Adhesion and Electron Properties of Quasi-2D Mo2C, Ti2C, and V2C MXene Flakes after Van Der Waals Adsorption of Alcohol Molecules: Influence of Humidity
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Atomistic Models of MXene 2D-Films
3.2. Electronic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Persson, I.; Lind, H.; Palisaitis, J.; Li, M.; Li, Y.; Chen, K.; Zhou, J.; Du, S.; Chai, Z.; et al. Tin + 1Cn MXenes with fully saturated and thermally stable Cl terminations. Nanoscale Adv. 2019, 1, 3680–3685. [Google Scholar] [CrossRef] [Green Version]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Rui, X.; Lagunas, F.; Wang, D.; Kile, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Mehdi Aghaei, S.; Aasi, A.; Panchapakesan, B. Experimental and Theoretical Advances in MXene-Based Gas Sensors. ACS Omega 2021, 6, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kim, D.-J. Recent Exploration of Two-Dimensional MXenes for Gas Sensing: From a Theoretical to an Experimental View. J. Electrochem. Soc. 2020, 167, 037515. [Google Scholar] [CrossRef] [Green Version]
- Grützmacher, P.G.; Suarez, S.; Tolosa, A.; Gachot, C.; Song, G.; Wang, B.; Presser, V.; Mücklich, F.; Anasori, B.; Rosenkranz, A. Superior Wear-Resistance of Ti3C2Tx Multilayer Coatings. ACS Nano 2021, 15, 8216–8224. [Google Scholar] [CrossRef]
- Marian, M.; Tremmel, S.; Wartzack, S.; Song, G.; Wang, B.; Yu, J.; Rosenkranz, A. MXene nanosheets as an emerging solid lubricant for machine elements—Towards increased energy efficiency and service life. Appl. Surf. Sci. 2020, 523, 146503. [Google Scholar] [CrossRef]
- Marian, M.; Song, G.C.; Wang, B.; Fuenzalida, V.M.; Krauß, S.; Merle, B.; Tremmel, S.; Wartzack, S.; Yu, J.; Rosenkranz, A. Effective usage of 2D MXene nanosheets as solid lubricant—Influence of contact pressure and relative humidity. Appl. Surf. Sci. 2020, 531, 147311. [Google Scholar] [CrossRef]
- Rosenkranz, A.; Grützmacher, P.; Espinoza-Gonzalez, R.; Fuenzalida, V.M.; Blanco, E.; Escalona, N.; Gracia, F.J.; Villarroel, R.; Guo, L.; Kang, R.; et al. Multi-layer Ti3C2Tx-nanoparticles (MXenes) as solid lubricants—Role of surface terminations and intercalated water. Appl. Surf. Sci. 2019, 494, 13–21. [Google Scholar] [CrossRef]
- Huang, S.; Mochalin, V.N. Hydrolysis of 2D Transition-Metal Carbides (MXenes) in Colloidal Solutions. Inorg. Chem. 2019, 58, 1958–1966. [Google Scholar] [CrossRef]
- Li, L.; Fu, X.; Chen, S.; Uzun, S.; Levitt, A.; Shuck, C.E.; Han, W.; Gogotsi, Y. Hydrophobic and Stable MXene-polymer Pressure Sensors for Wearable Electronics. ACS Appl. Mater. Interfaces 2020, 12, 15365–15369. [Google Scholar] [CrossRef]
- Pei, Y.; Zhang, X.; Hui, Z.; Zhou, J.; Huang, X.; Sun, G.; Huang, W. Ti3C2TX MXene for Sensing Applications: Recent Progress, Design Principles, and Future Perspectives. ACS Nano 2021, 15, 3996–4017. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; He, M.; Hu, Q.; Wu, Q.; Sun, G.; Xie, L.; Zhang, Z.; Zhu, Z.; Zhou, A. Ti3C2 MXene-Based Sensors with High Selectivity for NH3 Detection at Room Temperature. ACS Sens. 2019, 4, 2763. [Google Scholar] [CrossRef] [PubMed]
- Koh, H.-J.; Kim, S.J.; Maleski, K.; Cho, S.-Y.; Kim, Y.-J.; Ahn, C.W.; Gogotsi, Y.; Jung, H.-T. Enhanced Selectivity of MXene Gas Sensors through Metal Ion Intercalation: In Situ X-ray Diffraction Study. ACS Sens. 2019, 4, 1365. [Google Scholar] [CrossRef]
- Chae, Y.; Kim, S.J.; Cho, S.-Y.; Choi, J.; Maleski, K.; Lee, B.J.; Jung, H.-T.; Gogotsi, Y.; Lee, Y.; Ahn, C.W. An investigation into the factors governing the oxidation of two-dimensional Ti3C2 MXene. Nanoscale 2019, 11, 8387. [Google Scholar] [CrossRef]
- Halim, J.; Kota, S.; Lukatskaya, M.R.; Naguib, M.; Zhao, M.-Q.; Moon, E.J.; Pitock, J.; Nanda, J.; May, S.J.; Gogotsi, Y.; et al. Synthesis and Characterization of 2D Molybdenum Carbide (MXene). Adv. Funct. Mater. 2016, 26, 3118. [Google Scholar] [CrossRef]
- Guo, W.; Surya, S.G.; Babar, V.; Ming, F.; Sharma, S.; Alshareef, H.N.; Schwingenschlögl, U.; Salama, K.N. Selective Toluene Detection with Mo2CTx MXene at Room Temperature. ACS Appl. Mater. Interfaces 2020, 12, 57218. [Google Scholar] [CrossRef]
- Wu, M.; Wang, B.; Hu, Q.; Wang, L.; Zhou, A. The Synthesis Process and Thermal Stability of V2C MXene. Materials 2018, 11, 2112. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Jiang, Y.; Duan, Z.; Huang, Q.; Wu, Y.; Liu, B.; Zhao, Q.; Wang, Q.; Yuan, Z.; Tai, H. Highly sensitive and selective NO2 sensor of alkalized V2CT MXene driven by interlayer swelling. Sens. Actuators B Chem. 2021, 344, 130150. [Google Scholar] [CrossRef]
- Wu, M.; An, Y.; Yang, R.; Tao, Z.; Xia, Q.; Hu, Q.; Li, M.; Chen, K.; Zhang, Z.; Huang, Q.; et al. V2CTx and Ti3C2Tx MXenes Nanosheets for Gas Sensing. ACS Appl. Nano Mater. 2021, 4, 6257–6268. [Google Scholar] [CrossRef]
- Nahirniak, S.; Saruhan, B. MXene Heterostructures as Perspective Materials for Gas Sensing Applications. Sensors 2022, 22, 972. [Google Scholar] [CrossRef]
- Lee, E.; Vahid-Mohammadi, A.; Yoon, Y.S.; Beidaghi, M.; Kim, D.J. Two-dimensional vanadium carbide MXene for gas sensors with ultrahigh sensitivity toward nonpolar gases. ACS Sens. 2019, 4, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Soler, J.M.; Artacho, E.; Gale, J.D.; García, A.; Junquera, J.; Ordejón, P.; Sánchez-Portal, D. The SIESTA method for ab-initio order-N materials simulation. J. Phys. Condens. Matt. 2002, 14, 2745–2779. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; Papior, N.; Akhtar, A.; Artacho, E.; Blum, V.; Bosoni, E.; Brandimarte, P.; Brandbyge, M.; Cerda, J.I.; Corsetti, F.; et al. SIESTA: Recent developments and applica-tions. J. Chem. Phys. 2020, 152, 204108. [Google Scholar] [CrossRef] [PubMed]
- Berland, K.; Hyldgaard, P. Exchange functional that tests the robustness of the plasmon description of the van der Waals density functional. Phys. Rev. B 2014, 89, 035412. [Google Scholar] [CrossRef] [Green Version]
- Berland, K.; Arter, C.A.; Cooper, V.R.; Lee, K.; Lundqvist, B.I.; Schroder, E.; Thonhauser, T.; Hyldgaard, P. Van der Waals density functionals built upon the electron-gas tradition: Facing the challenge of competing interactions. J. Chem. Phys. 2014, 140, 18A539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Johnson, D.D. Modified Broyden’s method for accelerating convergence in self-consistent calculations. Phys. Rev. B 1988, 38, 12807. [Google Scholar] [CrossRef]
- Büttiker, M.; Imry, Y.; Landauer, R.; Pinhas, S. Generalized Many-Channel Conductance Formula with Application to Small Rings. Phys. Rev. B 1985, 31, 6207–6215. [Google Scholar] [CrossRef] [Green Version]
- Rao, D.; Zhang, L.; Wang, Y.; Meng, Z.; Qian, X.; Liu, J.; Shen, X.; Qiao, G.; Lu, R.J. Mechanism on the Improved Performance of Lithium Sulfur Batteries with MXene-Based Additives. Phys. Chem. C 2017, 121, 11047–11054. [Google Scholar] [CrossRef]
- Champagne, A.; Shi, L.; Ouisse, T.; Hackens, B.; Charlier, J.-C. Electronic and vibrational properties of V2C-based MXenes: From experiments to first-principles modeling. Phys. Rev. B 2018, 97, 115439. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Kutana, A.; Yakobson, B.I. Predicting stable phase monolayer Mo2C (MXene), a superconductor with chemically-tunable critical temperature. J. Mater. Chem. C 2017, 5, 3438–3444. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Dai, D.; Yanga, P.; Lic, L. Atomic orbitals in molecules: General electronegativity and improvement of Mulliken population analysis. Phys. Chem. Chem. Phys. 2006, 8, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Pazniak, H.; Varezhnikov, A.S.; Kolosov, D.A.; Plugin, I.A.; Di Vito, A.; Glukhova, O.E.; Sheverdyaeva, P.M.; Spasova, M.; Kaikov, I.; Kolesnikov, E.A.; et al. 2D Molybdenum Carbide MXenes for Enhanced Selective Detection of Humidity in Air. Adv. Mater. 2021, 33, 2104878. [Google Scholar] [CrossRef] [PubMed]

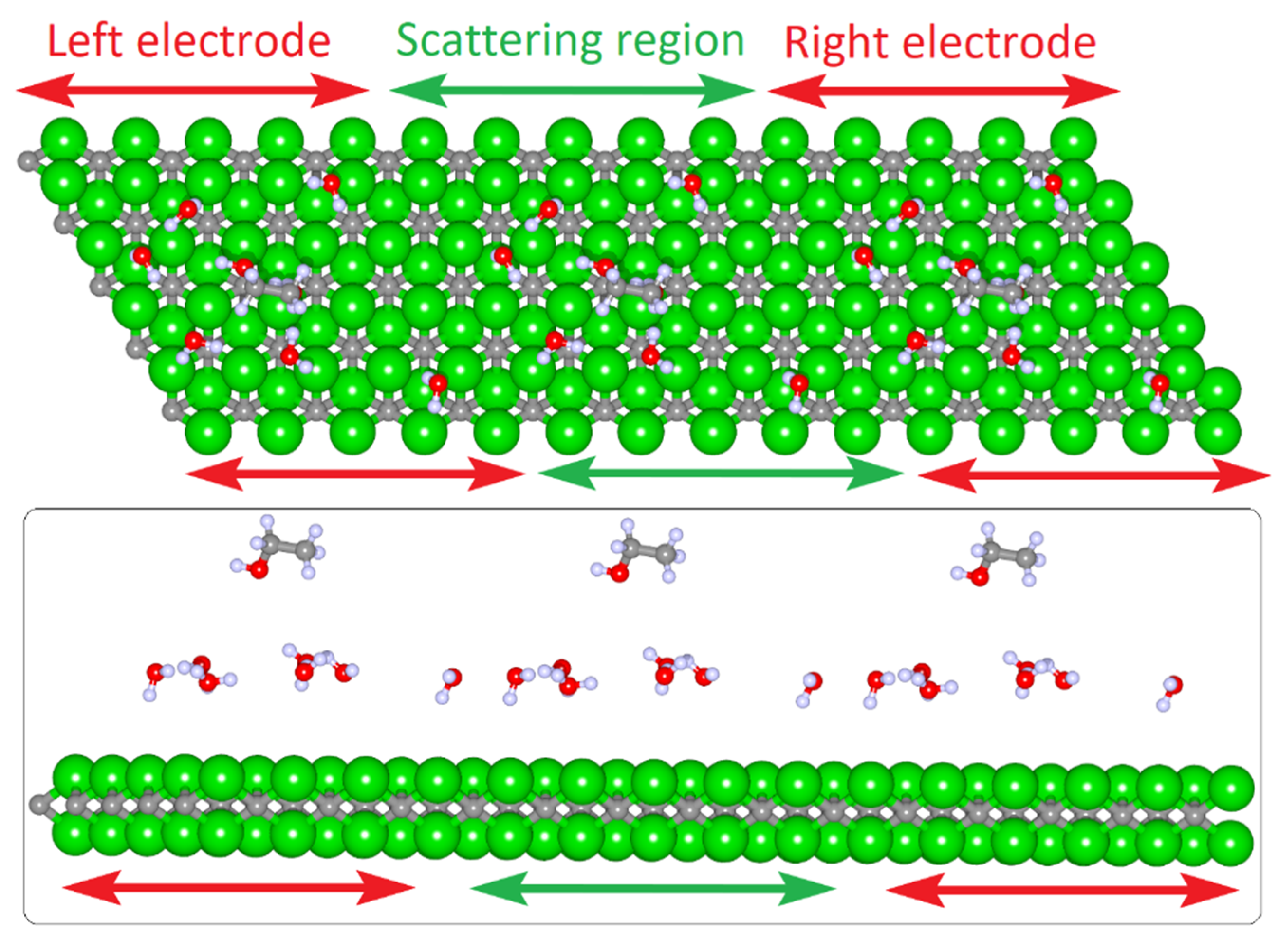
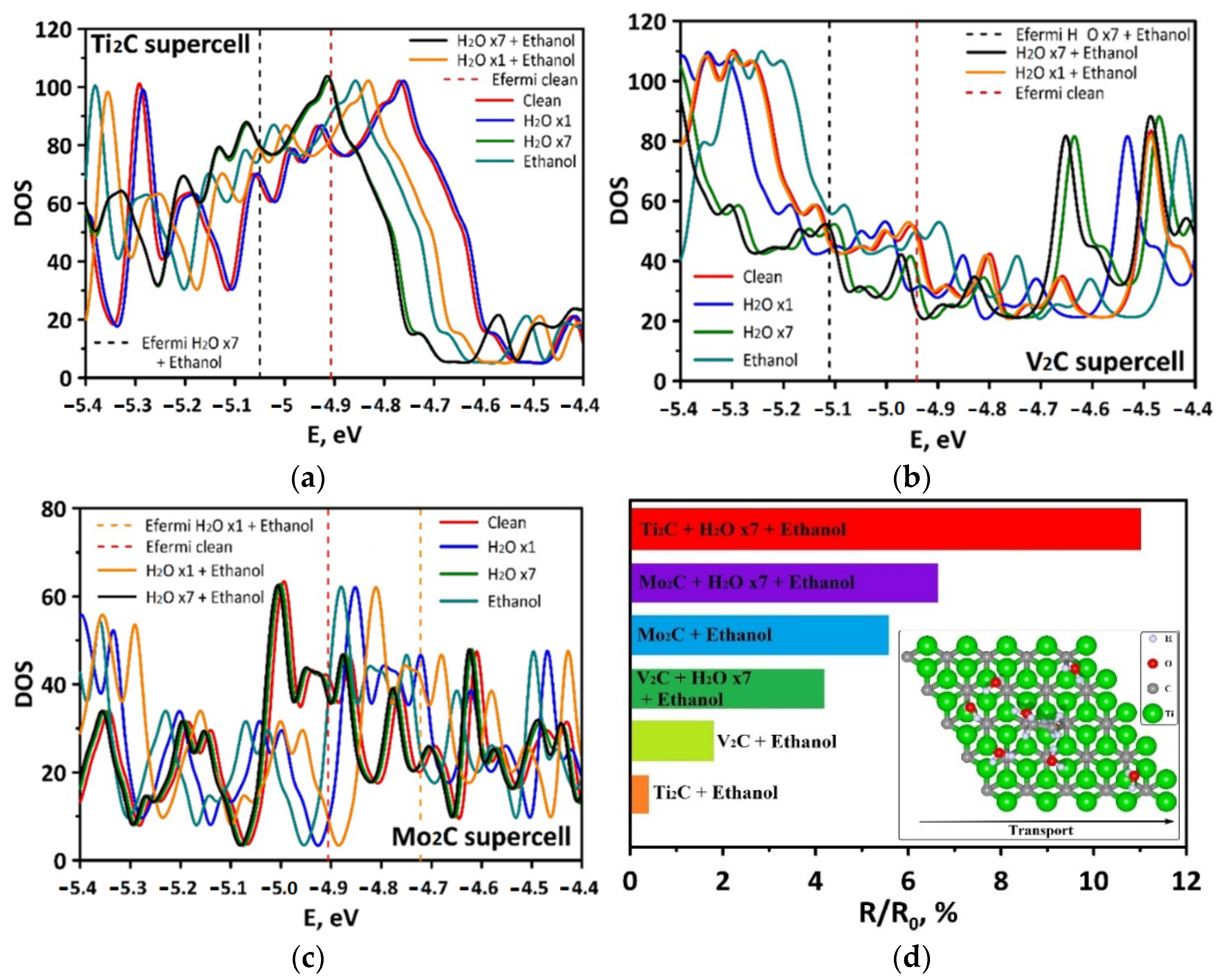


| Structure | EF, eV (Ti2C) | EF, eV (V2C) | EF, eV (Mo2C) |
|---|---|---|---|
| Pure MXene film | −4.907 | −4.941 | −4.906 |
| MXene + 1 water molecule | −4.899 | −4.992 | −4.764 |
| MXene + 7 water molecules | −5.048 | −5.094 | −4.913 |
| MXene + ethanol molecule | −4.996 | −4.887 | −4.791 |
| MXene + 1 water molecule + ethanol molecule | −4.970 | −4.947 | −4.722 |
| MXene + 7 water molecules + ethanol molecule | −5.050 | −5.111 | −4.918 |
| Structure | Excess Charge, e- (Ti2C) | Excess Charge, e- (V2C) | Excess Charge, e- (Mo2C) |
|---|---|---|---|
| MXene + 1 water molecule | −0.010 | −0.006 | −0.021 |
| MXene + 7 water molecules | 0.014 | 0.011 | 0.054 |
| MXene + ethanol molecule | −0.007 | −0.015 | −0.011 |
| MXene + 1 water molecule + ethanol molecule | (−0.01) H2O (0.012) Ethanol | (−0.004) H2O (0.006) Ethanol | (−0.006) H2O (−0.014) Ethanol |
| MXene + 7 water molecules + ethanol molecule | (−0.008) H2O x7 (0.006) Ethanol | (−0.009) H2O x7 (0.0011) Ethanol | (−0.079) H2O x7 (0.007) Ethanol |
| Structure | R, Ohm (Ti2C) | R, Ohm (V2C) | R, Ohm (Mo2C) |
|---|---|---|---|
| MXene + 1 water molecule | 886.1 | 2562.7 | 973.2 |
| MXene + 7 water molecules | 901.1 | 2604.8 | 1011.2 |
| MXene + ethanol molecule | 983.9 | 2670.3 | 1037.8 |
| MXene + 1 water molecule + ethanol molecule | 890.1 | 2609.5 | 1028.1 |
| MXene + 7 water molecules + ethanol molecule | 902.3 | 2613.3 | 1012.4 |
| Structure | Binding Energy, eV (Ti2C) | Binding Energy, eV (V2C) | Binding Energy, eV (Mo2C) |
|---|---|---|---|
| MXene + 1 water molecule | −0.068 | −0.054 | −0.132 |
| MXene + 7 water molecules | −0.841 | −0.601 | −1.287 |
| MXene + ethanol molecule | −0.129 | −0.137 | −0.051 |
| MXene + 1 water molecule + ethanol molecule | −0.226 | −0.136 | −0.109 |
| MXene + 7 water molecules + ethanol molecule | −1.132 | −0.625 | −1.375 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolosov, D.A.; Levitsky, S.G.; Glukhova, O.E. Adhesion and Electron Properties of Quasi-2D Mo2C, Ti2C, and V2C MXene Flakes after Van Der Waals Adsorption of Alcohol Molecules: Influence of Humidity. Lubricants 2022, 10, 159. https://doi.org/10.3390/lubricants10070159
Kolosov DA, Levitsky SG, Glukhova OE. Adhesion and Electron Properties of Quasi-2D Mo2C, Ti2C, and V2C MXene Flakes after Van Der Waals Adsorption of Alcohol Molecules: Influence of Humidity. Lubricants. 2022; 10(7):159. https://doi.org/10.3390/lubricants10070159
Chicago/Turabian StyleKolosov, Dmitry A., Semyon G. Levitsky, and Olga E. Glukhova. 2022. "Adhesion and Electron Properties of Quasi-2D Mo2C, Ti2C, and V2C MXene Flakes after Van Der Waals Adsorption of Alcohol Molecules: Influence of Humidity" Lubricants 10, no. 7: 159. https://doi.org/10.3390/lubricants10070159






