, B.; Yucel, A.; Biri, A.A.; Babur, C. Gingival crevicular fluid levels of osteoprotegerin (OPG) in premenopausal and postmenopausal women with or without chronic periodontitis. J. Dent. 2012, 40, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; Paolantonio, M.; D’Attilio, M.; D’Archivio, D.; Tripodi, D.; Femminella, B.; Festa, F.; Spoto, G. Alkaline phosphatase activity in gingival crevicular fluid during human orthodontic tooth movement. Am. J. Orthod. Dentofac. 2002, 122, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Perinetti, G.; D’Apuzzo, F.; Contardo, L.; Primozic, J.; Rupel, K.; Perillo, L. Gingival crevicular fluid alkaline phosphate activity during the retention phase of maxillary expansion in prepubertal subjects: A split-mouth longitudinal study. Am. J. Orthod. Dentofac. 2015, 148, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, D.; Avantaggiato, A.; Cura, F.; Girardi, A.; Carinci, F. Biomarkers of periodontl tissue in gingival crevicular fluid during orthodontic movement: An overview. OA Dent. 2014, 2013, 105873. [Google Scholar]
- Zheng, H.; Li, R.; Zhang, J.; Zhou, S.; Ma, Q.; Zhou, Y.; Chen, F.; Lin, J. Salivary biomarkers indicate obstructive sleep apnea patients with cardiovascular diseases. Sci. Rep. 2014, 4, 7046. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Yue, L.; Li, H.; Zhang, J.; Yan, Z.; Fan, Y.; Yang, H.; Liu, Q.; Zhang, D.; Xia, Z.; et al. Diagnostic and prognostic role of serum protein peak at 6449 m/z in gastric adenocarcinoma based on mass spectrometry. Br. J. Cancer 2016, 114, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Husi, H.; Skipworth, R.J.; Cronshaw, A.; Fearon, K.C.; Ross, J.A. Proteomic identification of potential cancer markers in human urine using subtractive analysis. Int. J. Oncol. 2016, 48, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Ma, Q.; Lin, J.; Chen, F. Salivary peptidomic analysis-The extension of Proteomics. J. Mol. Biomark. Diagn. 2013, 2. [Google Scholar] [CrossRef]
- Ngo, L.H.; Darby, I.B.; Veith, P.D.; Locke, A.G.; Reynolds, E.C. Mass spectrometric analysis of gingival crevicular fluid biomarkers can predict periodontal disease progression. J. Periodontal Res. 2013, 48, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Ngo, L.H.; Veith, P.D.; Chen, Y.; Chen, D.; Darby, I.B.; Reynolds, E.C. Mass Spectrometric Analyses of Peptides and Proteins in Human Gingival Crevicular Fluid. J. Proteome Res. 2010, 9, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Maggisano, G.; Lombardo, N.; Montalcini, T.; Paduano, S.; Pelaia, G.; Savino, R.; Terracciano, R. Influence of storage conditions on MALDI-TOF MS profiling of gingival crevicular fluid: Implications on the role of S100A8 and S100A9 for clinical and proteomic based diagnostic investigations. Proteomics 2016, 16, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Preianò, M.; Falcone, D.; Maggisano, G.; Montalcini, T.; Navarra, M.; Paduano, S.; Savino, R.; Terracciano, R. Assessment of pre-analytical and analytical variables affecting peptidome profiling of gingival crevicular fluid by MALDI-TOF mass spectrometry. Clin. Chim. Acta 2014, 437, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, T.; Franchi, L.; Mcnamara, J.J. An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar] [PubMed]
- Cozza, P.; Baccetti, T.; Franchi, L.; De Toffol, L.; Mcnamara, J.J. Mandibular changes produced by functional appliances in Class II malocclusion: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 591–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perinetti, G.; Primožič, J.; Franchi, L.; Contardo, L. Treatment effects of removable functional appliances in pre-pubertal and pubertal class II patients: A systematic review and meta-analysis of controlled studies. PLoS ONE 2015, 10, e141198. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Baccetti, T.; Mcnamara, J.A. Postpubertal assessment of treatment timing for maxillary expansion and protraction therapy followed by fixed appliances. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 555–568. [Google Scholar] [CrossRef]
- Zhao, X.; Lin, J.; Jiang, J.; Wang, Q.; Ng, S.H. Validity and reliability of a method for assessment of cervical vertebral maturation. Angle Orthod. 2012, 82, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Ramberg, P.; Wahlander, Å.; Grossman, J.; Jönsson, D.; Barnes, V.M.; Papapanou, P.N. Label-Free Quantitative Proteomics Reveals Differentially Regulated Proteins in Experimental Gingivitis. J. Proteome Res. 2013, 12, 657–678. [Google Scholar] [CrossRef] [PubMed]
- Baliban, R.C.; Sakellari, D.; Li, Z.; Dimaggio, P.A.; Garcia, B.A.; Floudas, C.A. Novel protein identification methods for biomarker discovery via a proteomic analysis of periodontally healthy and diseased gingival crevicular fluid samples. J. Clin. Periodontol. 2012, 39, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bostanci, N.; Heywood, W.; Mills, K.; Parkar, M.; Nibali, L.; Donos, N. Application of Label-Free Absolute Quantitative Proteomics in Human Gingival Crevicular Fluid by LC/MSE (Gingival Exudatome). J. Proteome Res. 2010, 9, 2191–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baliban, R.C.; Sakellari, D.; Li, Z.; Guzman, Y.A.; Garcia, B.A.; Floudas, C.A. Discovery of biomarker combinations that predict periodontal health or disease with high accuracy from GCF samples based on high-throughput proteomic analysis and mixed-integer linear optimization. J. Clin. Periodontol. 2013, 40, 131–139. [Google Scholar] [CrossRef] [PubMed]
| Pubertal Group | |||
| Gender | Number | Mean Age (Years) | Mean Concentration (ug/mL) |
| Male | 10 | 10.6 ± 1.5 | 275.3 ± 73.6 |
| Female | 10 | 10.8 ± 1.1 | 224.8 ± 78.8 |
| Post-Pubertal Group | |||
| Gender | Number | Mean Age (Years) | Mean Concentration (ug/mL) |
| Male | 10 | 23.3 ± 0.48 | 353.4 ± 86.6 |
| Female | 10 | 23.2 ± 0.78 | 237.8 ± 79.5 |
| Models | 1 | 2 | 3 | Average |
|---|---|---|---|---|
| Fishert | 84.62% (11/13) | 84.62% (11/13) | 85.7% (12/14) | 84.98% |
| KNN | 76.92% (10/13) | 84.62% (11/13) | 85.7% (12/14) | 82.41% |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, X.; Gu, Y.; Chen, F. Gingival Crevicular Fluid as a Novel Potential Source of Biomarkers Distinguishes Pubertal from Post-Pubertal Subjects. Diagnostics 2016, 6, 41. https://doi.org/10.3390/diagnostics6040041
Wen X, Gu Y, Chen F. Gingival Crevicular Fluid as a Novel Potential Source of Biomarkers Distinguishes Pubertal from Post-Pubertal Subjects. Diagnostics. 2016; 6(4):41. https://doi.org/10.3390/diagnostics6040041
Chicago/Turabian StyleWen, Xi, Yan Gu, and Feng Chen. 2016. "Gingival Crevicular Fluid as a Novel Potential Source of Biomarkers Distinguishes Pubertal from Post-Pubertal Subjects" Diagnostics 6, no. 4: 41. https://doi.org/10.3390/diagnostics6040041





