Enhancing the Performance of Rapeseed Oil Lubricant for Machinery Component Applications through Hybrid Blends of Nanoadditives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Characterization
2.2. Sample Preparation
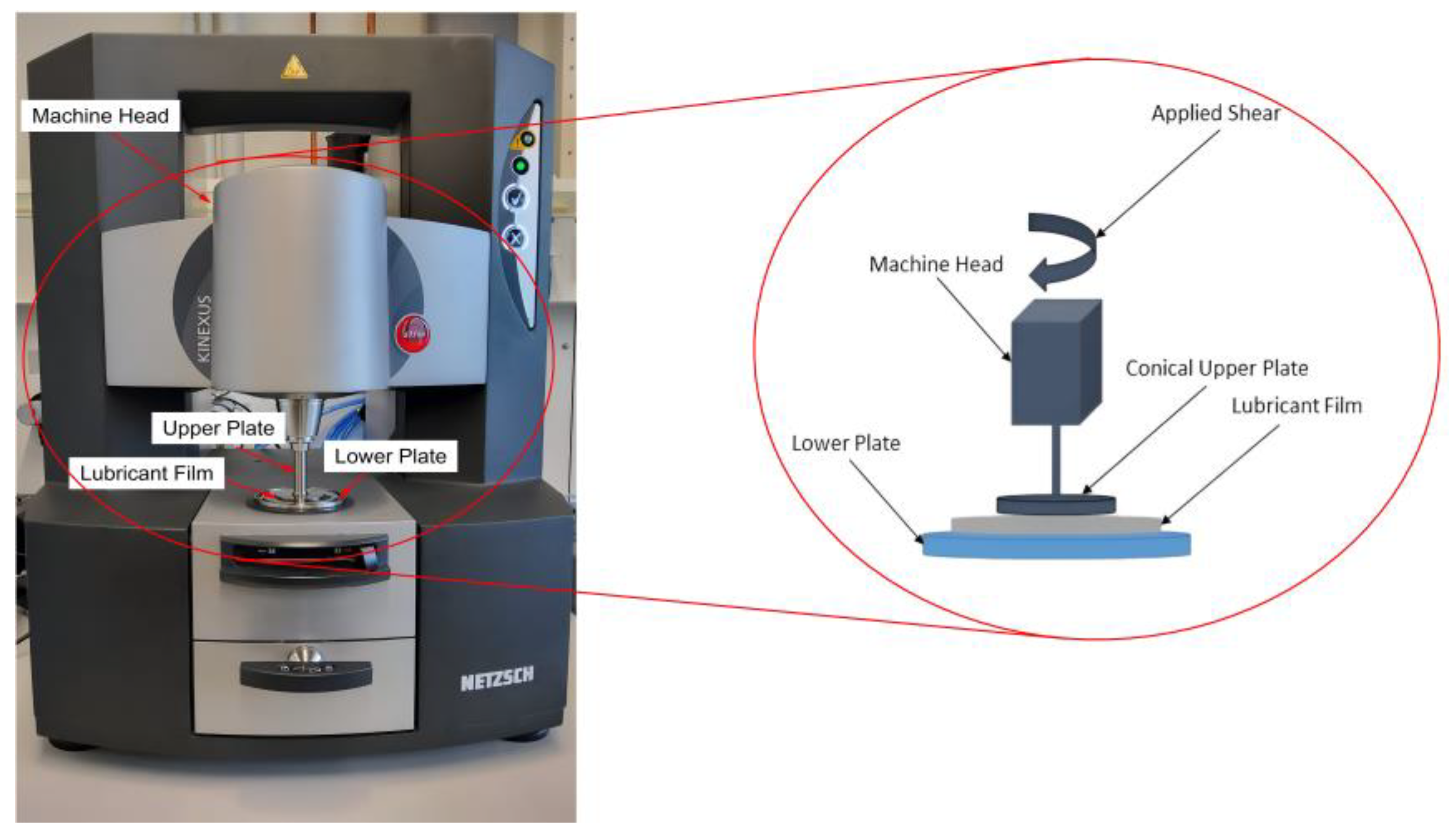
2.3. Rheological Behavior
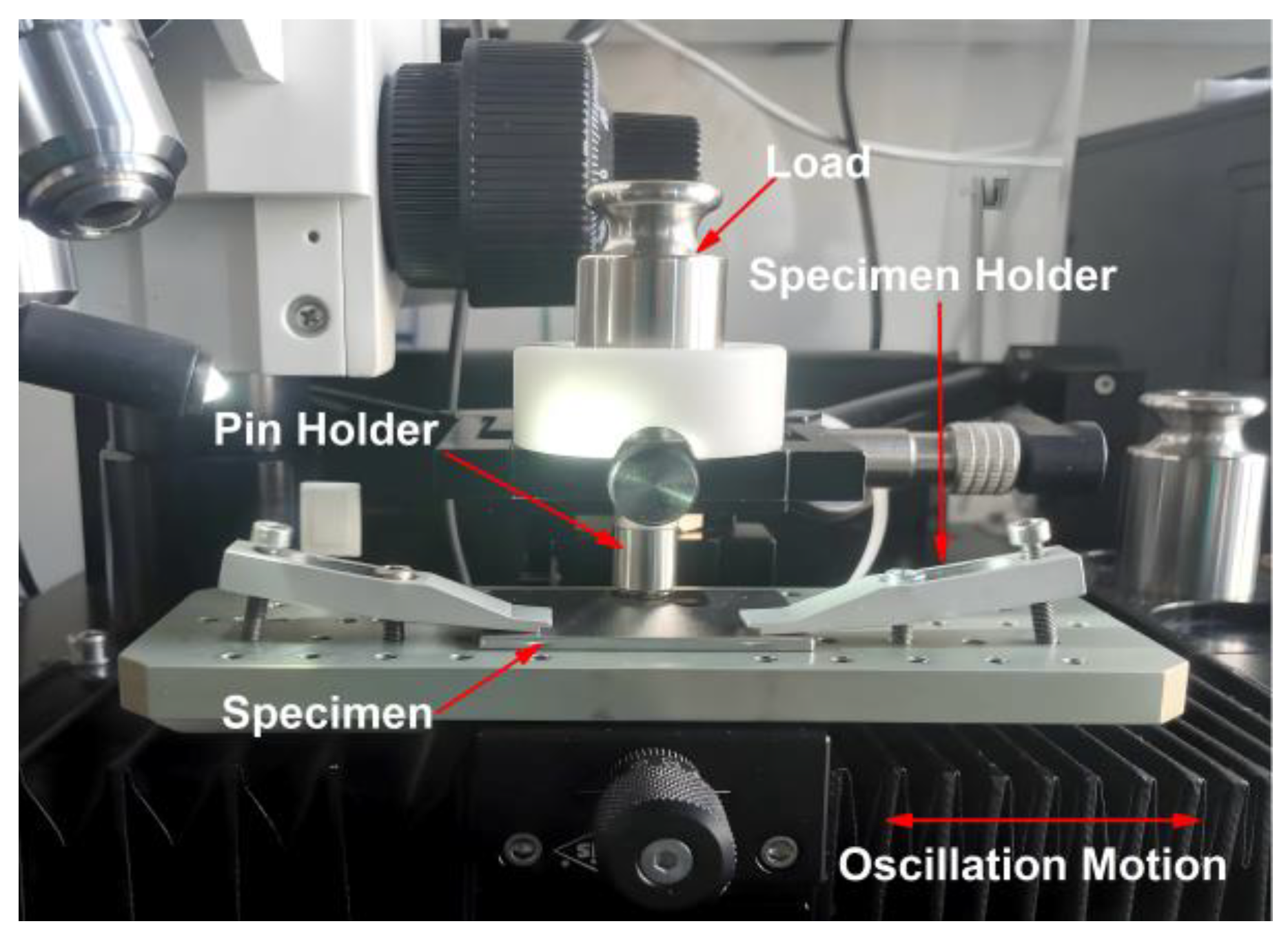
2.4. Tribological Properties
2.5. Wettability Analysis
3. Results and Discussion
3.1. Materials and Characterization
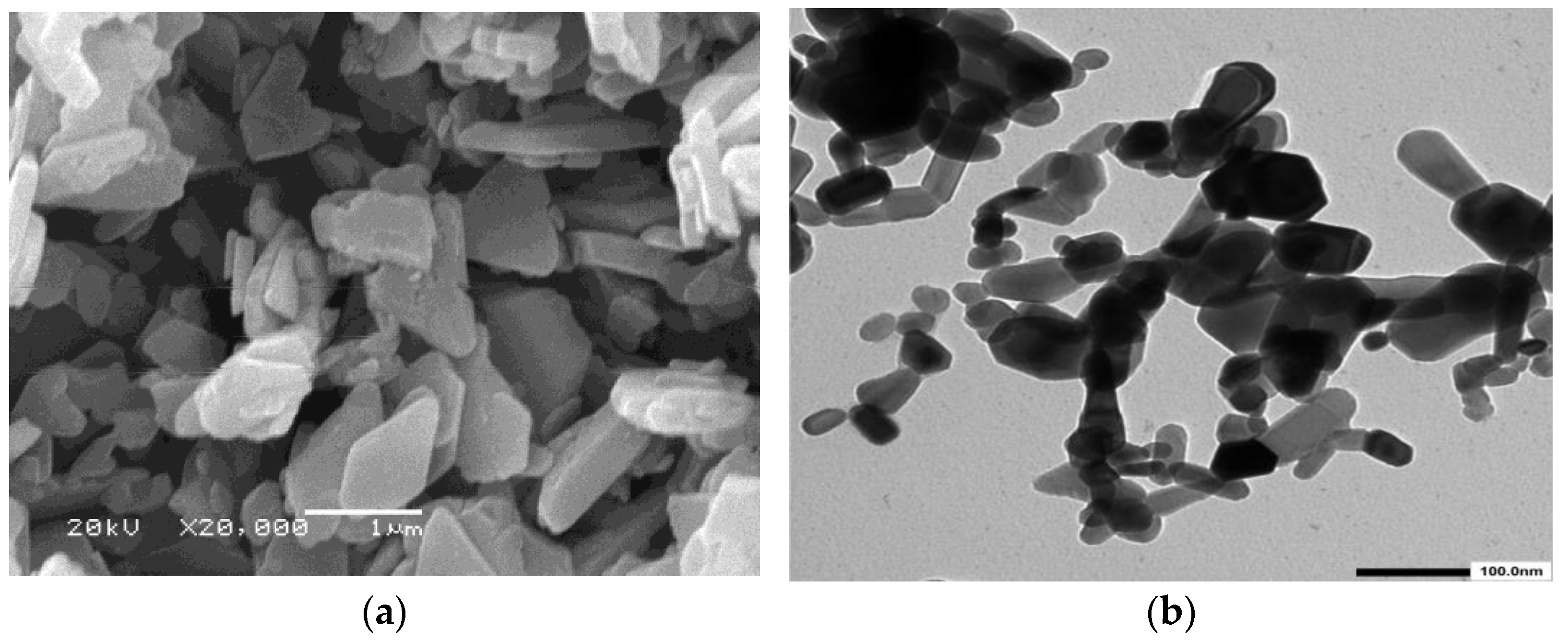
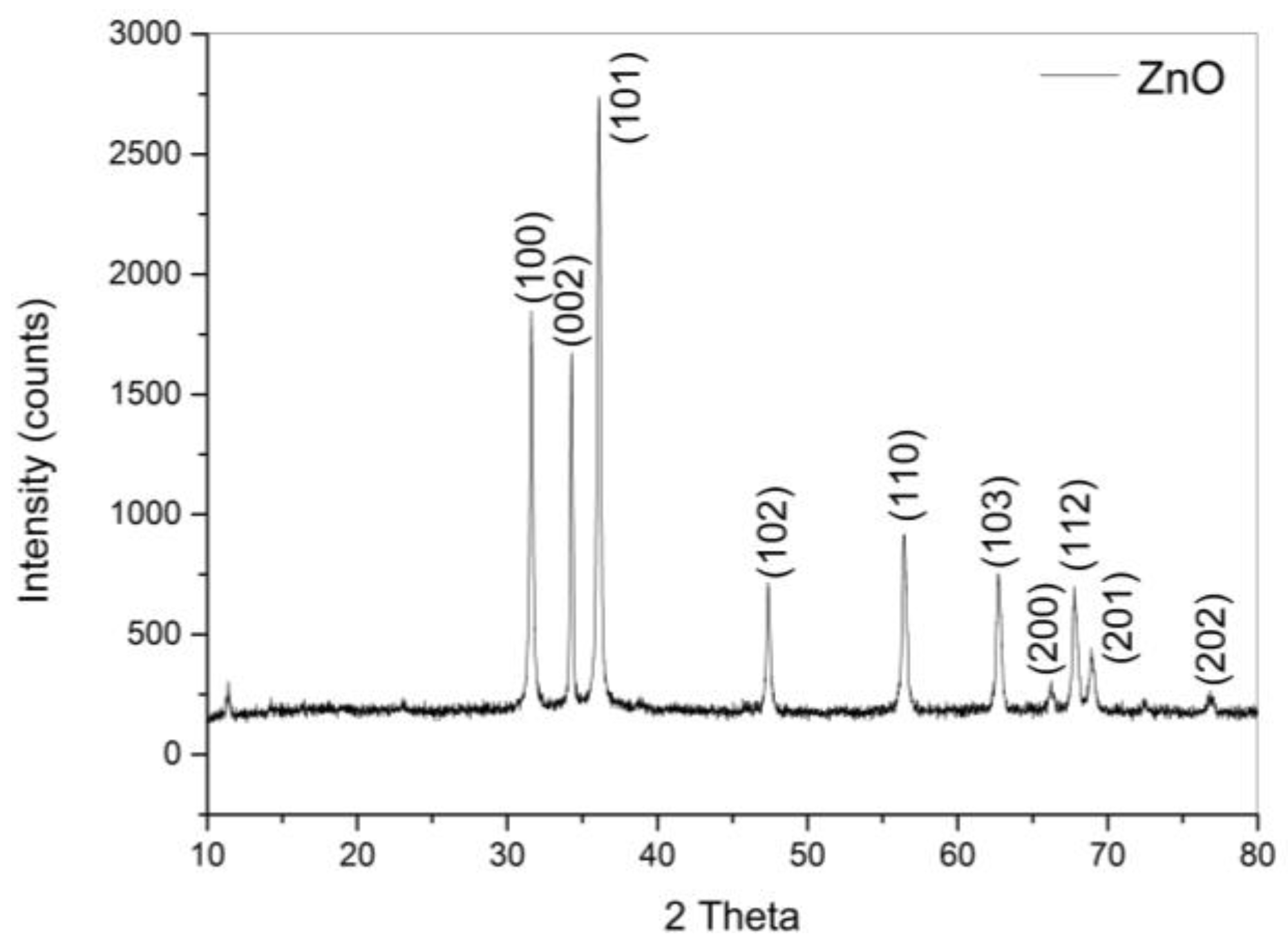
3.1.1. ZnO Characterization
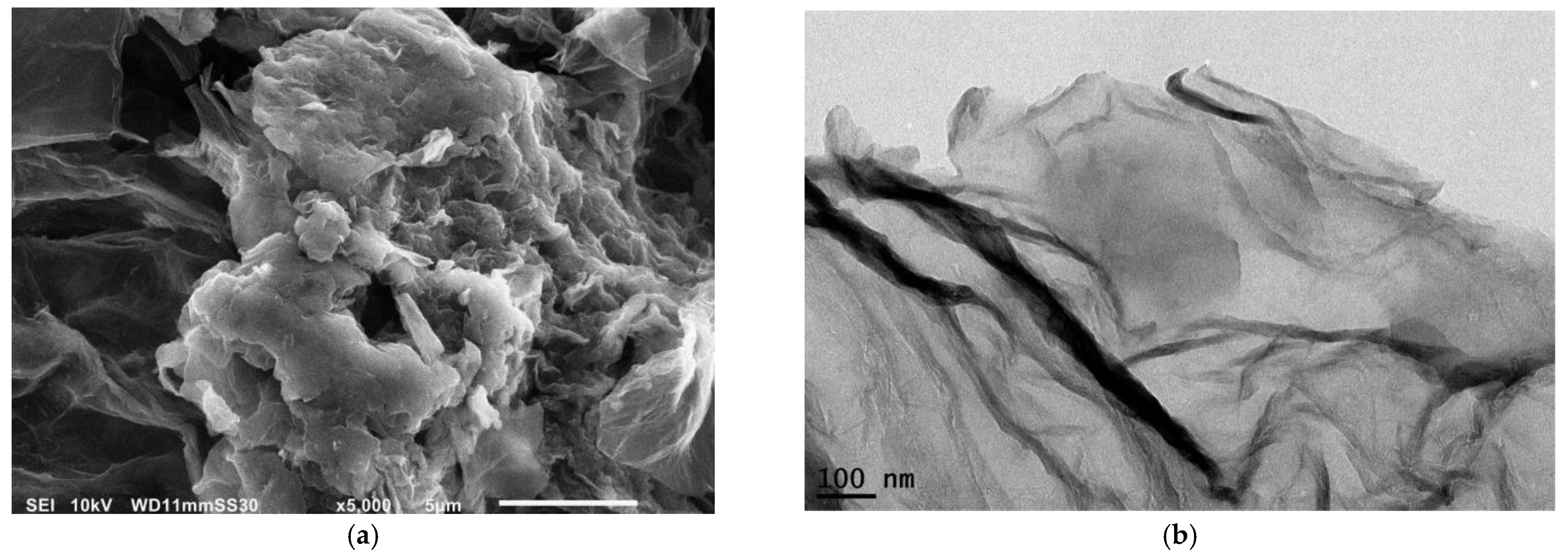
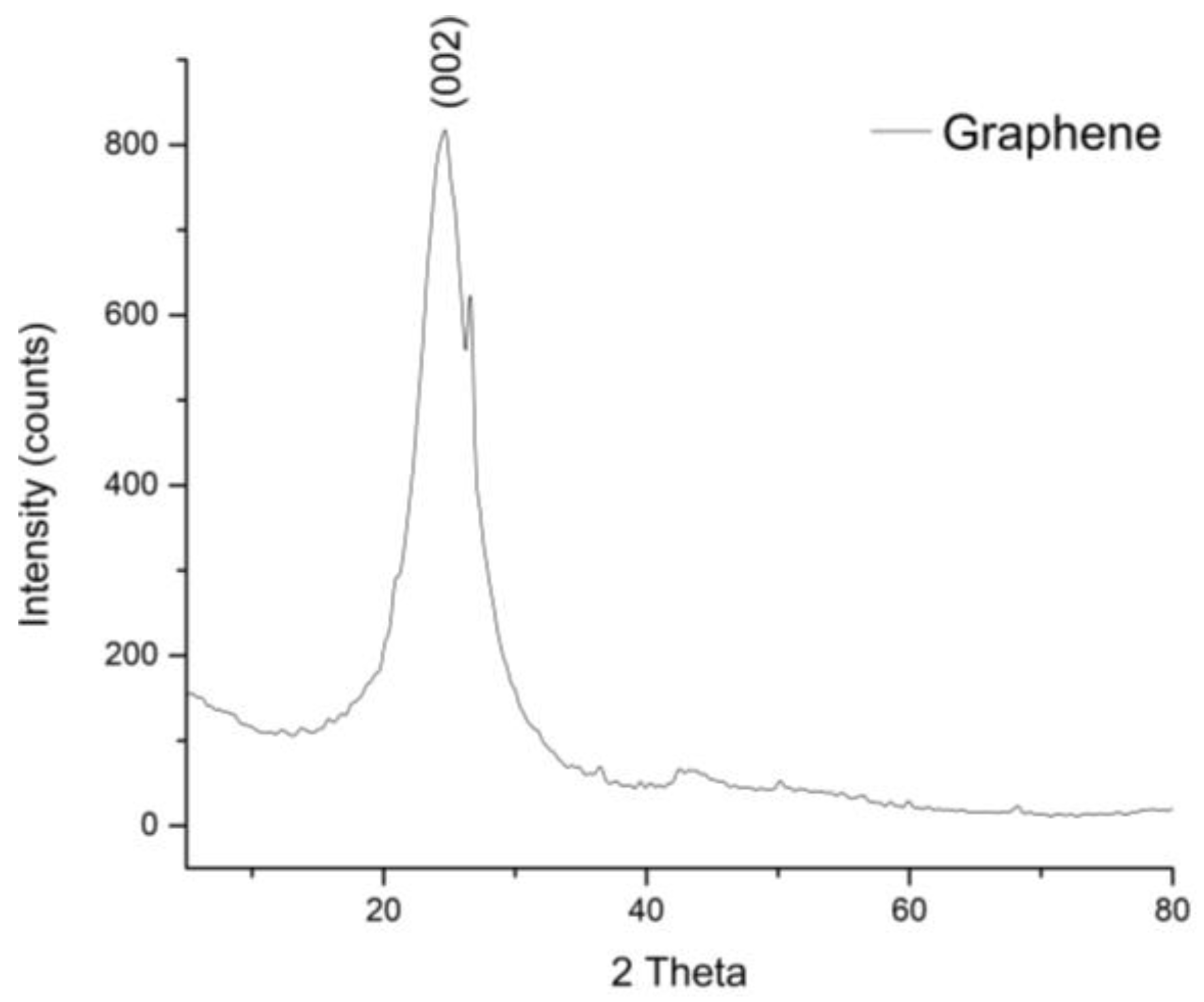
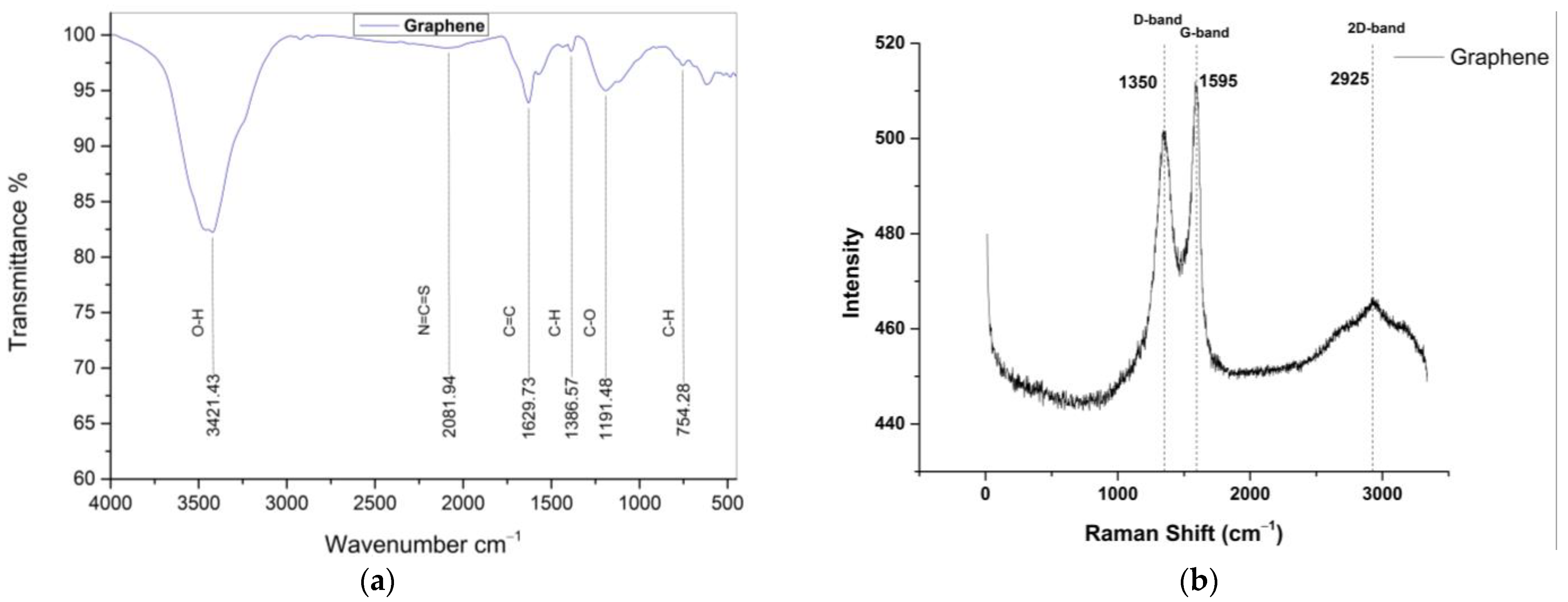
3.1.2. Graphene Characterization
3.2. Rheological Behavior
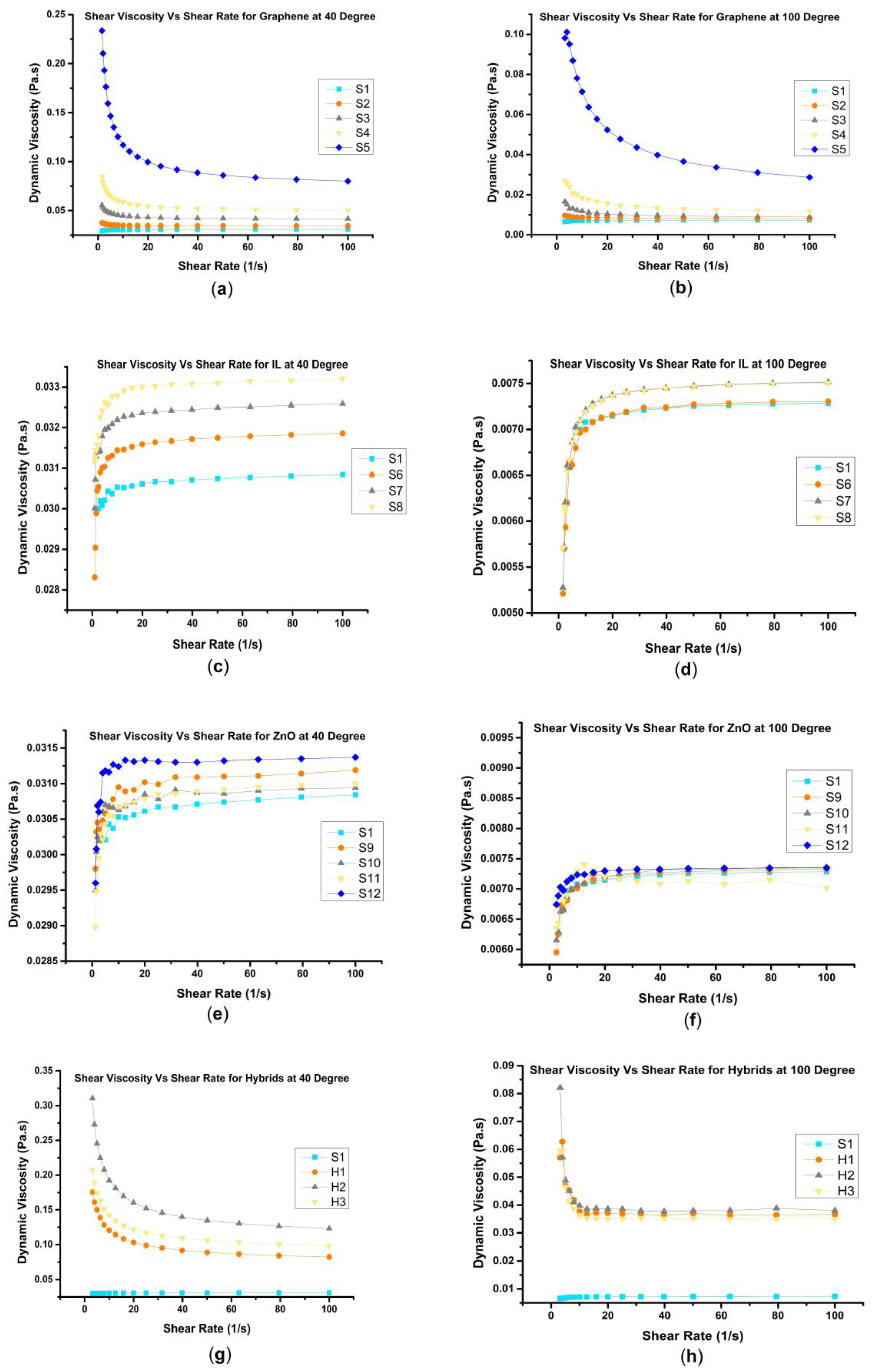
3.2.1. Effect of Shear Rate
3.2.2. Effect of Nanoadditive Concentration
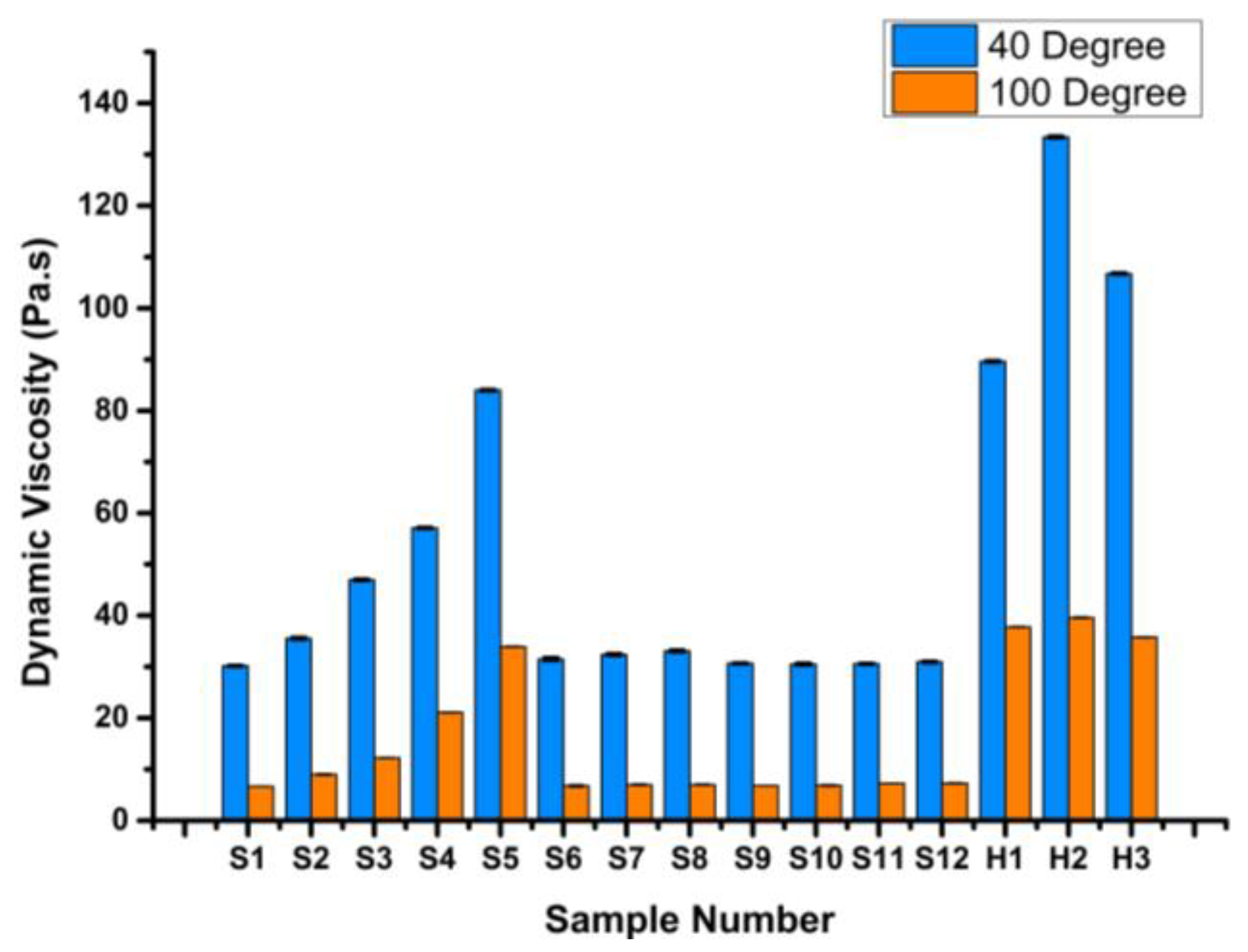
3.2.3. Viscosity Index
3.3. Tribological Properties
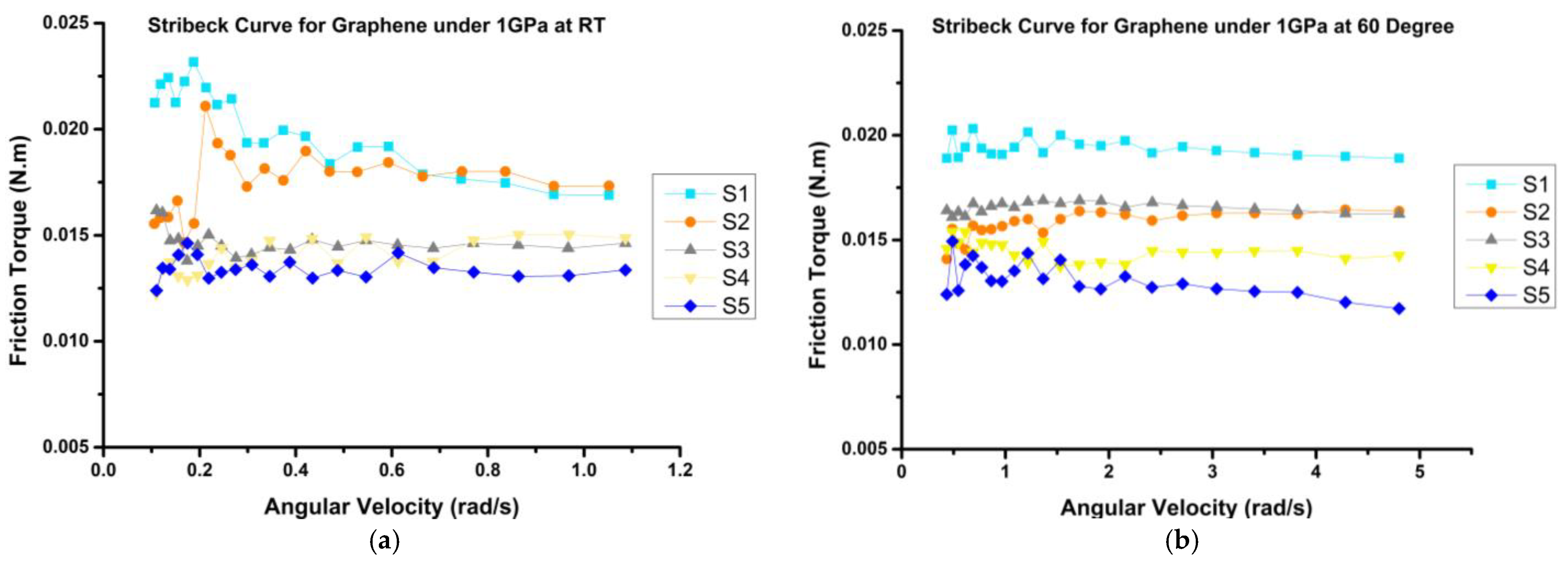
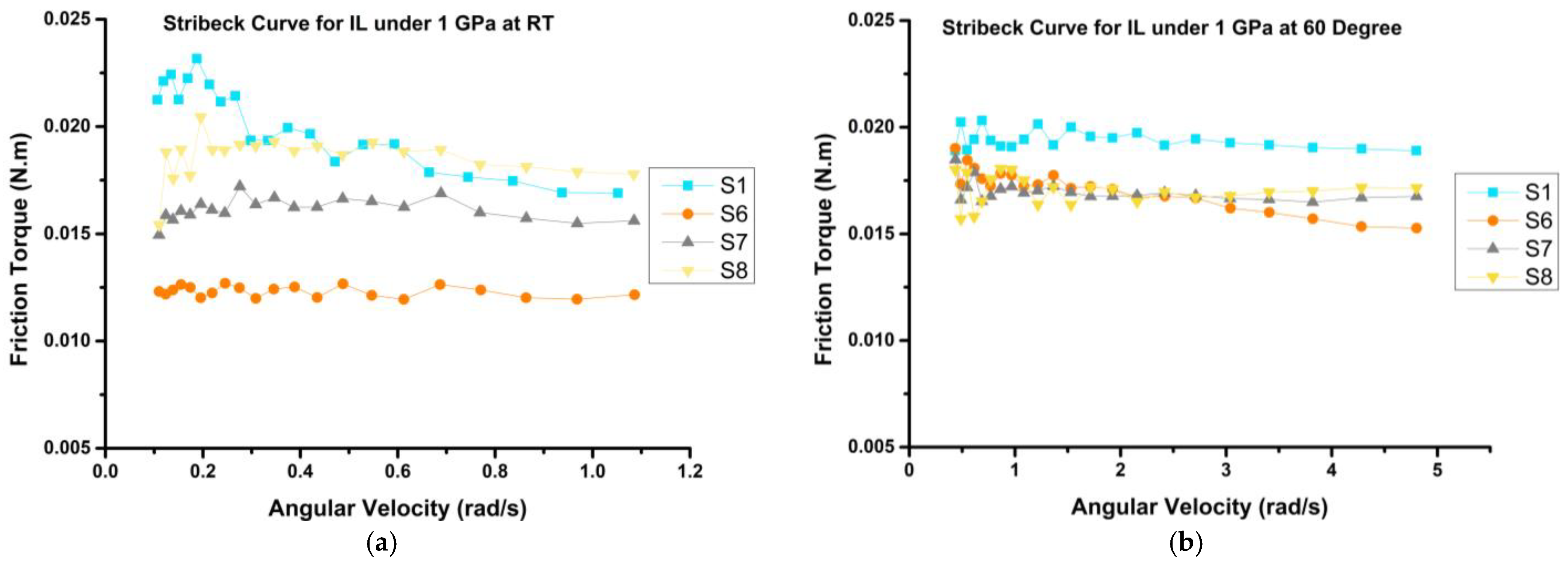
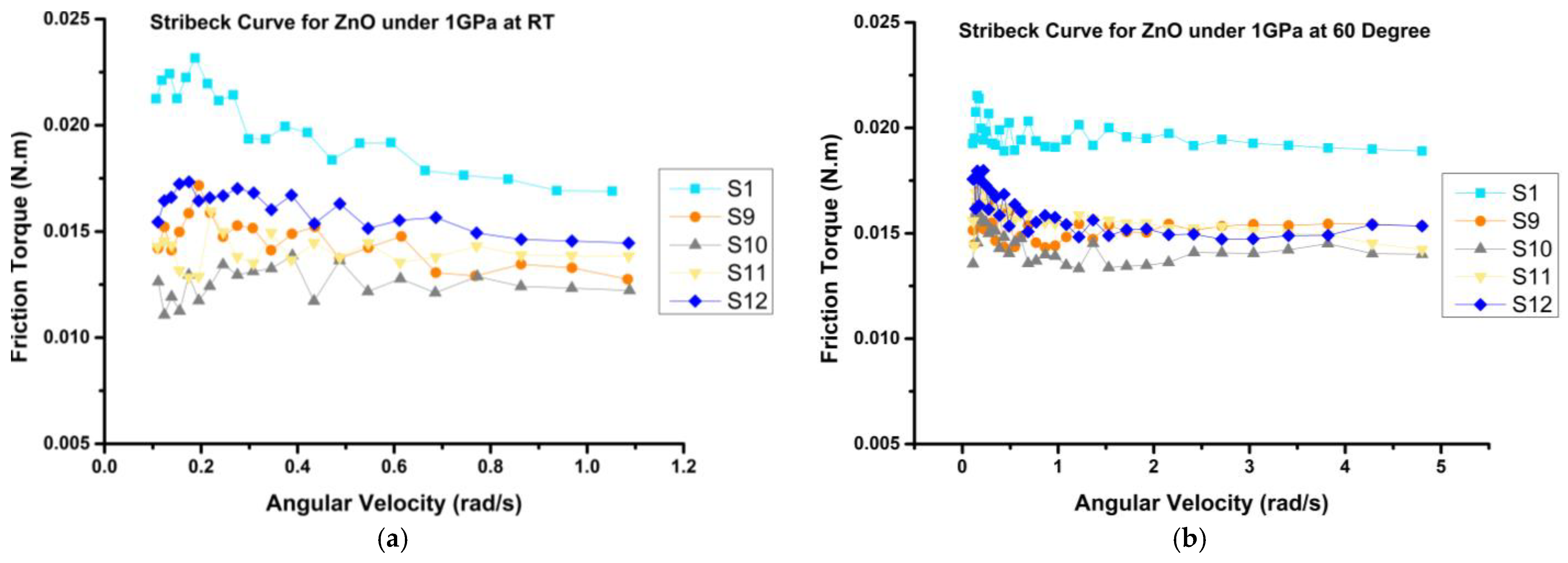
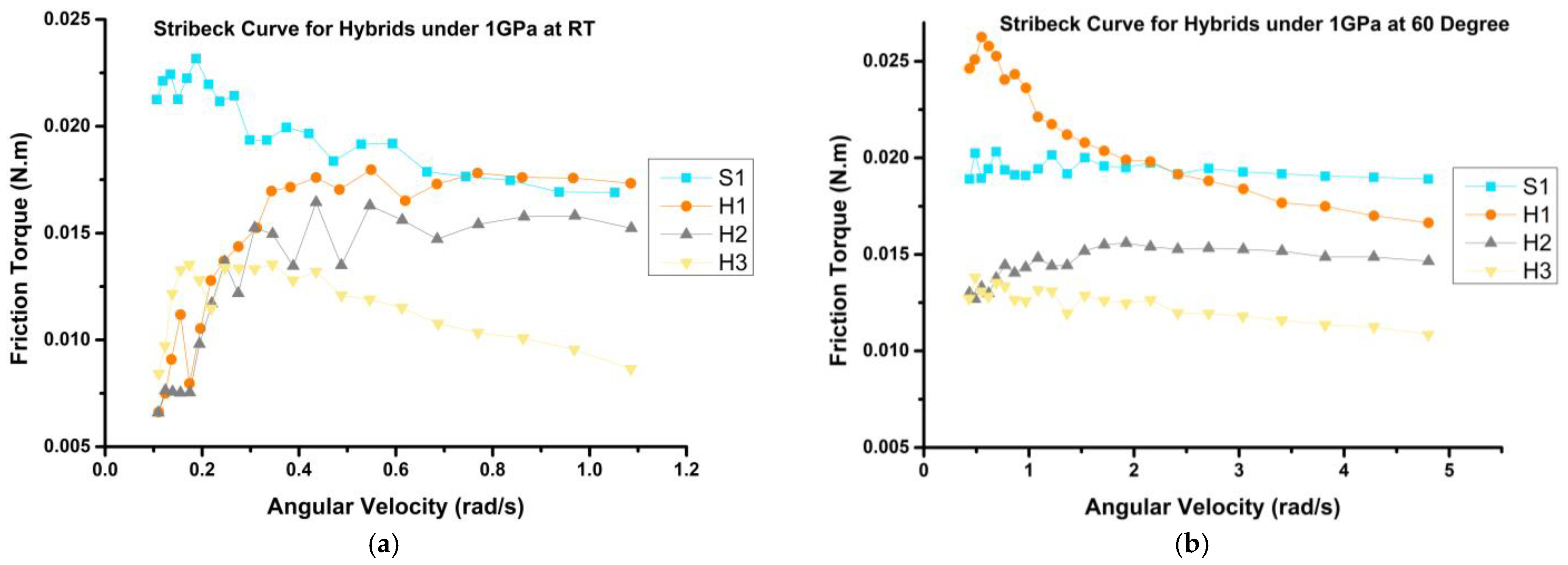
3.3.1. Frictional Behavior
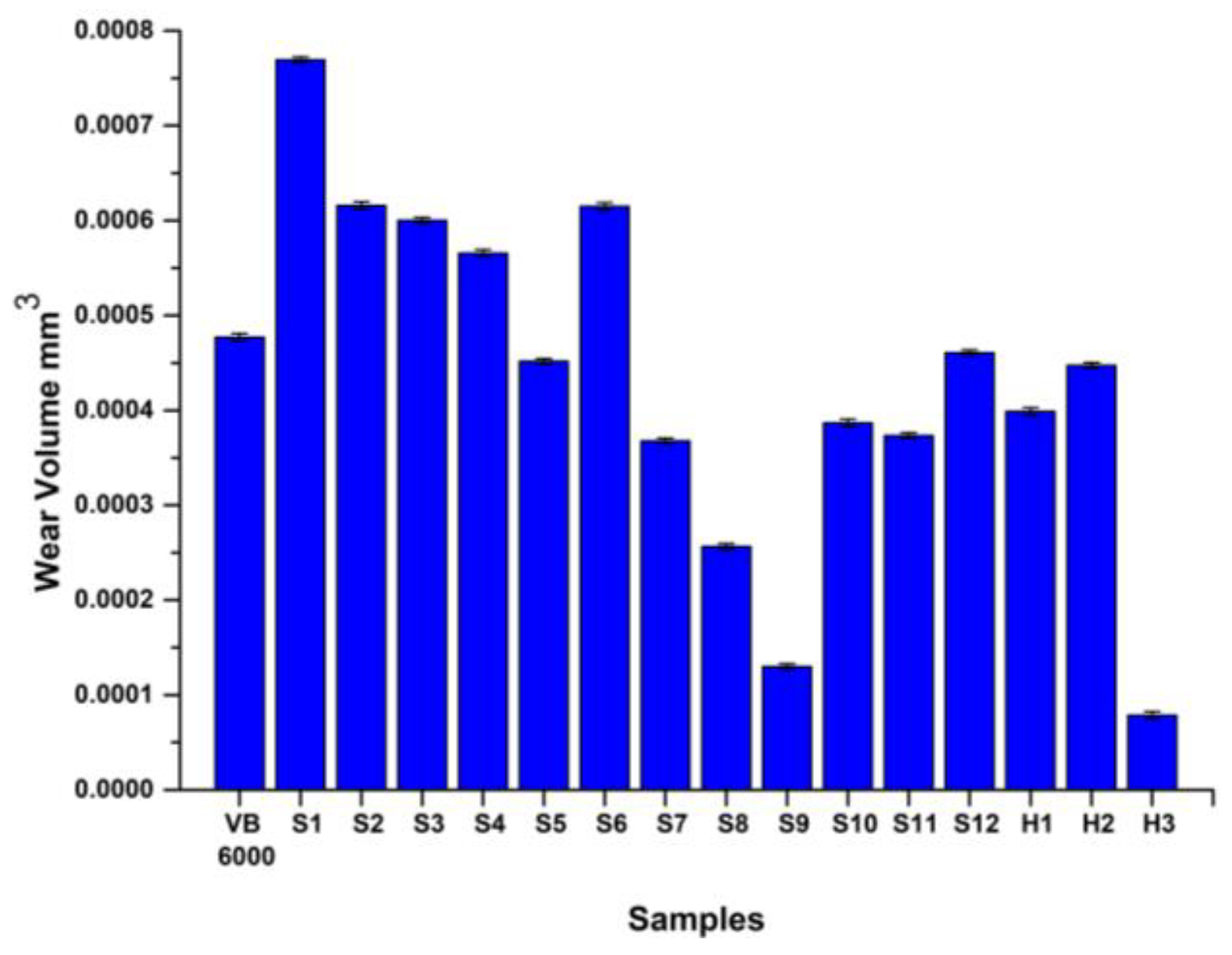
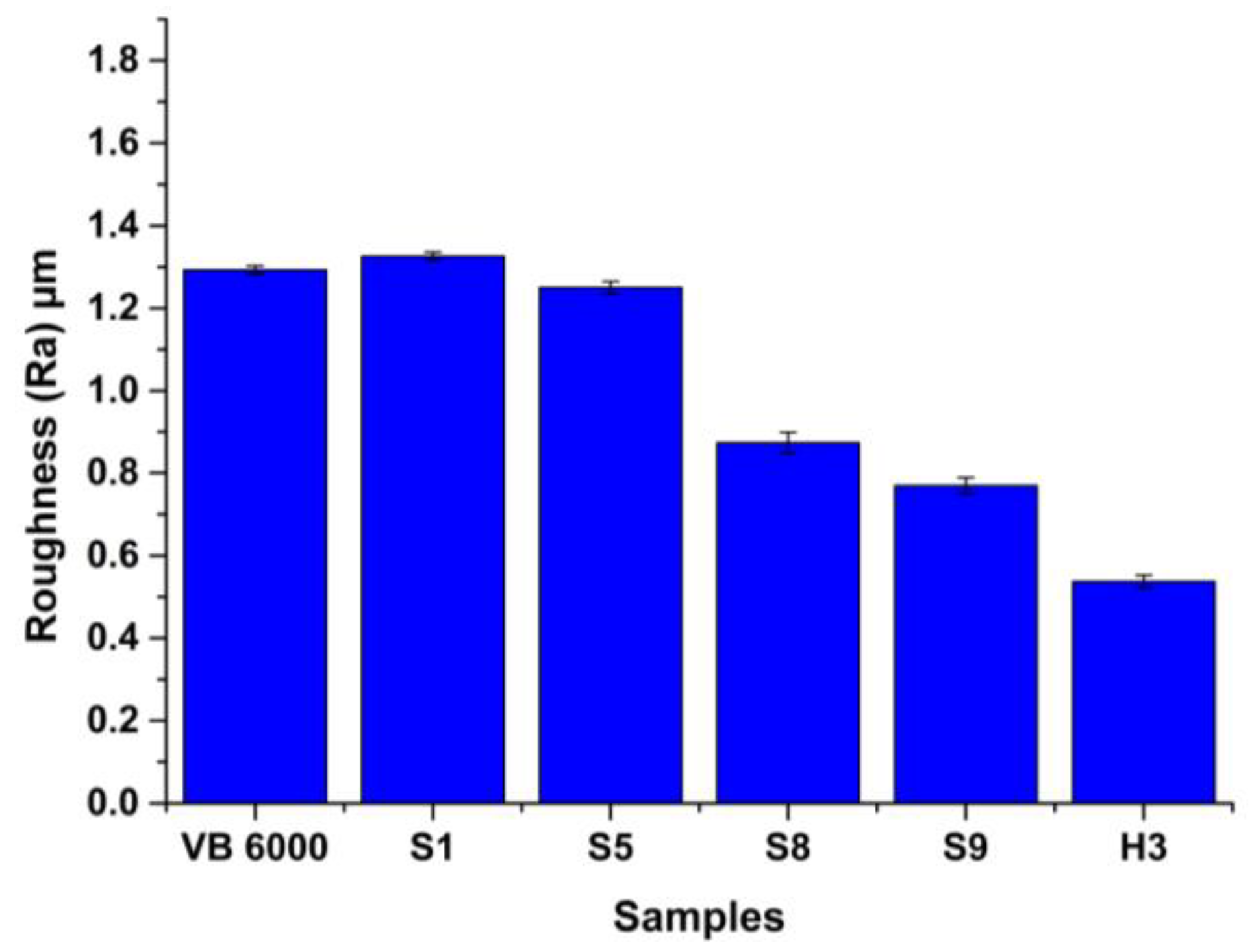
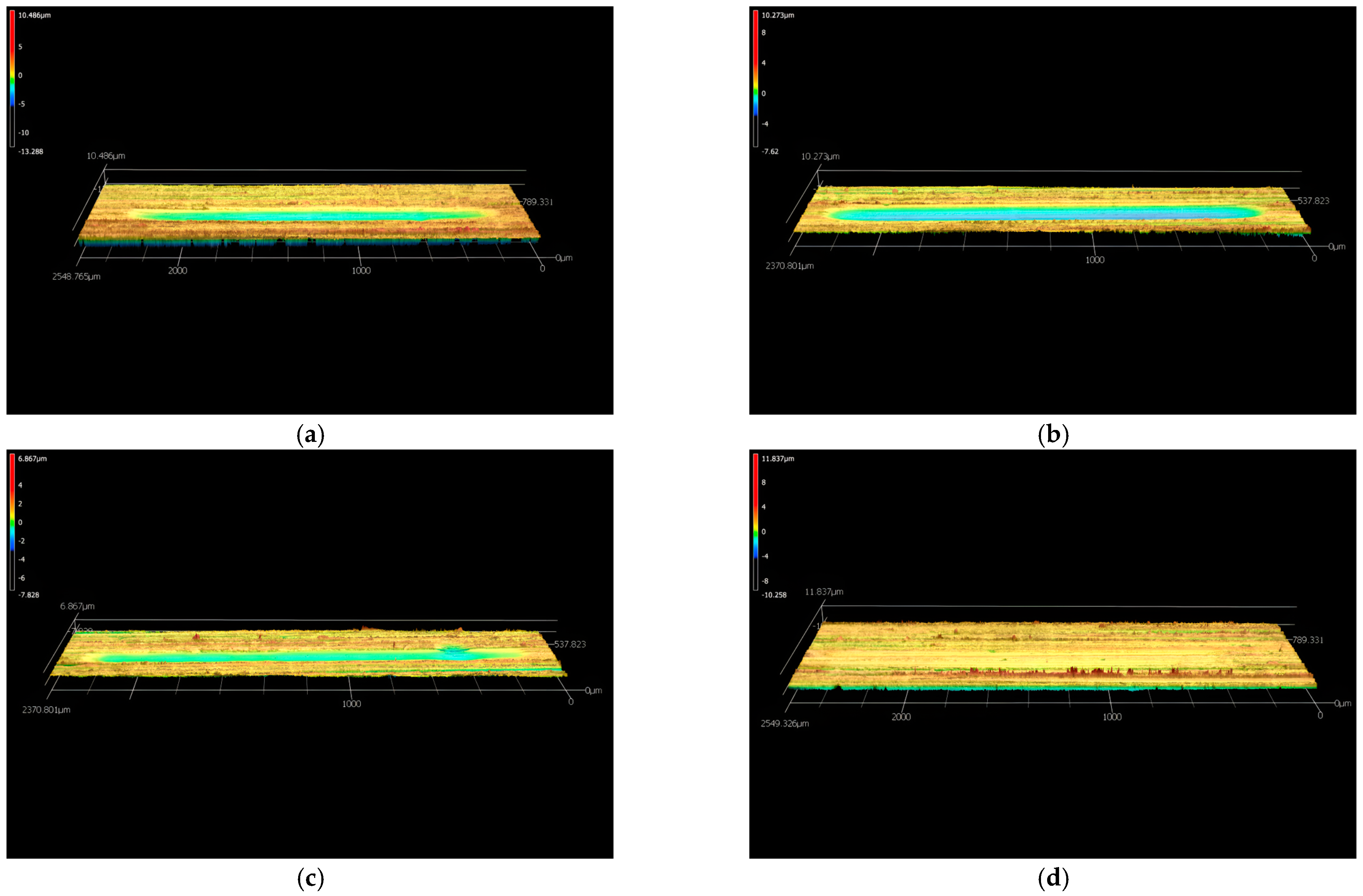
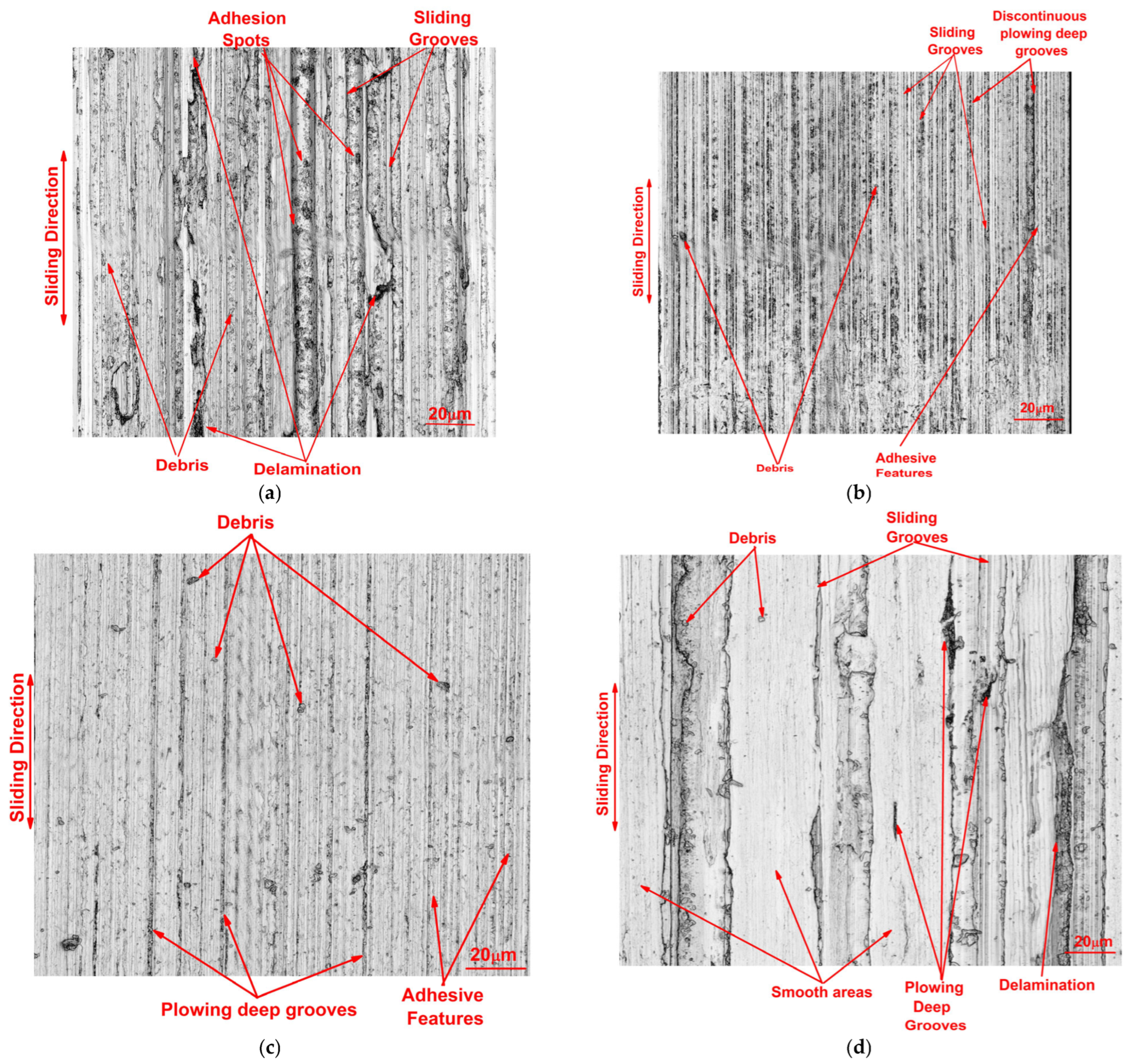
3.3.2. Wear Results
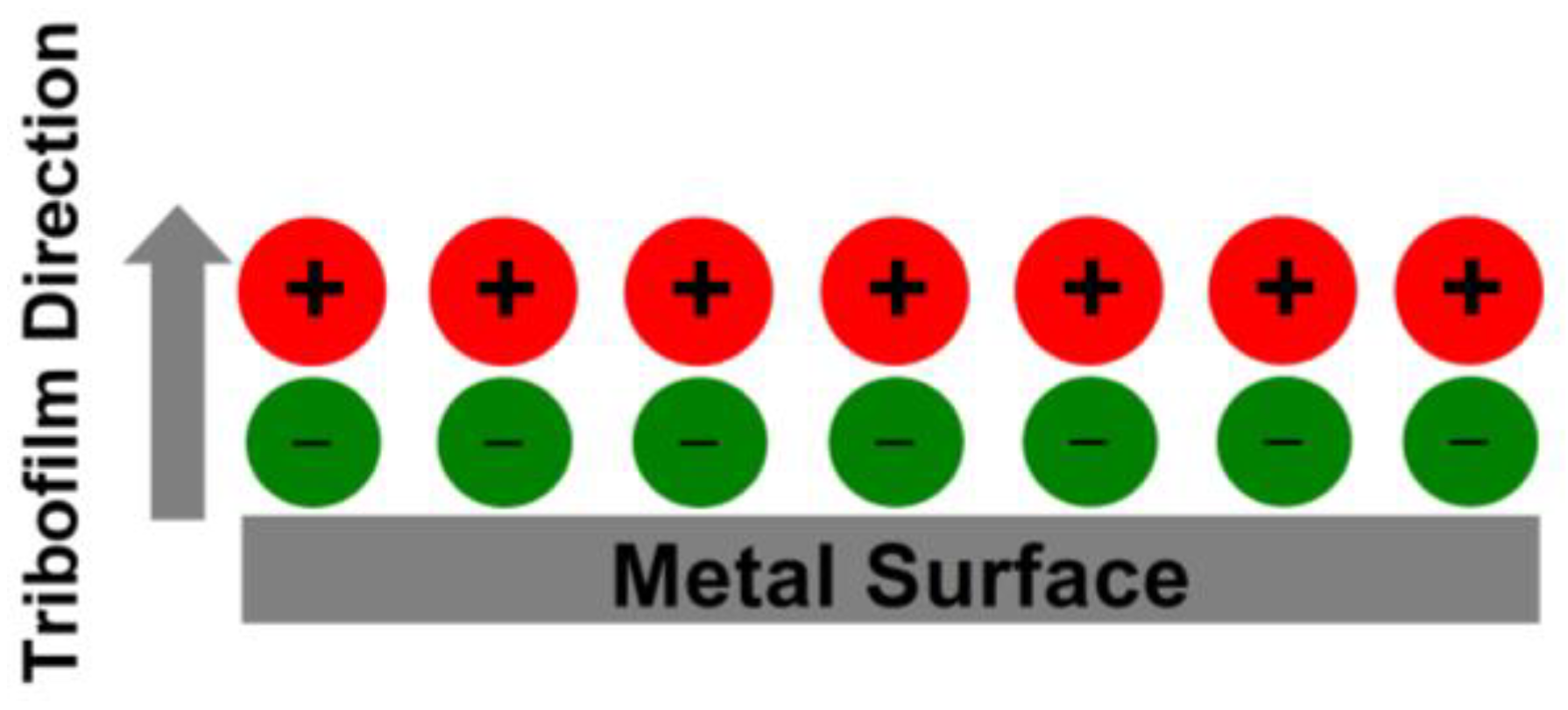
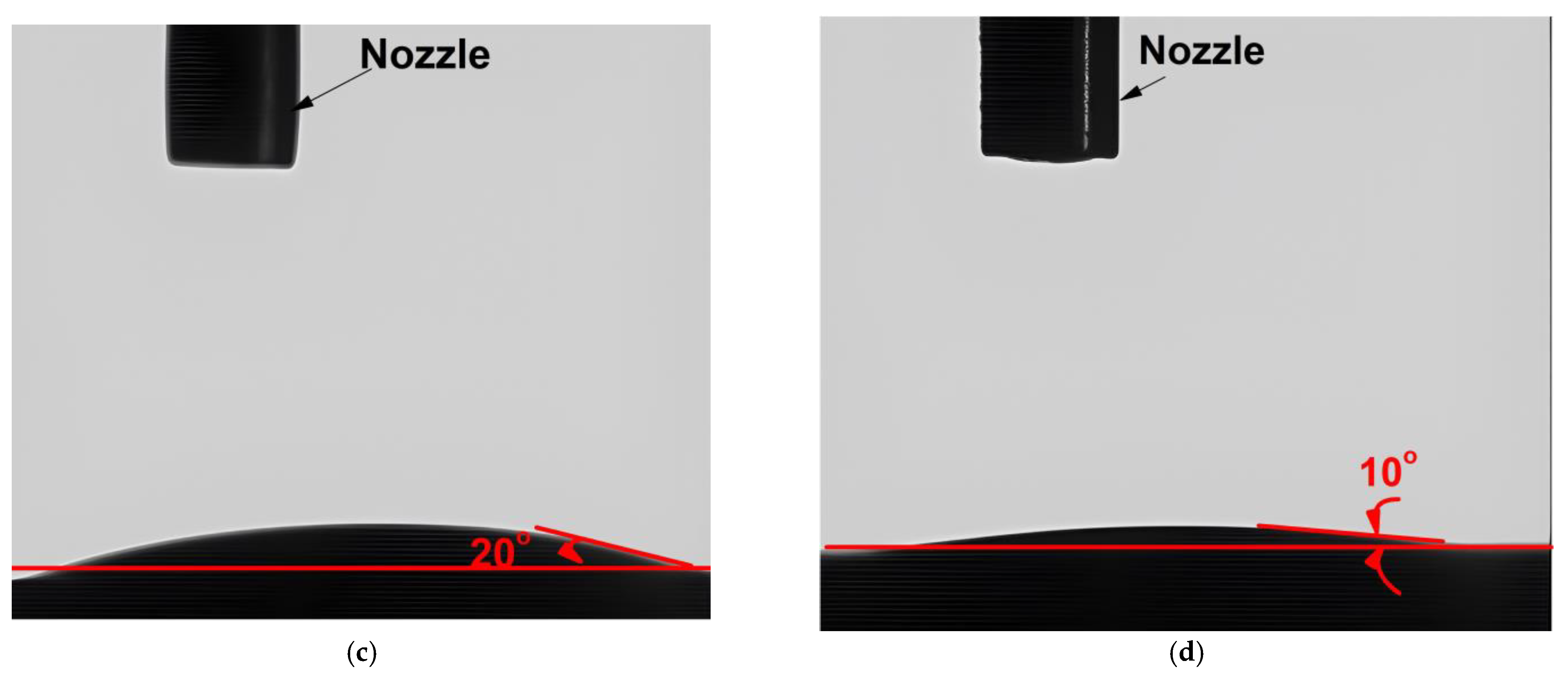
3.4. Wettability Analysis
4. Conclusions
- The addition of graphene to rapeseed oil resulted in shear thinning behavior, while ZnO and IL exhibited a shear thickening trend. When these nanoadditives were combined in hybrids, such as H1, H2, and H3, the shear thickening behavior of ZnO and IL transformed into a milder shear thinning behavior, approaching a near-Newtonian behavior, particularly in H1 and H3.
- VI values showed a drastic increase of up to 150% after adding graphene to rapeseed oil, emphasizing its superior thermal stability when compared to ZnO and IL, which exhibited enhancements in the range of 10% to 38% compared to VB 6000.
- The frictional behavior of the prepared mixtures demonstrated reductions of up to 41% and 35% at room temperature (RT) and 60 °C, respectively, when compared to the bio-oil sample (S1). Notably, H3 emerged as the optimal sample, exhibiting the most substantial decrease in coefficient of friction (COF) by up to 30%, as compared to VB 6000.
- Concerning wear behavior, H3 exhibited negligible wear scar, achieving a superior reduction in wear volume by 84% when compared to VB 6000. This highlights its enhanced wear resistance, further supported by its minimal average roughness value on the worn surface and the smoothest worn area.
- The physical behavior of the chosen optimal samples, S9 and H3, was evaluated by examining their wettability characteristics. H3 exhibited significantly superior wettability on a stainless-steel surface, surpassing VB 6000 by 63%.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Can Oil Be Recycled? 2009. Available online: https://www.scientificamerican.com/article/can-oil-be-recycled/#:~:text=Condela%3A%20There’s%20about%201.3%20billion,power%20plants%20or%20industrial%20boilers (accessed on 19 October 2023).
- Machine Oil: Reclaim, Recycle or Regenerate? Available online: https://www.skf.com/id/services/recondoil/knowledge-hub/recondoil-articles/machine-oil-reclaim-recycle-or-regenerate (accessed on 19 October 2023).
- Abere, J.O. Improved Performance of Bio-Lubricant by Nanoparticles Additives. Ph.D. Thesis, University of Sheffield, Sheffield, UK, 2017. [Google Scholar]
- Lee, C.T.; Lee, M.B.; Mong, G.R.; Chong, W.W.F. A bibliometric analysis on the tribological and physicochemical properties of vegetable oil–based bio-lubricants (2010–2021). Environ. Sci. Pollut. Res. 2022, 29, 56215–56248. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, C.; Usman, Z.; Agada, F. Biodiesel production from Ceiba pentandra seed oil using CaO derived from snail shell as catalyst. Pet. Sci. Eng. 2018, 2, 7–16. [Google Scholar] [CrossRef]
- Kumar, G.; Garg, H.C. Influence of a halogen free ionic liquid on the rheological and tribological characteristics of canola oil. Ind. Lubr. Tribol. 2022, 74, 914–921. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, X.; Yang, S.; Chen, H.; Wang, D. The study of epoxidized rapeseed oil used as a potential biodegradable lubricant. J. Am. Oil Chem. Soc. 2000, 77, 561–563. [Google Scholar] [CrossRef]
- Durak, E.r. A study on friction behavior of rapeseed oil as an environmentally friendly additive in lubricating oil. Ind. Lubr. Tribol. 2004, 56, 23–37. [Google Scholar] [CrossRef]
- Matthaus, B.; Özcan, M.M.; Al Juhaimi, F. Some rape/canola seed oils: Fatty acid composition and tocopherols. Z. Naturforschung C 2016, 71, 73–77. [Google Scholar] [CrossRef]
- Arnšek, A.; Vižintin, J. Lubricating properties of rapeseed-based oils. J. Synth. Lubr. 2000, 16, 281–296. [Google Scholar] [CrossRef]
- Kandeva, M.; Kalitchin, Z.; Zadorozhnaya, E.; Vencl, A. Performance characteristics of lubricant based on rapeseed oil containing different amounts of metal-containing additive. Ind. Lubr. Tribol. 2022, 74, 309–315. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative Stability of Selected Edible Oils. Molecules 2018, 23, 1746. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Mechanisms and Factors for Edible Oil Oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Parker, T.D.; Adams, D.; Zhou, K.; Harris, M.; Yu, L. Fatty acid composition and oxidative stability of cold-pressed edible seed oils. J. Food Sci. 2003, 68, 1240–1243. [Google Scholar] [CrossRef]
- Gertz, C.; Klostermann, S.; Kochhar, S.P. Testing and comparing oxidative stability of vegetable oils and fats at frying temperature. Eur. J. Lipid Sci. 2000, 102, 543–551. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, J. Ionic Liquids as Lubricant Additives: A Review. ACS Appl. Mater. Interfaces 2017, 9, 3209–3222. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, F.; Liang, Y.; Liu, W. Tribological performance of phosphonium based ionic liquids for an aluminum-on-steel system and opinions on lubrication mechanism. Wear 2006, 261, 1174–1179. [Google Scholar] [CrossRef]
- Gutierrez, M.A.; Haselkorn, M.; Iglesias, P. The Lubrication Ability of Ionic Liquids as Additives for Wind Turbine Gearboxes Oils. Lubricants 2016, 4, 14. [Google Scholar] [CrossRef]
- Presilla, R.; Leckner, J.; Glavatskih, S. Grease lubricity in the fretting contact: Are ionic liquids the solution? Tribol. Int. 2023, 185, 108509. [Google Scholar] [CrossRef]
- Mallach, D.; Pape, F.; Lipinsky, D.; Arlinghaus, H.F. ToF-SIMS analysis of boundary layers formed under zinc-free antiwear. Ind. Lubr. Tribol. 2020, 72, 1013–1017. [Google Scholar] [CrossRef]
- Kronberger, M.; Pagano, F.; Pejaković, V.; Igartua, A.; Urbistondo, E.; Kalin, M. Miscibility and tribological investigations of ionic liquids in biodegradable esters. Lubr. Sci. 2014, 26, 463–487. [Google Scholar] [CrossRef]
- González, R.; Viesca, J.L.; Battez, A.H.; Hadfield, M.; Fernández-González, A.; Bartolomé, M. Two phosphonium cation-based ionic liquids as lubricant additive to a polyalphaolefin base oil. J. Mol. Liq. 2019, 293, 111536. [Google Scholar] [CrossRef]
- Rohlmann, P.; Munavirov, B.; Furó, I.; Antzutkin, O.; Rutland, M.W.; Glavatskih, S. Non-halogenated Ionic Liquid Dramatically Enhances Tribological Performance of Biodegradable Oils. Front Chem. 2019, 7, 98. [Google Scholar] [CrossRef]
- Bambam, A.K.; Alok, A.; Rajak, A.; Gajrani, K.K. Tribological performance of phosphonium-based halogen-free ionic liquids as lubricant additives. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2023, 237, 881–893. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, D.; Wong, J.S.; Cai, M. Interactions between ZDDP and an oil-soluble ionic liquid additive. Tribol. Int. 2021, 158, 106938. [Google Scholar] [CrossRef]
- Sanes, J.; Avilés, M.-D.; Saurín, N.; Espinosa, T.; Carrión, F.-J.; Bermúdez, M.-D. Synergy between graphene and ionic liquid lubricant additives. Tribol. Int. 2017, 116, 371–382. [Google Scholar] [CrossRef]
- Nasser, K.I.; del Río, J.M.L.; Lopez, E.R.; Fernandez, J. Synergistic effects of hexagonal boron nitride nanoparticles and phosphonium ionic liquids as hybrid lubricant additives. J. Mol. Liq. 2020, 311, 113343. [Google Scholar] [CrossRef]
- José, M.; del Río, L.; López, E.R.; Fernández, J. Synergy between boron nitride or graphene nanoplatelets and tri(butyl)ethylphosphonium diethylphosphate ionic liquid as lubricant additives of triisotridecyltrimellitate oil. J. Mol. Liq. 2020, 301, 112442. [Google Scholar] [CrossRef]
- Maurya, U.; Vasu, V.; Kashinath, D. Ionic Liquid-Nanoparticle-Based Hybrid-Nanolubricant Additives for Potential Enhancement of Tribological Properties of Lubricants and Their Comparative Study with ZDDP. Tribol. Lett. 2022, 70, 11. [Google Scholar] [CrossRef]
- Sikdar, S.; Rahman, M.H.; Menezes, P.L. Synergistic Study of Solid Lubricant Nano-Additives Incorporated in canola oil for Enhancing Energy Efficiency and Sustainability. Sustainability 2022, 14, 290. [Google Scholar] [CrossRef]
- Seymour, B.T.; Fu, W.; Wright, R.A.; Luo, H.; Qu, J.; Dai, S.; Zhao, B. Improved lubricating performance by combining oil-soluble hairy silica nanoparticles and an ionic liquid as an additive for a synthetic base oil. ACS Appl. Mater. Interfaces 2018, 10, 15129–15139. [Google Scholar] [CrossRef]
- Nasser, K.I.; Liñeira del Río, J.M.; López, E.R.; Fernández, J. Hybrid combinations of graphene nanoplatelets and phosphonium ionic liquids as lubricant additives for a polyalphaolefin. J. Mol. Liq. 2021, 336, 116266. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, M.; Mo, Y.; Bai, P.; Wei, Q.; Jin, L.; You, S.; Wang, M.; Li, L.; Chen, X.; et al. Synergistic lubricating effect of graphene/ionic liquid composite material used as an additive. Friction 2021, 9, 1568–1579. [Google Scholar] [CrossRef]
- Pape, F.; Poll, G. Investigations on Graphene Platelets as Dry Lubricant and as Grease Additive for Sliding Contacts and Rolling Bearing Application. Lubricants 2020, 8, 3. [Google Scholar] [CrossRef]
- Nassef, B.G.; El-Labban, H.; Al-Oufy, A.; Daha, M. Effect of Graphene Addition to Lithium-Based Grease on the Performance of Rolling Element Bearings. Key Eng. Mater. 2022, 913, 279–284. [Google Scholar] [CrossRef]
- Ho, C.Y.; Yusup, S.; Soon, C.V.; Arpin, M.T. Rheological Behaviour of Graphene Nano-sheets in Hydrogenated Oil-based Drilling Fluid. Procedia Eng. 2016, 148, 49–56. [Google Scholar] [CrossRef]
- Hasan, W.; Khan, M.N. Rheological characterization of vegetable oil blends: Effect of shear rate, temperature, and short-term heating. J. Food Process Eng. 2020, 43, e13396. [Google Scholar] [CrossRef]
- Läuger, J.; Pondicherry, K. New Insights into the Use of a Rotational Rheometer as Tribometer. Annu. Trans. Nord. Rheol. Soc. 2017, 25, 1–10. [Google Scholar]
- Amann, T.; Gatti, F.; Li, K.; Demirel, Y.; Kailer, A.; Feng, H.; Yuan, C. Investigation of ionic liquids with and without graphene as lubricant additive for metal/metal and metal/PEEK contacts over a wide temperature range. Lubr. Sci. 2021, 33, 100–111. [Google Scholar] [CrossRef]
- Cao, D.; Gong, S.; Shu, X.; Zhu, D.; Liang, S. Preparation of ZnO Nanoparticles with High Dispersibility Based on Oriented Attachment (OA) Process. Nanoscale Res. Lett. 2019, 14, 210. [Google Scholar] [CrossRef]
- Gavhane, R.S.; Kate, A.M.; Pawar, A.; Safaei, M.R.; Soudagar, M.E.M.; Abbas, M.M.; Ali, H.M.; Banapurmath, N.R.; Goodarzi, M.; Badruddin, I.A.; et al. Effect of Zinc Oxide Nano-Additives and Soybean Biodiesel at Varying Loads and Compression Ratios on VCR Diesel Engine Characteristics. Symmetry 2020, 12, 1042. [Google Scholar] [CrossRef]
- Maher, S.; Nisar, S.; Aslam, S.M.; Saleem, F.; Behlil, F.; Imran, M.; Assiri, M.A.; Nouroz, A.; Naheed, N.; Khan, Z.A.; et al. Synthesis and Characterization of ZnO Nanoparticles Derived from Biomass (Sisymbrium Irio) and Assessment of Potential Anticancer Activity. ACS Omega 2023, 8, 15920–15931. [Google Scholar] [CrossRef]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, characterization, and spectroscopic properties of ZnO nanoparticles. Int. Sch. Res. Notices 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Taufiq, A.; Ulya, H.N.; Utomo, J.; Sunaryono; Hidayat, N.; Susanto, H.; Mufti, N.; Munasir; Soontaranon, S. Structural, Optical, and Antifungal Characters of Zinc Oxide Nanoparticles Prepared by Sol-gel Method. J. Phys. Conf. Ser. 2018, 1093, 012001. [Google Scholar] [CrossRef]
- Kołodziejczak-Radzimska, A.; Markiewicz, E.; Jesionowski, T. Structural Characterisation of ZnO Particles Obtained by the Emulsion Precipitation Method. J. Nanomater. 2012, 2012, 656353. [Google Scholar] [CrossRef]
- Zandi, S.; Kameli, P.; Salamati, H.; Ahmadvand, H.; Hakimi, M. Microstructure and optical properties of ZnO nanoparticles prepared by a simple method. Phys. B Condens. 2011, 406, 3215–3218. [Google Scholar] [CrossRef]
- Xiong, G.; Pal, U.; Serrano, J.; Ucer, K.; Williams, R. Photoluminesence and FTIR study of ZnO nanoparticles: The impurity and defect perspective. Phys. Status Solidi C 2006, 3, 3577–3581. [Google Scholar] [CrossRef]
- Vafaee, M.; Ghamsari, M.S. Preparation and characterization of ZnO nanoparticles by a novel sol–gel route. Mater. Lett. 2007, 61, 3265–3268. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Heris, S.Z.; Estellé, P. Experimental comparison between ZnO and MoS2 nanoparticles as additives on performance of diesel oil-based nano lubricant. Sci. Rep. 2020, 10, 5813. [Google Scholar] [CrossRef]
- Jamaluddin, N.A.; Talib, N.; Sani, A.S.A. Tribological Assessment of Modified Jatropha Oil with hBN and Graphene Nanoparticles as a New Preference for the Metalworking fluid. Int. J. Adv. Eng. Technol. 2020, 9, 3144–3149. [Google Scholar] [CrossRef]
- Yan, Y.D.; Dhont, J.K.G.; Smits, C.; Lekkerkerker, H.N.W. Oscillatory-shear-induced order in nonaqueous dispersions of charged colloidal spheres. Phys. A Stat. Mech. Appl. 1994, 202, 68–80. [Google Scholar] [CrossRef]
- López-Barrón, C.R.; Wagner, N.J.; Porcar, L. Layering, melting, and recrystallization of a close-packed micellar crystal under steady and large-amplitude oscillatory shear flows. J. Rheol. 2015, 59, 793–820. [Google Scholar] [CrossRef]
- Moghaddam, M.B.; Goharshadi, E.K.; Entezari, M.H.; Nancarrow, P. Preparation, characterization, and rheological properties of graphene–glycerol nanofluids. Chem. Eng. J. 2013, 231, 365–372. [Google Scholar] [CrossRef]
- Bao, J.; Heyd, R.; Régnier, G.; Ammar, A.; Peixinho, J. Viscosity of graphene in lubricating oil, ethylene glycol and glycerol. J. Therm. Anal. Calorim. 2023. [Google Scholar] [CrossRef]
- Crawford, N.C.; Popp, L.B.; Johns, K.E.; Caire, L.M.; Peterson, B.N.; Liberatore, M.W. Shear thickening of corn starch suspensions: Does concentration matter? J. Colloid Interface Sci. 2013, 396, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.; Jaeger, H.M. Shear thickening in concentrated suspensions: Phenomenology, mechanisms and relations to jamming. Rep. Prog. Phys. 2014, 77, 046602. [Google Scholar] [CrossRef] [PubMed]
- Chow, M.K.; Zukoski, C.F. Nonequilibrium behavior of dense suspensions of uniform particles: Volume fraction and size dependence of rheology and microstructure. J. Rheol. 1995, 39, 33–59. [Google Scholar] [CrossRef]
- Wei, M.; Lin, K.; Sun, L. Shear thickening fluids and their applications. Mater. Des. 2022, 216, 110570. [Google Scholar] [CrossRef]
- Küçüksönmez, E.; Servantie, J. Shear thinning and thickening in dispersions of spherical nanoparticles. Phys. Rev. E 2020, 102, 012604. [Google Scholar] [CrossRef]
- Sakthivel, S.; Velusamy, S. Effect of ammonium based ionic liquids on the rheological behavior of the heavy crude oil for high pressure and high temperature conditions. Petroleum 2022, 8, 552–566. [Google Scholar] [CrossRef]
- Mozes, R.; Cooper, P.K.; Atkin, R.; Li, H. Ionic Liquids as Grease Base Liquids. Lubricants 2017, 5, 31. [Google Scholar] [CrossRef]
- Burrell, G.L.; Dunlop, N.F.; Separovic, F. Non-Newtonian viscous shear thinning in ionic liquids. Soft Matter 2010, 6, 2080–2086. [Google Scholar] [CrossRef]
- Bossis, G.; Brady, J. The rheology of Brownian suspensions. J. Chem. Phys. 1989, 91, 1866–1874. [Google Scholar] [CrossRef]
- Egres, R.G.; Nettesheim, F.; Wagner, N.J. Rheo-SANS investigation of acicular-precipitated calcium carbonate colloidal suspensions through the shear thickening transition. J. Rheol. 2006, 50, 685–709. [Google Scholar] [CrossRef]
- Hoffman, R.L. Explanations for the cause of shear thickening in concentrated colloidal suspensions. J. Rheol. 1998, 42, 111–123. [Google Scholar] [CrossRef]
- Wittmar, A.; Gautam, D.; Schilling, C.; Dörfler, U.; Mayer-Zaika, W.; Winterer, M.; Ulbricht, M. Stable zinc oxide nanoparticle dispersions in ionic liquids. J. Nanopart. Res. 2014, 16, 2341. [Google Scholar] [CrossRef]
- Xie, M.; Cheng, J.; Huo, C.; Zhao, G. Improving the lubricity of a bio-lubricating grease with the multilayer graphene additive. Tribol. Int. 2020, 150, 106386. [Google Scholar] [CrossRef]
- Beheshti, A.; Huang, Y.; Ohno, K.; Blakey, I.; Stokes, J.R. Improving tribological properties of oil-based lubricants using hybrid colloidal additives. Tribol. Int. 2020, 144, 106130. [Google Scholar] [CrossRef]
- Wittmar, A.; Ruiz-Abad, D.; Ulbricht, M. Dispersions of silica nanoparticles in ionic liquids investigated with advanced rheology. J. Nanopart. Res. 2012, 14, 651. [Google Scholar] [CrossRef]
- Sawada, H.; Kodama, S.; Tsunashima, K.; Sugiya, M. Preparation and properties of novel phosphonium-type ionic liquids/silica gel nanocomposites. J. Mater. Sci. 2007, 42, 2532–2535. [Google Scholar] [CrossRef]
- He, Z.; Alexandridis, P. Nanoparticles in ionic liquids: Interactions and organization. Phys. Chem. Chem. Phys. 2015, 17, 18238–18261. [Google Scholar] [CrossRef]
- Azman, S.S.N.; Zulkifli, N.W.M.; Masjuki, H.; Gulzar, M.; Zahid, R. Study of tribological properties of lubricating oil blend added with graphene nanoplatelets. J. Mater. Res. 2016, 31, 1932–1938. [Google Scholar] [CrossRef]
- Bader, A.; Hoziefa, W.; Hussein Mohammed Abdel, M.; Hamoud, M.; Salunkhe, S.; Abou Bakr, E.; Abdel-Mottaleb, M.; Davim, J.P. Tribological Performance and Rheological Properties of Engine Oil with Graphene Nano-Additives. Lubricants 2022, 10, 137. [Google Scholar] [CrossRef]
- Cai, Z.; Tian, M.; Zhang, G. Experimental Study on the Flow and Heat Transfer of Graphene-Based Lubricants in a Horizontal Tube. Processes 2020, 8, 1675. [Google Scholar] [CrossRef]
- Singh, Y.; Singh, N.K.; Sharma, A.; Singla, A.; Singh, D.; Abd Rahim, E. Effect of ZnO nanoparticles concentration as additives to the epoxidized Euphorbia Lathyris oil and their tribological characterization. Fuel 2021, 285, 119148. [Google Scholar] [CrossRef]
- Bambam, A.K.; Dhanola, A.; Gajrani, K.K. Machining of titanium alloys using phosphonium-based halogen-free ionic liquid as lubricant additives. Ind. Lubr. Tribol. 2022, 74, 722–728. [Google Scholar] [CrossRef]
- Zhao, J.; He, Y.; Wang, Y.; Wang, W.; Yan, L.; Luo, J. An investigation on the tribological properties of multilayer graphene and MoS2 nanosheets as additives used in hydraulic applications. Tribol. Int. 2016, 97, 14–20. [Google Scholar] [CrossRef]
- Del Río, J.M.L.; Guimarey, M.J.; Prado, J.I.; Lugo, L.; López, E.R.; Comuñas, M.J. Improving the tribological performance of a biodegradable lubricant adding graphene nanoplatelets as additives. J. Mol. Liq. 2022, 345, 117797. [Google Scholar] [CrossRef]
- Nassef, M.G.A.; Soliman, M.; Nassef, B.G.; Daha, M.A.; Nassef, G.A. Impact of Graphene Nano-Additives to Lithium Grease on the Dynamic and Tribological Behavior of Rolling Bearings. Lubricant 2022, 10, 29. [Google Scholar] [CrossRef]
- Sarno, M.; Scarpa, D.; Senatore, A.; Ahmed Abdalglil Mustafa, W. rGO/GO Nanosheets in Tribology: From the State of the Art to the Future Prospective. Lubricants 2020, 8, 31. [Google Scholar] [CrossRef]
- Kumar, P.; Wani, M.F. Effect of temperature on the friction and wear properties of graphene nano-platelets as lubricant additive on Al-25Si alloy. Mater. Res. Express 2019, 6, 046513. [Google Scholar] [CrossRef]
- Zhu, Y.; Lin, S.; Wang, H.; Liu, D. Study on the tribological properties of porous sweating PEEK composites under ionic liquid lubricated condition. J. Appl. Polym. Sci. 2014, 131, 40989. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, J.; Cai, C. Tribological Performance of Phosphonium Ionic Liquids as Additives in Lithium Lubricating Grease. Lubricants 2018, 6, 23. [Google Scholar] [CrossRef]
- Elagouz, A.; Ali, M.K.A.; Xianjun, H.; Abdelkareem, M.A.A.; Hassan, M.A. Frictional performance evaluation of sliding surfaces lubricated by zinc-oxide nano-additives. Surf. Eng. 2020, 36, 144–157. [Google Scholar] [CrossRef]
- Bhaumik, S.; Maggirwar, R.; Datta, S.; Pathak, S.D. Analyses of anti-wear and extreme pressure properties of castor oil with zinc oxide nano friction modifiers. Appl. Surf. Sci. 2018, 449, 277–286. [Google Scholar] [CrossRef]
- Hao, L.; Hao, W.; Li, P.; Liu, G.; Li, H.; Aljabri, A.; Xie, Z. Friction and Wear Properties of a Nanoscale Ionic Liquid-like GO@SiO2 Hybrid as a Water-Based Lubricant Additive. Lubricants 2022, 10, 125. [Google Scholar] [CrossRef]
- Murthy, N.; Rai, A.K.; Berkebile, S. Improved Loss-of-Lubrication Performance with Lubricants Containing Nano-Graphene Platelets and Ionic Liquids. Appl. Sci. 2020, 10, 7958. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Xiang, X.; Zheng, G.; Zeng, X.; Li, Z.; Ren, T.; Zhang, Y. Tribological performances of highly dispersed graphene oxide derivatives in vegetable oil. Tribol. Int. 2018, 126, 39–48. [Google Scholar] [CrossRef]
- Somers, A.E.; Howlett, P.C.; MacFarlane, D.R.; Forsyth, M. A Review of Ionic Liquid Lubricants. Lubricants 2013, 1, 3–21. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A review of ionic liquids for green molecular lubrication in nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Carvalho, D.O.A.; da Silva, L.R.R.; Sopchenski, L.; Jackson, M.J.; Machado, Á.R. Performance evaluation of vegetable-based cutting fluids in turning of AISI 1050 steel. Int. J. Adv. Manuf. Technol. 2019, 103, 1603–1619. [Google Scholar] [CrossRef]
- Wasan, D.T.; Nikolov, A.D. Spreading of nanofluids on solids. Nature 2003, 423, 156–159. [Google Scholar] [CrossRef]
- Sefiane, K.; Skilling, J.; MacGillivray, J. Contact line motion and dynamic wetting of nanofluid solutions. Adv. Colloid Interface Sci. 2008, 138, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Quraishi, M.A. Ionic liquids-metal surface interactions: Effect of alkyl chain length on coordination capabilities and orientations. Adv. Electr. Electron. Eng. 2022, 2, 100070. [Google Scholar] [CrossRef]






| Item | Rapeseed Oil | Blaser Vasco 6000 |
|---|---|---|
| Viscosity at 40 °C | 32 mm2/s | 42 mm2/s |
| Viscosity at 100 °C | 7 mm2/s | 8 mm2/s |
| Structure | Fatty acid structure | Water-miscible, Ester oil base, boron, formaldehyde and chlorine-free |
| Flash Point °C | 218 | 129 |
| Sample Designation | Content |
|---|---|
| S1 | Pure rapeseed oil |
| S2 | Rapeseed oil + 0.5 wt.% Gr |
| S3 | Rapeseed oil + 1 wt.% Gr |
| S4 | Rapeseed oil + 2 wt.% Gr |
| S5 | Rapeseed oil + 4 wt.% Gr |
| S6 | Rapeseed oil + 1 wt.% Ionic liquid |
| S7 | Rapeseed oil + 2 wt.% Ionic liquid |
| S8 | Rapeseed oil + 5 wt.% Ionic liquid |
| S9 | Rapeseed oil + 0.05 wt.% ZnO |
| S10 | Rapeseed oil + 0.1 wt.% ZnO |
| S11 | Rapeseed oil + 0.25 wt.% ZnO |
| S12 | Rapeseed oil + 0.5 wt.% ZnO |
| H1 | Rapeseed oil + 1% IL + 4% Gr |
| H2 | Rapeseed oil + 0.1% ZnO + 4% Gr |
| H3 | Rapeseed oil + 1% IL + 0.1% ZnO + 4% Gr |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nassef, B.G.; Pape, F.; Poll, G. Enhancing the Performance of Rapeseed Oil Lubricant for Machinery Component Applications through Hybrid Blends of Nanoadditives. Lubricants 2023, 11, 479. https://doi.org/10.3390/lubricants11110479
Nassef BG, Pape F, Poll G. Enhancing the Performance of Rapeseed Oil Lubricant for Machinery Component Applications through Hybrid Blends of Nanoadditives. Lubricants. 2023; 11(11):479. https://doi.org/10.3390/lubricants11110479
Chicago/Turabian StyleNassef, Belal G., Florian Pape, and Gerhard Poll. 2023. "Enhancing the Performance of Rapeseed Oil Lubricant for Machinery Component Applications through Hybrid Blends of Nanoadditives" Lubricants 11, no. 11: 479. https://doi.org/10.3390/lubricants11110479





