Complex Organics in Space: A Changing View of the Cosmos
Abstract
:1. Introduction
2. Inorganic Mineral Solids in Space
3. Molecules in Space
4. Organics in the Solar System
5. Organics as Carriers of Unexplained Spectral Phenomena in the Interstellar Medium
5.1. Diffuse Interstellar Bands
5.2. 220 nm Feature
5.3. Extended Red Emission
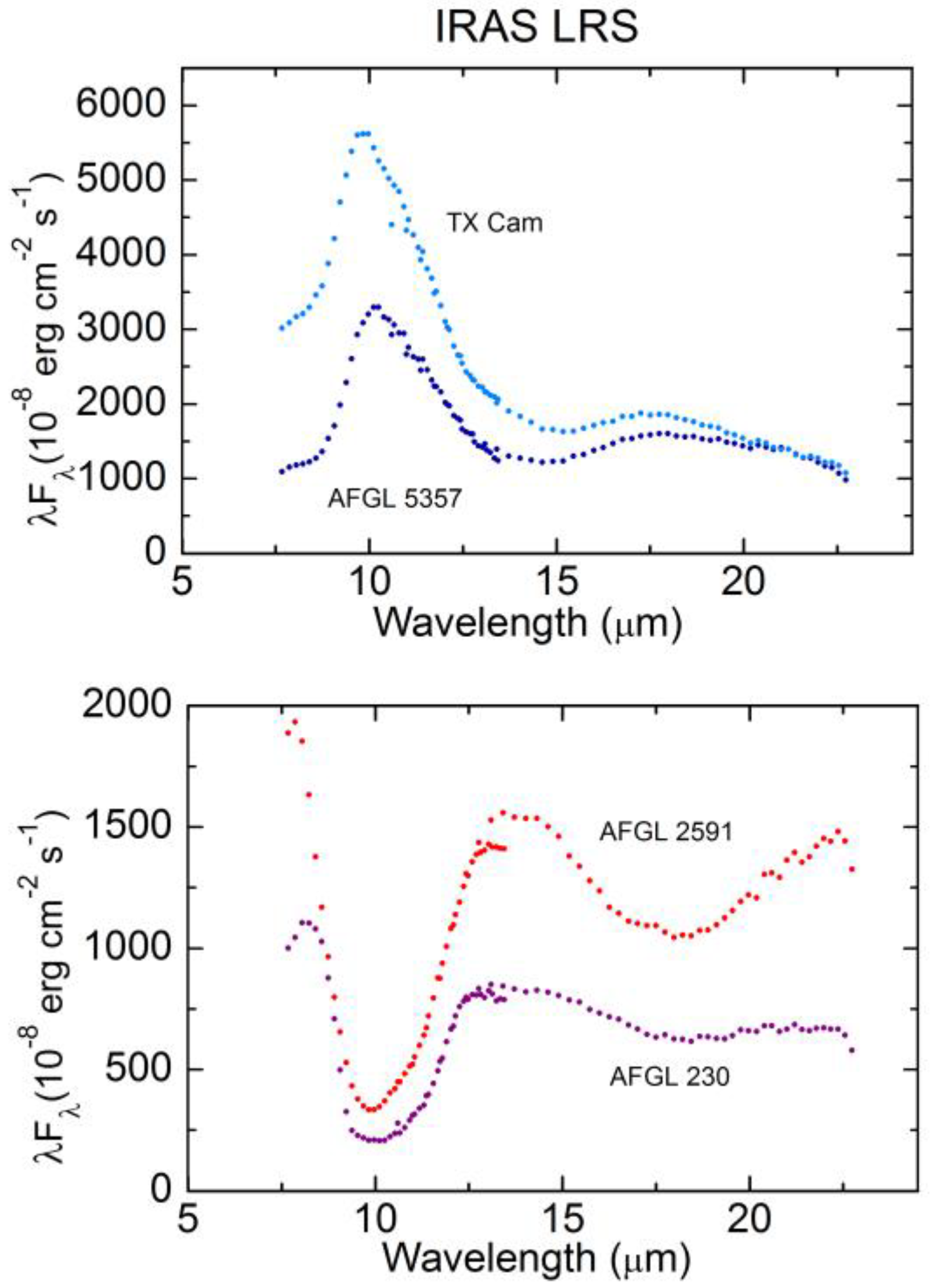
5.4. Bands of 21 and 30 µm
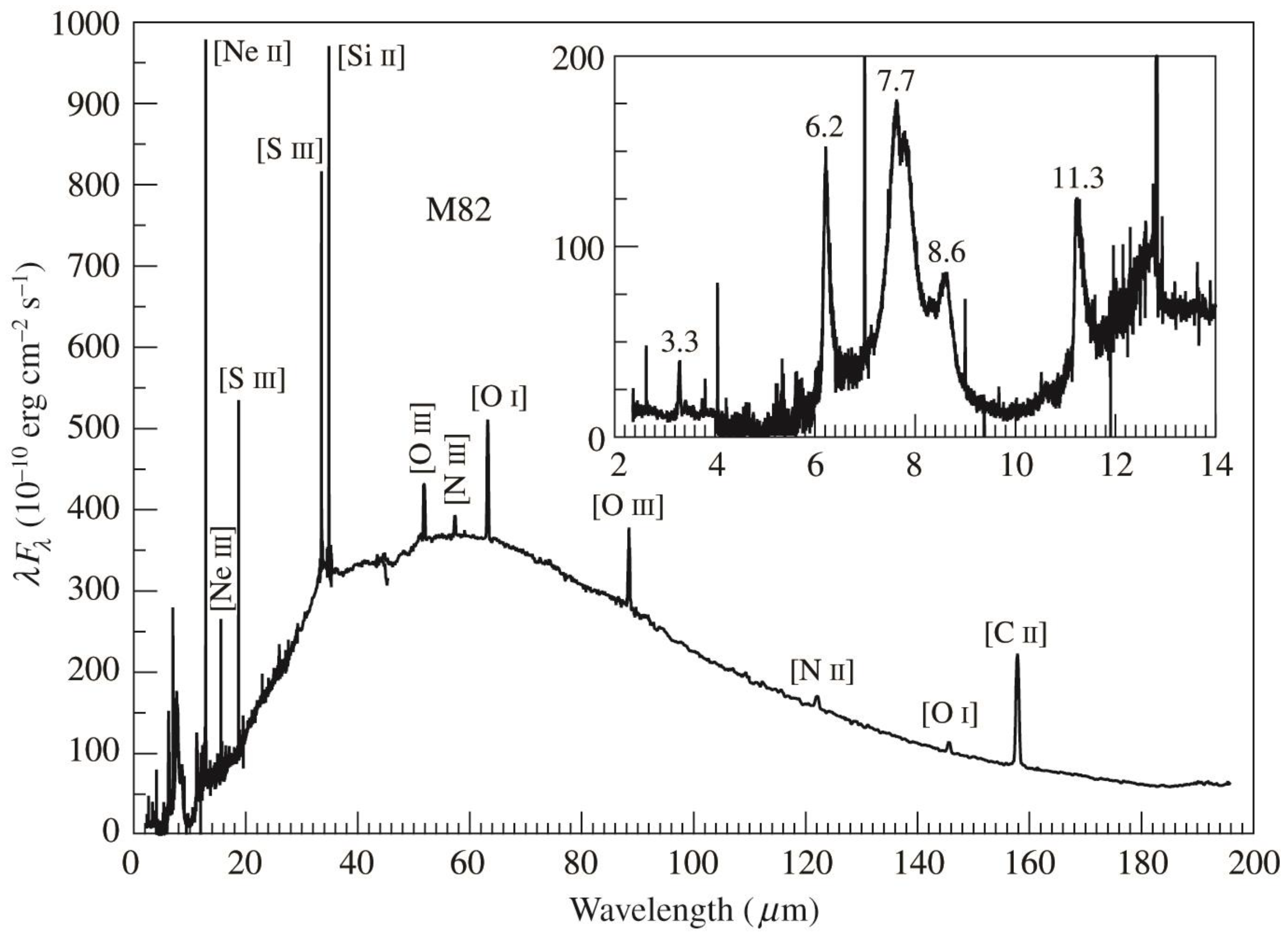
5.5. Unidentified Infrared Emission Bands
6. Aliphatic Organics in the Diffuse Interstellar Medium
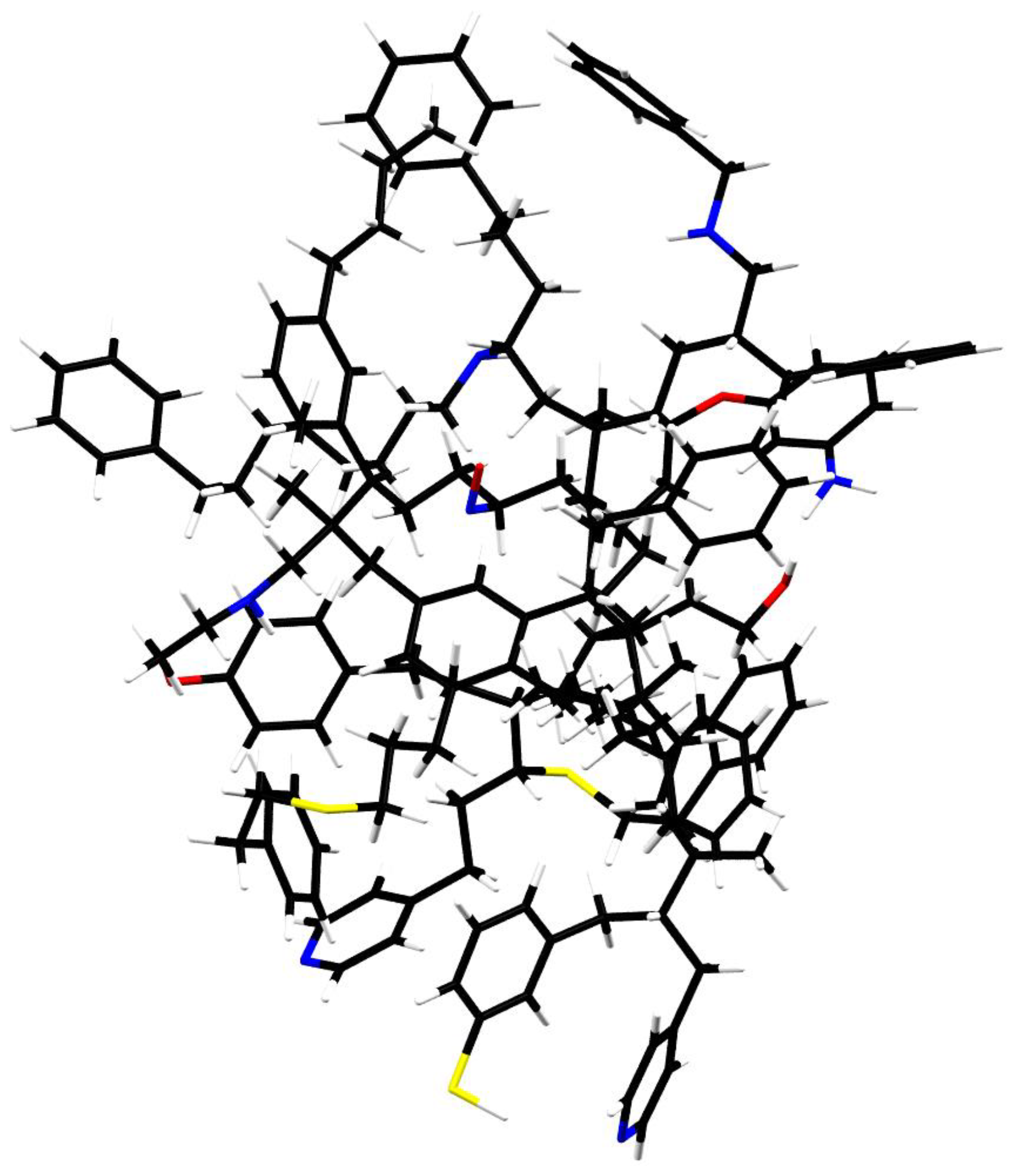
7. Circumstellar Synthesis of Mixed Aromatic/Aliphatic Nanoparticles
8. Abiotic Synthesis of Complex Organics
9. Organics beyond the Milky Way
10. Relationship between Terrestrial and Cosmic Organics
11. Conclusions and Future Prospects
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
| 1 | For details on the radiation mechanisms of atoms, molecules, and solids, see the following publication: Kwok, S. Physics and Chemistry of the Interstellar Medium; University Science Books: 2006. |
References
- Groves, B.; Dopita, M.A.; Sutherland, R.S.; Kewley, L.J.; Fischera, J.; Leitherer, C.; Brandl, B.; van Breugel, W. Modeling the Pan-Spectral Energy Distribution of Starburst Galaxies. IV. The Controlling Parameters of the Starburst SED. Astrophys. J. Suppl. Ser. 2008, 176, 438–456. [Google Scholar] [CrossRef]
- Woolf, N.J.; Ney, E.P. Circumstellar Infrared Emission from Cool Stars. Astrophys. J. 1969, 155, L181–L184. [Google Scholar] [CrossRef]
- Treffers, R.; Cohen, M. High-resolution spectra of cool stars in the 10- and 20-micron regions. Astrophys. J. 1974, 188, 545–552. [Google Scholar] [CrossRef]
- Kwok, S.; Volk, K.; Bidelman, W.P. Classification and Identification of IRAS Sources with Low-Resolution Spectra. Astrophys. J. Suppl. Ser. 1997, 112, 557–584. [Google Scholar] [CrossRef]
- Donn, B. Polycyclic Hydrocarbons, Platt Particles, and Interstellar Extinction. Astrophys. J. 1968, 152, L129. [Google Scholar] [CrossRef]
- Hoyle, F.; Wickramasinghe, N.C. Polysaccharides and infrared spectra of galactic sources. Nature 1977, 268, 610–612. [Google Scholar] [CrossRef]
- Sagan, C.; Khare, B.N. Tholins: Organic chemistry of interstellar grains and gas. Nature 1979, 277, 102–107. [Google Scholar] [CrossRef]
- McKellar, A. Evidence for the Molecular Origin of Some Hitherto Unidentified Interstellar Lines. Publ. Astron. Soc. Pac. 1940, 52, 187. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C(60): Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Cami, J.; Bernard-Salas, J.; Peeters, E.; Malek, S.E. Detection of C60 and C70 in a Young Planetary Nebula. Science 2010, 329, 1180–1182. [Google Scholar] [CrossRef]
- Sellgren, K.; Werner, M.W.; Ingalls, J.G.; Smith, J.D.T.; Carleton, T.M.; Joblin, C. C60 in Reflection Nebulae. Astrophys. J. Lett. 2010, 722, L54–L57. [Google Scholar] [CrossRef]
- Dame, T.M.; Hartmann, D.; Thaddeus, P. The Milky Way in Molecular Clouds: A New Complete CO Survey. Astrophys. J. 2001, 547, 792. [Google Scholar] [CrossRef]
- Morokuma-Matsui, K.; Bekki, K.; Wang, J.; Serra, P.; Koyama, Y.; Morokuma, T.; Egusa, F.; For, B.-Q.; Nakanishi, K.; Koribalski, B.S.; et al. CO(J = 1–0) Mapping Survey of 64 Galaxies in the Fornax Cluster with the ALMA Morita Array. Astrophys. J. Suppl. Ser. 2022, 263, 40. [Google Scholar] [CrossRef]
- Heyer, M.; Dame, T.M. Molecular Clouds in the Milky Way. Annu. Rev. Astron. Astrophys. 2015, 53, 583–629. [Google Scholar] [CrossRef]
- Kwok, S. The synthesis of organic and inorganic compounds in evolved stars. Nature 2004, 430, 985–991. [Google Scholar] [CrossRef]
- Nagy, B.; Meinschein, W.G.; Hennessy, D.J. Mass spectroscopic analysis of the Orgueil meteorite: Evidence for biogenic hydrocarbons. Ann. N. Y. Acad. Sci. 1961, 93, 27–35. [Google Scholar] [CrossRef]
- Schmitt-Kopplin, P.; Gabelica, Z.; Gougeon, R.D.; Fekete, A.; Kanawati, B.; Harir, M.; Gebefuegi, I.; Eckel, G.; Hertkorn, N. High molecular diversity of extraterrestrial organic matter in Murchison meteorite revealed 40 years after its fall. Proc. Natl. Acad. Sci. USA 2010, 107, 2763–2768. [Google Scholar] [CrossRef]
- Pizzarello, S.; Shock, E. The Organic Composition of Carbonaceous Meteorites: The Evolutionary Story Ahead of Biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef]
- Cody, G.D.; Heying, E.; Alexander, C.M.O.; Nittler, L.R.; Kilcoyne, A.L.D.; Sandford, S.A.; Stroud, R.M. Establishing a molecular relationship between chrondritic and cometary organic solids. Proc. Natl. Acad. Sci. USA 2011, 108, 19171–19176. [Google Scholar] [CrossRef]
- Sandford, S.A.; Aleon, J.; Alexander, C.M.O.D.; Araki, T.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Brucato, J.R.; et al. Organics captured from comet 81P/Wild 2 by the Stardust cpacecraft. Science 2006, 314, 1720–1724. [Google Scholar] [CrossRef]
- Fray, N.; Bardyn, A.; Cottin, H.; Altwegg, K.; Baklouti, D.; Briois, C.; Colangeli, L.; Engrand, C.; Fischer, H.; Glasmachers, A.; et al. High-molecular-weight organic matter in the particles of comet 67P/Churyumov-Gerasimenko. Nature 2016, 538, 72–74. [Google Scholar] [CrossRef] [PubMed]
- Flynn, G.J.; Keller, L.P.; Feser, M.; Wirick, S.; Jacobsen, C. The origin of organic matter in the solar system: Evidence from the interplanetary dust particles. Geochim. Cosmochim. Acta 2003, 67, 4791–4806. [Google Scholar] [CrossRef]
- Dalle Ore, C.M.; Fulchignoni, M.; Cruikshank, D.P.; Barucci, M.A.; Brunetto, R.; Campins, H.; de Bergh, C.; Debes, J.H.; Dotto, E.; Emery, J.P.; et al. Organic materials in planetary and protoplanetary systems: Nature or nurture? A&A 2011, 533, A98. [Google Scholar]
- De Sanctis, M.C.; Ammannito, E.; McSween, H.Y.; Raponi, A.; Marchi, S.; Capaccioni, F.; Capria, M.T.; Carrozzo, F.G.; Ciarniello, M.; Fonte, S.; et al. Localized aliphatic organic material on the surface of Ceres. Science 2017, 355, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Yada, T.; Abe, M.; Okada, T.; Nakato, A.; Yogata, K.; Miyazaki, A.; Hatakeda, K.; Kumagai, K.; Nishimura, M.; Hitomi, Y.; et al. Preliminary analysis of the Hayabusa2 samples returned from C-type asteroid Ryugu. Nat. Astron. 2021, 6, 214–220. [Google Scholar] [CrossRef]
- Kebukawa, Y.; Quirico, E.; Dartois, E.; Yabuta, H.; Bejach, L.; Bonal, L.; Dazzi, A.; Deniset-Besseau, A.; Duprat, J.; Engrand, C.; et al. Infrared absorption spectra from organic matter in the asteroid Ryugu samples: Some unique properties compared to unheated carbonaceous chondrites. Meteorit. Planet. Sci. [CrossRef]
- Cruikshank, D.P.; Clemett, S.J.; Grundy, W.M.; Stern, S.A.; Olkin, C.B.; Binzel, R.P.; Cook, J.C.; Dalle Ore, C.M.; Earle, A.M.; Smith-Ennico, K.; et al. Pluto and Charon: The Non-Ice Surface Component. In Proceedings of the 47th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 1 March 2016; p. 1700. [Google Scholar]
- Lorenz, R.D.; Mitchell, K.L.; Kirk, R.L.; Hayes, A.G.; Aharonson, O.; Zebker, H.A.; Paillou, P.; Radebaugh, J.; Lunine, J.I.; Janssen, M.A.; et al. Titan’s inventory of organic surface materials. Geophys. Res. Lett. 2008, 35, 2206. [Google Scholar] [CrossRef]
- Eigenbrode, J.L.; Summons, R.E.; Steele, A.; Freissinet, C.; Millan, M.; Navarro-González, R.; Sutter, B.; McAdam, A.C.; Franz, H.B.; Glavin, D.P.; et al. Organic matter preserved in 3-billion-year-old mudstones at Gale crater, Mars. Science 2018, 360, 1096–1101. [Google Scholar] [CrossRef]
- Postberg, F.; Khawaja, N.; Abel, B.; Choblet, G.; Glein, C.R.; Gudipati, M.S.; Henderson, B.L.; Hsu, H.-W.; Kempf, S.; Klenner, F.; et al. Macromolecular organic compounds from the depths of Enceladus. Nature 2018, 558, 564–568. [Google Scholar] [CrossRef]
- Koga, T.; Naraoka, H. A new family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef]
- Rubin, A.E.; Trigo-Rodriguez, J.M.; Kunihiro, T.; Kallemeyn, G.W.; Wasson, J.T. Carbon-rich chondritic clast PV1 from the Plainview H-chondrite regolith breccia: Formation from H3 chondrite material by possible cometary impact. Geochim. Cosmochim. Acta 2005, 69, 3419–3430. [Google Scholar] [CrossRef]
- Rotelli, L.; Trigo-Rodríguez, J.M.; Moyano-Cambero, C.E.; Carota, E.; Botta, L.; Di Mauro, E.; Saladino, R. The key role of meteorites in the formation of relevant prebiotic molecules in a formamide/water environment. Sci. Rep. 2016, 6, 38888. [Google Scholar] [CrossRef] [PubMed]
- Caselli, P.; Ceccarelli, C. Our astrochemical heritage. A&A Rev. 2012, 20, 56. [Google Scholar] [CrossRef]
- Bowen, I.S. The origin of the nebular lines and the structure of the planetary nebulae. Astrophys. J. 1928, 67, 1. [Google Scholar] [CrossRef]
- Zanstra, H. An Application of the Quantum Theory to the Luminosity of Diffuse Nebulae. Astrophys. J. 1927, 65, 50. [Google Scholar] [CrossRef]
- Heger, M.L. Further study of the sodium lines in class B stars. Lick Obs. Bull. 1922, 10, 141. [Google Scholar] [CrossRef]
- Snow, T.P. Diffuse Interstellar Bands: Past and Present. Proc. IAU Symp. 2014, 9, 3–12. [Google Scholar] [CrossRef]
- Foing, B.H.; Ehrenfreund, P. Detection of two interstellar absorption bands coincident with spectral features of C+60. Nature 1994, 369, 296–298. [Google Scholar] [CrossRef]
- Campbell, E.K.; Holz, M.; Gerlich, D.; Maier, J.P. Laboratory confirmation of C60+ as the carrier of two diffuse interstellar bands. Nature 2015, 523, 322–323. [Google Scholar] [CrossRef]
- Walker, G.A.H.; Bohlender, D.A.; Maier, J.P.; Campbell, E.K. Identification of More Interstellar C60+ Bands. Astrophys. J. Lett. 2015, 812, L8. [Google Scholar] [CrossRef]
- García-Hernández, D.A.; Díaz-Luis, J.J. Diffuse interstellar bands in fullerene planetary nebulae: The fullerenes—Diffuse interstellar bands connection. Astron. Astrophys. 2013, 550, L6. [Google Scholar] [CrossRef]
- Douglas, A.E. Origin of diffuse interstellar lines. Nature 1977, 269, 130–132. [Google Scholar] [CrossRef]
- Leger, A.; D’Hendecourt, L. Are polycyclic aromatic hydrocarbons the carriers of the diffuse interstellar bands in the visible? Astron. Astrophys. 1985, 146, 81–85. [Google Scholar]
- Salama, F.; Galazutdinov, G.A.; Krełowski, J.; Biennier, L.; Beletsky, Y.; Song, I.-O. Polycyclic Aromatic Hydrocarbons and the Diffuse Interstellar Bands: A Survey. Astrophys. J. 2011, 728, 154. [Google Scholar] [CrossRef]
- Zanolli, Z.; Malcıoğlu, O.B.; Charlier, J.-C. Carbynes connected to polycyclic aromatic hydrocarbons as potential carriers of diffuse interstellar bands. Astron. Astrophys. 2023, 675, L9. [Google Scholar] [CrossRef]
- Sarre, P.J. The diffuse interstellar bands: A major problem in astronomical spectroscopy. J. Mol. Spectrosc. 2006, 238, 1–10. [Google Scholar] [CrossRef]
- Cami, J.; Cox, N.L.J. The Diffuse Interstellar Bands. In The Diffuse Interstellar Bands; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Stecher, T.P. Interstellar Ectinction in the Ultraviolet. Astrophys. J. 1965, 142, 1683. [Google Scholar] [CrossRef]
- Bless, R.C.; Savage, B.D. Ultraviolet Photometry from the Orbiting Astronomical Observatory. II. Interstellar Extinction. Astrophys. J. 1972, 171, 293. [Google Scholar] [CrossRef]
- York, D.G.; Drake, J.F.; Jenkins, E.B.; Morton, D.C.; Rogerson, J.B.; Spitzer, L. Spectrophotometric Results from the Copernicus Satellite. VI. Extinction by Grains at Wavelengths between 1200 and 1000 Å. Astrophys. J. 1973, 182, L1. [Google Scholar] [CrossRef]
- Fitzpatrick, E.L.; Clayton, G.C.; Draine, B.T. Interstellar Extinction in the Milky Way Galaxy. In arXiv; 2004. [Google Scholar]
- Joblin, C.; Leger, A.; Martin, P. Contribution of polycyclic aromatic hydrocarbon molecules to the interstellar extinction curve. Astrophys. J. 1992, 393, L79–L82. [Google Scholar] [CrossRef]
- Duley, W.W.; Seahra, S. Graphite, Polycyclic Aromatic Hydrocarbons, and the 2175 Å Extinction Feature. Astrophys. J. 1998, 507, 874–888. [Google Scholar] [CrossRef]
- Mennella, V.; Colangeli, L.; Bussoletti, E.; Palumbo, P.; Rotundi, A. A New Approach to the Puzzle of the Ultraviolet Interstellar Extinction Bump. Astrophys. J. Lett. 1998, 507, L177–L180. [Google Scholar] [CrossRef]
- Iglesias-Groth, S. Fullerenes and Buckyonions in the Interstellar Medium. Astrophys. J. Lett. 2004, 608, L37–L40. [Google Scholar] [CrossRef]
- Cataldo, F.; Iglesias-Groth, S. On the action of UV photons on hydrogenated fulleranes C60H36 and C60D36. Mon. Not. R. Astron. Soc. 2009, 400, 291–298. [Google Scholar] [CrossRef]
- Papoular, R.J.; Papoular, R. A polycrystalline graphite model for the 2175 Å interstellar extinction band. Mon. Not. R. Astron. Soc. 2009, 394, 2175–2181. [Google Scholar] [CrossRef]
- Schmidt, G.D.; Cohen, M.; Margon, B. Discovery of optical molecular emission from the bipolar nebula surrounding HD 44179. Astrophys. J. Lett. 1980, 239, L133–L138. [Google Scholar] [CrossRef]
- Schmidt, G.D.; Witt, A.N. X marks the SPOT—Distribution and excitation of unidentified molecules in the Red Rectangle. Astrophys. J. 1991, 383, 698–704. [Google Scholar] [CrossRef]
- Vijh, U.P.; Witt, A.N.; Gordon, K.D. Discovery of Blue Luminescence in the Red Rectangle: Possible Fluorescence from Neutral Polycyclic Aromatic Hydrocarbon Molecules? Astrophys. J. 2004, 606, L65–L68. [Google Scholar] [CrossRef]
- Duley, W.W. Evidence for hydrogenated amorphous carbon in the Red Rectangle. Mon. Not. R. Astron. Soc. 1985, 215, 259–263. [Google Scholar] [CrossRef]
- Sakata, A.; Wada, S.; Narisawa, T.; Asano, Y.; Iijima, Y.; Onaka, T.; Tokunaga, A.T. Quenched carbonaceous composite—Fluorescence spectrum compared to the extended red emission observed in reflection nebulae. Astrophys. J. 1992, 393, L83–L86. [Google Scholar] [CrossRef]
- Webster, A. The Extended Red Emission and the Fluorescence of C/60. Mon. Not. R. Astron. Soc. 1993, 264, L1. [Google Scholar] [CrossRef]
- Chang, H.-C.; Chen, K.; Kwok, S. Nanodiamond as a Possible Carrier of Extended Red Emission. Astrophys. J. 2006, 639, L63–L66. [Google Scholar] [CrossRef]
- Ledoux, G.; Guillois, O.; Huisken, F.; Kohn, B.; Porterat, D.; Reynaud, C. Crystalline silicon nanoparticles as carriers for the Extended Red Emission. Astron. Astrophys. 2001, 377, 707–720. [Google Scholar] [CrossRef]
- Zagury, F. Raman Scattering by Atomic Hydrogen in Photodissociation Regions: An Alternative to the Polycyclic Aromatic Hydrocarbons Hypothesis. Astrophys. J. 2023, 952, 116. [Google Scholar] [CrossRef]
- Forrest, W.J.; Houck, J.R.; McCarthy, J.F. A far-infrared emission feature in carbon-rich stars and planetary nebulae. Astrophys. J. 1981, 248, 195–200. [Google Scholar] [CrossRef]
- Hony, S.; Waters, L.B.F.M.; Tielens, A.G.G.M. The carrier of the “30” mu m emission feature in evolved stars. A simple model using magnesium sulfide. Astron. Astrophys. 2002, 390, 533–553. [Google Scholar] [CrossRef]
- Gładkowski, M.; Szczerba, R.; Sloan, G.C.; Lagadec, E.; Volk, K. 30-micron sources in galaxies with different metallicities. Astron. Astrophys. 2019, 626, A92. [Google Scholar] [CrossRef]
- Hrivnak, B.J.; Volk, K.; Kwok, S. 2-45 Micron Infrared Spectroscopy of Carbon-rich Proto-Planetary Nebulae. Astrophys. J. 2000, 535, 275–292. [Google Scholar] [CrossRef]
- Kwok, S.; Volk, K.M.; Hrivnak, B.J. A 21 micron emission feature in four proto-planetary nebulae. Astrophys. J. 1989, 345, L51–L54. [Google Scholar] [CrossRef]
- Volk, K.; Kwok, S.; Hrivnak, B.J. High-Resolution Infrared Space Observatory Spectroscopy of the Unidentified 21 Micron Feature. Astrophys. J. 1999, 516, L99–L102. [Google Scholar] [CrossRef]
- Buss, R.H.; Cohen, M.; Tielens, A.; Werner, M.W.; Bregman, J.D.; Witteborn, F.C.; Rank, D.; Sandford, S.A. Hydrocarbon Emission Features In The Infrared-Spectra Of Warm Supergiants. Astrophys. J. 1990, 365, L23–L26. [Google Scholar] [CrossRef]
- Webster, A. The Lowest of the Strongly Infrared Active Vibrations of the Fulleranes and Astronomical Emission Band at a Wavelength of 21-MICRONS. R. Astron. Soc. Mon. Not. 1995, 277, 1555. [Google Scholar] [CrossRef]
- Hill, H.G.M.; Jones, A.P.; d’Hendecourt, L.B. Diamonds in carbon-rich proto-planetary nebulae. Astron. Astrophys. 1998, 336, L41–L44. [Google Scholar]
- von Helden, G.; Tielens, A.G.G.M.; van Heijnsbergen, D.; Duncan, M.A.; Hony, S.; Waters, L.B.F.M.; Meijer, G. Titanium Carbide Nanocrystals in Circumstellar Environments. Science 2000, 288, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Papoular, R. The contribution of Oxygen to the “30”, “26” and “20” µm features. Astron. Astrophys. 2000, 362, L9–L12. [Google Scholar]
- Posch, T.; Mutschke, H.; Andersen, A. Reconsidering the origin of the 21 micron feature: Oxides in carbon-rich protoplanetary nebulae? Astrophys. J. 2004, 616, 1167–1180. [Google Scholar] [CrossRef]
- Speck, A.K.; Hofmeister, A.M. Processing of presolar grains around post-asymptotic giant branch stars: Silicon carbide as the carrier of the 21 micron feature. Astrophys. J. 2004, 600, 986–991. [Google Scholar] [CrossRef]
- Volk, K.; Sloan, G.C.; Kraemer, K.E. The 21 μm and 30 μm emission features in carbon-rich objects. Astrophys. Space Sci. 2020, 365, 88. [Google Scholar] [CrossRef]
- Ootsubo, T.; Kawakita, H.; Shinnaka, Y.; Watanabe, J.-i.; Honda, M. Unidentified infrared emission features in mid-infrared spectrum of comet 21P/Giacobini-Zinner. Icarus 2020, 338, 113450. [Google Scholar] [CrossRef]
- Knacke, R.F. Carbonaceous compounds in interstellar dust. Nature 1977, 269, 132–134. [Google Scholar] [CrossRef]
- Duley, W.W.; Williams, D.A. The infrared spectrum of interstellar dust: Surface functional groups on carbon. Mon. Not. R. Astron. Soc. 1981, 196, 269–274. [Google Scholar] [CrossRef]
- Leger, A.; Puget, J.L. Identification of the ’unidentified’ IR emission features of interstellar dust? Astron. Astrophys. 1984, 137, L5–L8. [Google Scholar]
- Allamandola, L.J.; Tielens, A.G.G.M.; Barker, J.R. Interstellar polycyclic aromatic hydrocarbons: The infrared emission bands, the excitation/emission mechanism and the astrophysical implications. Astrophys. J. Suppl. Ser. 1989, 71, 733–775. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, K. The near-infrared continuum emission of visual reflection nebulae. Astrophys. J. 1984, 277, 623–633. [Google Scholar] [CrossRef]
- Bernstein, L.S.; Lynch, D.K. Small Carbonaceous Molecules, Ethylene Oxide (c-C2H4O) and Cyclopropenylidene (c-C3H2): Sources of the Unidentified Infrared Bands? Astrophys. J. 2009, 704, 226–239. [Google Scholar] [CrossRef]
- Jones, A.P.; Duley, W.W.; Williams, D.A. The structure and evolution of hydrogenated amorphous carbon grains and mantles in the interstellar medium. QJRAS 1990, 31, 567–582. [Google Scholar]
- Pino, T.; Dartois, E.; Cao, A.-T.; Carpentier, Y.; Chamaillé, T.; Vasquez, R.; Jones, A.P.; D’Hendecourt, L.; Bréchignac, P. The 6.2 μm band position in laboratory and astrophysical spectra: A tracer of the aliphatic to aromatic evolution of interstellar carbonaceous dust. Astron. Astrophys. 2008, 490, 665–672. [Google Scholar] [CrossRef]
- Hu, A.; Duley, W.W. Spectra of Carbon Nanoparticles: Laboratory Simulation of the Aromatic CH Emission Feature at 3.29 μm. Astrophys. J. Lett. 2008, 677, L153–L156. [Google Scholar] [CrossRef]
- Sakata, A.; Wada, S.; Onaka, T.; Tokunaga, A.T. Infrared spectrum of quenched carbonaceous composite (QCC). II—A new identification of the 7.7 and 8.6 micron unidentified infrared emission bands. Astrophys. J. 1987, 320, L63–L67. [Google Scholar] [CrossRef]
- Papoular, R.; Conrad, J.; Giuliano, M.; Kister, J.; Mille, G. A coal model for the carriers of the unidentified IR bands. Astron. Astrophys. 1989, 217, 204–208. [Google Scholar]
- Papoular, R. The use of kerogen data in understanding the properties and evolution of interstellar carbonaceous dust. Astron. Astrophys. 2001, 378, 597–607. [Google Scholar] [CrossRef]
- Cataldo, F.; Keheyan, Y.; Heymann, D. A new model for the interpretation of the unidentified infrared bands (UIBS) of the diffuse interstellar medium and of the protoplanetary nebulae. Int. J. Astrobiol. 2002, 1, 79–86. [Google Scholar] [CrossRef]
- Kwok, S.; Zhang, Y. Mixed aromatic-aliphatic organic nanoparticles as carriers of unidentified infrared emission features. Nature 2011, 479, 80–83. [Google Scholar] [CrossRef]
- Kwok, S.; Zhang, Y. Unidentified Infrared Emission Bands: PAHs or MAONs? Astrophys. J. 2013, 771, 5. [Google Scholar] [CrossRef]
- Hou, G.-L.; Lushchikova, O.V.; Bakker, J.M.; Lievens, P.; Decin, L.; Janssens, E. Buckyball-metal Complexes as Potential Carriers of Astronomical Unidentified Infrared Emission Bands. Astrophys. J. 2023, 952, 13. [Google Scholar] [CrossRef]
- Kwok, S. The mystery of unidentified infrared emission bands. Astrophys. Space Sci. 2022, 367, 16. [Google Scholar] [CrossRef]
- Wickramasinghe, D.T.; Allen, D.A. The 3.4-micron interstellar absorption feature. Nature 1980, 287, 518. [Google Scholar] [CrossRef]
- Sandford, S.A.; Allamandola, L.J.; Tielens, A.G.G.M.; Sellgren, K.; Tapia, M.; Pendleton, Y. The interstellar C-H stretching band near 3.4 microns—Constraints on the composition of organic material in the diffuse interstellar medium. Astrophys. J. 1991, 371, 607–620. [Google Scholar] [CrossRef]
- Pendleton, Y.J.; Sandford, S.A.; Allamandola, L.J.; Tielens, A.G.G.M.; Sellgren, K. Near-infrared absorption spectroscopy of interstellar hydrocarbon grains. Astrophys. J. 1994, 437, 683–696. [Google Scholar] [CrossRef]
- Chiar, J.E.; Tielens, A.G.G.M.; Whittet, D.C.B.; Schutte, W.A.; Boogert, A.C.A.; Lutz, D.; van Dishoeck, E.F.; Bernstein, M.P. The Composition and Distribution of Dust along the Line of Sight toward the Galactic Center. Astrophys. J. 2000, 537, 749–762. [Google Scholar] [CrossRef]
- Günay, B.; Burton, M.G.; Afşar, M.; Schmidt, T.W. Mapping the aliphatic hydrocarbon content of interstellar dust in the Galactic plane. Mon. Not. R. Astron. Soc. 2022, 515, 4201–4216. [Google Scholar] [CrossRef]
- Mason, R.E.; Wright, G.; Pendleton, Y.; Adamson, A. Hydrocarbon Dust Absorption in Seyfert Galaxies and Ultraluminous Infrared Galaxies. Astrophys. J. 2004, 613, 770–780. [Google Scholar] [CrossRef]
- Rosas, V.G.; Tielens, A.G.G.M.; van der Werf, P.; Jaffe, W.; Leftley, J.H.; Burtscher, L.; Petrov, R.; Isbell, J.W.; López, B.; Millour, F.; et al. The carbonaceous dust at sub-parsec scales in the nucleus of NGC 1068. Mon. Not. R. Astron. Soc. 2023. [Google Scholar] [CrossRef]
- Imanishi, M.; Nakagawa, T.; Shirahata, M.; Ohyama, Y.; Onaka, T. AKARI IRC Infrared 2.5-5 μm Spectroscopy of a Large Sample of Luminous Infrared Galaxies. Astrophys. J. 2010, 721, 1233–1261. [Google Scholar] [CrossRef]
- Yamagishi, M.; Kaneda, H.; Ishihara, D.; Kondo, T.; Onaka, T.; Suzuki, T.; Minh, Y.C. AKARI near-infrared spectroscopy of the aromatic and aliphatic hydrocarbon emission features in the galactic superwind of M 82. Astron. Astrophys. 2012, 541, 10. [Google Scholar] [CrossRef]
- Kwok, S.; Volk, K.; Bernath, P. On the Origin of Infrared Plateau Features in Proto-Planetary Nebulae. Astrophys. J. 2001, 554, L87–L90. [Google Scholar] [CrossRef]
- Kwok, S. The Origin and Evolution of Planetary Nebulae; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar] [CrossRef]
- Geballe, T.R.; Tielens, A.G.G.M.; Kwok, S.; Hrivnak, B.J. Unusual 3 micron emission features in three proto-planetary nebulae. Astrophys. J. 1992, 387, L89–L91. [Google Scholar] [CrossRef]
- Hrivnak, B.J.; Geballe, T.R.; Kwok, S. A Study of the 3.3 and 3.4 μm Emission Features in Proto-Planetary Nebulae. Astrophys. J. 2007, 662, 1059–1066. [Google Scholar] [CrossRef]
- Keller, L.P.; Bajt, S.; Baratta, G.A.; Borg, J.; Bradley, J.P.; Brownlee, D.E.; Busemann, H.; Brucato, J.R.; Burchell, M.; Colangeli, L.; et al. Infrared Spectroscopy of Comet 81P/Wild 2 Samples Returned by Stardust. Science 2006, 314, 1728–1731. [Google Scholar] [CrossRef]
- Kim, S.J.; Jung, A.; Sim, C.K.; Courtin, R.; Bellucci, A.; Sicardy, B.; Song, I.O.; Minh, Y.C. Retrieval and tentative indentification of the 3 μm spectral feature in Titan’s haze. Planet. Space Sci. 2011, 59, 699–704. [Google Scholar] [CrossRef]
- Dischler, B.; Bubenzer, A.; Koidl, P. Hard carbon coatings with low optical absorption. Appl. Phys. Lett. 1983, 42, 636–638. [Google Scholar] [CrossRef]
- Herlin, N.; Bohn, I.; Reynaud, C.; Cauchetier, M.; Galvez, A.; Rouzaud, J.-N. Nanoparticles produced by Laser Pyrolysis of hydrocarbons: Analogy with carbon cosmic dust. Astron. Astrophys. 1998, 330, 1127–1135. [Google Scholar]
- Sadjadi, S.; Zhang, Y.; Kwok, S. A Theoretical Study on the Vibrational Spectra of Polycyclic Aromatic Hydrocarbon Molecules with Aliphatic Sidegroups. Astrophys. J. 2015, 801, 34. [Google Scholar] [CrossRef]
- Sadjadi, S.; Kwok, S.; Zhang, Y. Theoretical infrared spectra of MAON molecules. J. Phys. Conf. Ser. 2016, 728, 062003. [Google Scholar] [CrossRef]
- Godard, M.; Féraud, G.; Chabot, M.; Carpentier, Y.; Pino, T.; Brunetto, R.; Duprat, J.; Engrand, C.; Bréchignac, P.; D’Hendecourt, L.; et al. Ion irradiation of carbonaceous interstellar analogues. Effects of cosmic rays on the 3.4 μm interstellar absorption band. Astron. Astrophys. 2011, 529, 146. [Google Scholar] [CrossRef]
- Colangeli, L.; Mennella, V.; Palumbo, P.; Rotundi, A.; Bussoletti, E. Mass extinction coefficients of various submicron amorphous carbon grains: Tabulated values from 40 NM to 2 mm. Astron. Astrophys. Suppl. Ser. 1995, 113, 561. [Google Scholar]
- Mennella, V.; Baratta, G.A.; Esposito, A.; Ferini, G.; Pendleton, Y.J. The Effects of Ion Irradiation on the Evolution of the Carrier of the 3.4 Micron Interstellar Absorption Band. Astrophys. J. 2003, 587, 727–738. [Google Scholar] [CrossRef]
- Scott, A.; Duley, W.W. The Decomposition of Hydrogenated Amorphous Carbon: A Connection with Polycyclic Aromatic Hydrocarbon Molecules. Astrophys. J. 1996, 472, L123–L125. [Google Scholar] [CrossRef]
- Mennella, V.; Brucato, J.R.; Colangeli, L.; Palumbo, P. Activation of the 3.4 Micron Band in Carbon Grains by Exposure to Atomic Hydrogen. Astrophys. J. 1999, 524, L71–L74. [Google Scholar] [CrossRef]
- Jäger, C.; Huisken, F.; Mutschke, H.; Jansa, I.L.; Henning, T.H. Formation Of Polycyclic Aromatic Hydrocarbons And Carbonaceous Solids In Gas-Phase Condensation Experiments. Astrophys. J. 2009, 696, 706–712. [Google Scholar] [CrossRef]
- Dartois, E.; Muñoz Caro, G.M.; Deboffle, D.; d’Hendecourt, L. Diffuse interstellar medium organic polymers. Photoproduction of the 3.4, 6.85 and 7.25 μm features. Astron. Astrophys. 2004, 423, L33–L36. [Google Scholar] [CrossRef]
- Carpentier, Y.; Féraud, G.; Dartois, E.; Brunetto, R.; Charon, E.; Cao, A.-T.; d’Hendecourt, L.; Bréchignac, P.; Rouzaud, J.-N.; Pino, T. Nanostructuration of carbonaceous dust as seen through the positions of the 6.2 and 7.7 μm AIBs. Astron. Astrophys. 2012, 548, 40. [Google Scholar] [CrossRef]
- Martínez, L.; Santoro, G.; Merino, P.; Accolla, M.; Lauwaet, K.; Sobrado, J.; Sabbah, H.; Pelaez, R.J.; Herrero, V.J.; Tanarro, I.; et al. Prevalence of non-aromatic carbonaceous molecules in the inner regions of circumstellar envelopes. Nat. Astron. 2020, 4, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Helton, L.A.; Evans, A.; Woodward, C.E.; Gehrz, R.D.; Vacca, W. The Dusty Nova: An Examination of Dust Production and Processing in the Ejecta of Classical Novae. Proceedings of Stellar Novae: Past and Future Decades, Cape Town, South Africa, 1 December 2014; p. 261. [Google Scholar]
- Endo, I.; Sakon, I.; Onaka, T.; Kimura, Y.; Kimura, S.; Wada, S.; Helton, L.A.; Lau, R.M.; Kebukawa, Y.; Muramatsu, Y.; et al. On the Nature of Organic Dust in Novae. Astrophys. J. 2021, 917, 103. [Google Scholar] [CrossRef]
- Cordiner, M.A. Extragalactic Diffuse Interstellar Bands: A Universal Problem. In Proceedings of the Diffuse Interstellar Bands, Haarlem, The Netherlands, 1 February 2014; pp. 41–50. [Google Scholar]
- Junkkarinen, V.T.; Cohen, R.D.; Beaver, E.A.; Burbidge, E.M.; Lyons, R.W.; Madejski, G. Dust and Diffuse Interstellar Bands in the za = 0.524 Absorption System toward AO 0235+164. Astrophys. J. 2004, 614, 658–670. [Google Scholar] [CrossRef]
- Elíasdóttir, Á.; Fynbo, J.P.U.; Hjorth, J.; Ledoux, C.; Watson, D.J.; Andersen, A.C.; Malesani, D.; Vreeswijk, P.M.; Prochaska, J.X.; Sollerman, J.; et al. Dust Extinction in High-z Galaxies with Gamma-Ray Burst Afterglow Spectroscopy: The 2175 Å Feature at z = 2.45. Astrophys. J. 2009, 697, 1725–1740. [Google Scholar] [CrossRef]
- Witstok, J.; Shivaei, I.; Smit, R.; Maiolino, R.; Carniani, S.; Curtis-Lake, E.; Ferruit, P.; Arribas, S.; Bunker, A.J.; Cameron, A.J.; et al. Carbonaceous dust grains seen in the first billion years of cosmic time. arXiv 2023, arXiv:2302.05468. [Google Scholar] [CrossRef]
- Volk, K.; Hrivnak, B.J.; Matsuura, M.; Bernard-Salas, J.; Szczerba, R.; Sloan, G.C.; Kraemer, K.E.; van Loon, J.T.; Kemper, F.; Woods, P.M.; et al. DISCOVERY AND ANALYSIS OF 21 μm FEATURE SOURCES IN THE MAGELLANIC CLOUDS. Astrophys. J. 2011, 735, 127. [Google Scholar] [CrossRef]
- Smith, J.D.T.; Draine, B.T.; Dale, D.A.; Moustakas, J.; Kennicutt, R.C.; Helou, G.; Armus, L.; Roussel, H.; Sheth, K.; Bendo, G.J.; et al. The mid-infrared spectrum of star-forming galaxies: Global properties of polycyclic aromatic hydrocarbon emission. Astrophys. J. 2007, 656, 770–791. [Google Scholar] [CrossRef]
- Spilker, J.S.; Phadke, K.A.; Aravena, M.; Archipley, M.; Bayliss, M.B.; Birkin, J.E.; Béthermin, M.; Burgoyne, J.; Cathey, J.; Chapman, S.C.; et al. Spatial variations in aromatic hydrocarbon emission in a dust-rich galaxy. Nature 2023, 618, 708–711. [Google Scholar] [CrossRef]
- Robinson, R. The Origins of Petroleum. Nature 1966, 212, 1291–1295. [Google Scholar] [CrossRef]
- Abelson, P.H. Organic Matter in the Earth’s Crust. Annu. Rev. Earth Planet. Sci. 1978, 6, 325. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Westgate, T.D.; Ward, J.A.; Slater, G.F.; Lacrampe-Couloume, G. Abiogenic formation of alkanes in the Earth’s crust as a minor source for global hydrocarbon reservoirs. Nature 2002, 416, 522–524. [Google Scholar] [CrossRef] [PubMed]
- Sephton, M.A.; Hazen, R.M. On the Origins of Deep Hydrocarbons. Rev. Mineral. Geochem. 2013, 75, 449–465. [Google Scholar] [CrossRef]
- Kutcherov, V.; Kolesnikov, A.; Dyuzheva, T.; Brazhkin, V. Synthesis of hydrocarbons under upper mantle conditions: Evidence for the theory of abiotic deep petroleum origin. J. Phys. Conf. Ser. 2010, 215, 012103. [Google Scholar] [CrossRef]
- Kenney, J.F.; Kutcherov, V.A.; Bendeliani, N.A.; Alekseev, V.A. The evolution of multicomponent systems at high pressures: VI. The thermodynamic stability of the hydrogen–carbon system: The genesis of hydrocarbons and the origin of petroleum. Proc. Natl. Acad. Sci. USA 2002, 99, 10976–10981. [Google Scholar] [CrossRef] [PubMed]
- Kwok, S. Abiotic synthesis of complex organics in the Universe. Nat. Astron. 2017, 1, 642. [Google Scholar] [CrossRef]
- Marty, B.; Alexander, C.M.O.D.; Raymond, S.N. Primordial Origins of Earth’s Carbon. Rev. Mineral. Geochem. 2013, 75, 149–181. [Google Scholar] [CrossRef]
- Kwok, S. Organic Matter in the Universe; Wiley: New York, NY, USA, 2011. [Google Scholar]
- Kwok, S. Stardust: The Cosmic Seeds of Life; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Pizzarello, S.; Shock, E. Carbonaceous Chondrite Meteorites: The Chronicle of a Potential Evolutionary Path between Stars and Life. Orig. Life Evol. Biosph. 2017, 47, 249–260. [Google Scholar] [CrossRef]
- Sephton, M.A. Organic compounds in carbonaceous meteorites. Nat. Prod. Rep. 2002, 19, 292–311. [Google Scholar] [CrossRef]
- Martins, Z.; Botta, O.; Fogel, M.L.; Sephton, M.A.; Glavin, D.P.; Watson, J.S.; Dworkin, J.P.; Schwartz, A.W.; Ehrenfreund, P. Extraterrestrial nucleobases in the Murchison meteorite. Earth Planet. Sci. Lett. 2008, 270, 130–136. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Furukawa, Y.; Koga, T.; Glavin, D.P.; Dworkin, J.P.; Naraoka, H. Identifying the wide diversity of extraterrestrial purine and pyrimidine nucleobases in carbonaceous meteorites. Nat. Commun. 2022, 13, 2008. [Google Scholar] [CrossRef]
- Cruikshank, D.P.; Materese, C.K.; Pendleton, Y.J.; Boston, P.J.; Grundy, W.M.; Schmitt, B.; Lisse, C.M.; Runyon, K.D.; Keane, J.T.; Beyer, R.A.; et al. Prebiotic Chemistry of Pluto. Astrobiology 2019, 19, 831–848. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Yabuta, H.; Williams, L.B.; Cody, G.D.; Alexander, C.M.O.D.; Pizzarello, S. The insoluble carbonaceous material of CM chondrites: A possible source of discrete organic compounds under hydrothermal conditions. Meteorit. Planet. Sci. 2007, 42, 37–48. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwok, S. Complex Organics in Space: A Changing View of the Cosmos. Galaxies 2023, 11, 104. https://doi.org/10.3390/galaxies11050104
Kwok S. Complex Organics in Space: A Changing View of the Cosmos. Galaxies. 2023; 11(5):104. https://doi.org/10.3390/galaxies11050104
Chicago/Turabian StyleKwok, Sun. 2023. "Complex Organics in Space: A Changing View of the Cosmos" Galaxies 11, no. 5: 104. https://doi.org/10.3390/galaxies11050104




